Cancer immunotherapy has shown remarkable progress in recent years. Nanocarriers, such as liposomes, have favorable advantages with the potential to further improve cancer immunotherapy and even stronger immune responses by improving cell type-specific delivery and enhancing drug efficacy. Liposomes can offer solutions to common problems faced by several cancer immunotherapies, including the following: (1) Vaccination: Liposomes can improve the delivery of antigens and other stimulatory molecules to antigen-presenting cells or T cells; (2) Tumor normalization: Liposomes can deliver drugs selectively to the tumor microenvironment to overcome the immune-suppressive state; (3) Rewiring of tumor signaling: Liposomes can be used for the delivery of specific drugs to specific cell types to correct or modulate pathways to facilitate better anti-tumor immune responses; (4) Combinational therapy: Liposomes are ideal vehicles for the simultaneous delivery of drugs to be combined with other therapies, including chemotherapy, radiotherapy, and phototherapy.
- liposome
- drug delivery
- cancer immunotherapy
- immunomodulation
1. The Potential of Immunotherapy for the Treatment of Cancer
Cancer immunotherapy has been widely explored because of its durable and robust effects [1]. Tumors are more than just insular masses consisting of proliferating cancer cells; they have a complex composition built by multiple cell types, which participate in heterotypic interactions with each other [2]. Sustained antitumor responses triggered by immunotherapeutic treatments have been demonstrated via the active stimulation of specific targets such as immune cells, normalization of the tumor microenvironment (TME), and other mechanisms.
1.1. The Generation and Regulation of Tumor Immunity
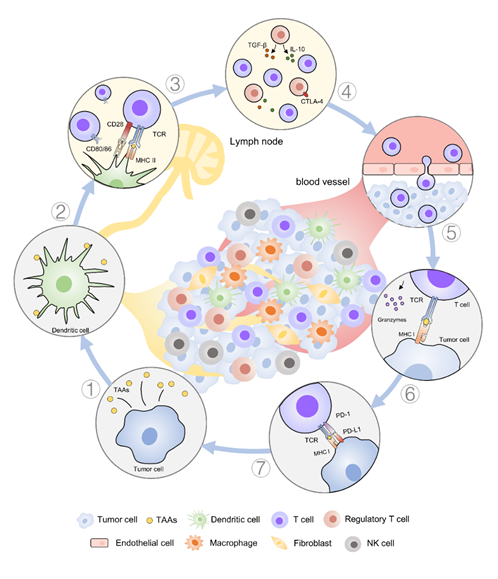
The generation of clinically effective antitumor responses normally requires the successful execution of several immune processes (Figure 1). Firstly, numerous cancer antigens, either tumor-specific or tumor-associated antigens (TAAs), are released during the process of tumor growth. These cancer antigens are phagocytosed, processed, and presented by antigen-presenting cells (APCs) such as dendritic cells (DCs). Then, the cancer antigens can be presented into the major histocompatibility complex (MHC) class II molecules or cross-presented into the MHC class I molecules on DCs that migrate to draining lymph nodes to initiate T cell activation [3]. During this process, DCs mature and costimulatory molecules (such as CD40, CD80, and CD86) are upregulated when specific cues are present, such as damage-associated molecular pattern molecules or pathogen-associated molecular pattern molecules present in the TME or provided by means of treatment. Upon maturation, DCs remodel their membranes to form dendrites to increase the membrane surface area and enhance T cell interactions [4]. Accordingly, higher numbers of DCs present in the TME are beneficial and can improve T cell activation [5,6][5][6].
Figure 1. Overview of a typical build-up of an immune response against cancer cells in solid tumors. (1) Cancer cells can release tumor-associated antigens (TAAs) in the tumor microenvironment (TME). (2) Anti-tumor response is initiated with the recognition of TAAs presented by antigen-presenting cells (APCs) such as dendritic cells (DCs). (3) The cognate T cell receptor (TCR) binds to major histocompatibility complex (MHC) I/II receptor containing the epitope peptide from TAAs. The priming of T cells generally occurs in lymphoid tissue. (4) During priming, T cells are susceptible to immune negative/positive factors that prevent/promote their full activation mediated by cytokines (e.g., transforming growth factor-β (TGF-β) and interleukin 10 (IL-10)) and costimulatory receptors, such as cytotoxic T-lymphocyte-associated protein 4 (CTLA-4). (5) Once activated successfully, effector T cells proliferate, secrete inflammatory cytokines, acquire cytolytic properties, and migrate to tumor sites. (6) Cytotoxic T cells can identify cancer cells and bind to cognate cancer antigens presented on MHC I on cancer cells and initiate T cell-mediated killing (e.g., release granzymes). (7) T cell function can also be stimulated or inhibited in the tumor. Negative costimulatory signals (e.g., programmed cell death protein 1 ligand 1, PD-L1) inhibit the function of T cells and induce anergy and the exhaustion of T cells.
Next, productive T cell responses are generated in lymphoid organs [7]. During this process, tolerance can still be promoted by regulatory T cells (Treg), and inhibitory receptors would oppose anti-tumor efficacy. As the potential site for therapeutic intervention, stimulatory adjuvants can be used to skew the magnitude and type of T cell response. Agonistic antibodies to secondary immune checkpoint molecules such as 4-1BB, OX40, and glucocorticoid-induced TNFR-related protein could amplify anti-tumor immune responses.
Once activated, effector T cells must migrate to the tumor site and infiltrate the TME to perform their killing job. Here, negative regulatory signals that dampen T cell activation or induce anergy and exhaustion must be avoided as much as possible [8]. Typically, cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed cell death protein 1 (PD-1) expressed on activated T cells are major suppressive costimulatory molecules, and therapeutic disruption with antagonistic antibodies has shown strong therapeutic potential [9,10][9][10]. Inside the abnormal TME, tumor populations, stromal cells, and multitudes of innate and adaptive immune cells together build up a complicated network to help tumor escape immune attacks through a variety of mechanisms [11–14][11][12][13][14]. Hence, an interesting strategy is to augment the anti-tumor immune response to overcome diverse immunosuppressive signals, which may be driven by both suppressive mediators and regulatory cell populations [15,16][15][16]. In this review, we have summarized the therapeutic strategies of immunomodulation in recent years and discuss the different mechanisms used to intervene with tumor immunity through the application of liposome technology.
1.2. Recent Development of Cancer Immunotherapy
Immunotherapy refers to the approach to treat cancer through generating or regulating an immune response against it [1]. Recently, harnessing immunotherapy has been a fundamental strategy in cancer therapy. In last two decades, various types of immunotherapies were developed to improve anti-tumor response through the modulation of stimulatory, suppressive, or regulatory mechanisms. These strategies include vaccines, monoclonal antibodies, immunomodulatory small molecules, as well as the exploration of the immunomodulatory functions of chemotherapy and radiation therapy [17,18][17][18]. In order to generate a successful and powerful immune response, cancer vaccine is normally given to enhance the immune system. In accordance with the immune response steps against cancer, this approach focuses on (1) enhancing antigen uptake, processing and presentation to T cells, and hence enhancing the activation and expansion of naïve T cells, e.g., antigen/adjuvant vaccines or cytokines that promote APC functions; and (2) intensifying the effector phase of immune responses, such as infusing back ex vivo stimulated and expanded tumor infiltrate T cells to patients. It is noted that this strategy shows great supply to the immune activation process in patients, but it might also push the immune system to a supraphysiological level with an increased risk of immune-related adverse events [19]. For antibody-targeted therapy, various strategies including using antibodies to target cancer directly, altering the host response to cancer, and delivering cytotoxic substances to cancer have been investigated. Oncologists now see monoclonal antibody (mAb)-based cancer therapies as a vital component of state-of-the-art cancer care; one typical example is using mAbs to block B7-H1 and PD-1 interactions [20]. There are arrays of molecular pathways that cause immune defects in the TME that can be targeted to reset or reprogram anti-tumor immunity. Molecular entities and the mechanisms of these pathways could be potential targets for cancer immunotherapy and provide an alternative for patients that cannot benefit from current immunotherapy [21].
2. The Emergence of Liposomes as Drug Delivery Vehicles in Cancer Immunotherapy
The increasing research on the applicability of nanotechnology in cancer therapy is based on its unique hallmarks from the fields of drug delivery, diagnosis, and imaging [22]. Nanocarriers that incorporate these features are very promising for clinical applications, and a variety of them have been explored in (pre) clinical research stages. There are several different types of nanocarriers including liposomes, polymer micelles, inorganic nanoparticles, drug conjugates, and virus-like nanoparticles, which have been used for enhancing the drug delivery of chemotherapeutics, radiation therapy, gene therapy, and immunotherapy. Along with the enormous progress achieved in the field of immun otherapy, nanotechnology-based immunotherapy has gradually displayed potential to improve the limitation associated with therapeutic monoclonal antibodies, tyrosine kinase inhibitors, and cancer vaccines [23–25][23][24][25]. It has distinctive features such as improving drug efficacy, reducing toxicity, better physicochemical properties, the capacity to deliver macromolecular drugs, and the ability to bypass tumor-driven resistance mechanisms [26–28][26][27][28]. One such nanotechnology-based system for cancer immunotherapy is liposomes. Liposomal formulations have been used in many clinical trials [29] for different purposes (e.g., for cancer targeting and vaccination). One of these formulations is being used in the clinic for cancer treatment (i.e., Doxil®).
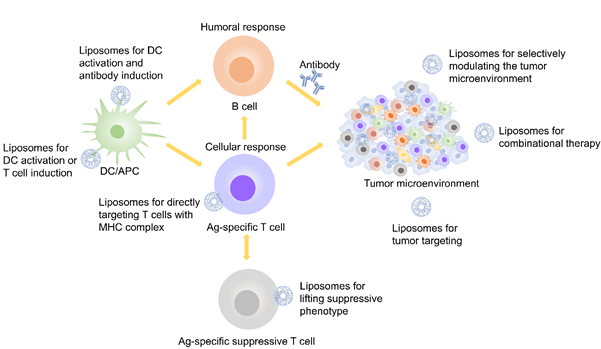
Liposomes are lipid-based nanoparticles with high potential to improve cancer immunotherapies, since they can incorporate and/or associate a high variety of cancer drug molecules (e.g., peptides, proteins, antibodies, low-molecular weight chemotherapeutics) [30,31][30][31]. Liposomes are very versatile because they can be used for different kinds of immunotherapeutic cancer treatments (e.g., vaccination and checkpoint blockade), as Figure 2 showed [32]. They are popular platforms for the controlled release of antigens, immunomodulators, and low-molecular-weight anti-cancer drugs [33]. The usage of liposomal-based drug delivery systems based in immunotherapy can be grouped into five different categories (Figure 3): (1) Vaccination: harnessing liposomes for the coordinated delivery of antigens and other stimulatory molecules to APCs or T cells, which employs the power of modern nanotechnology and yields improved outcomes as compared to conventional tumor antigen vaccination; (2) Tumor normalization: overcoming tumor-driven immunosuppressive signals (e.g., checkpoint blockade) in the TME by liposomes to improve selectivity and decrease systemic toxicity, which provides preliminary evidence of efficacy; (3) Tumor modulation: correcting or modulating an existing or known pathway during the development of the anti-tumor response; (4) Tumor targeting: targeting overexpressed surface molecules on cancer cells (may also be self-antigens) via B cell/antibody route or cancer-specific peptides presented on MHC-I on the cancer cells via Ag-specific T-cells, especially cytotoxic T-cells; and (5) Combinational therapy: exploring the combinational strategies between immunotherapy and others (e.g., chemotherapy, radiotherapy and phototherapy et al.), which provides opportunities for liposomes to co-load molecules with different properties.
Liposomes, in particular polyethylene glycol (PEG)ylated liposomes, tend to passively accumulate in tumor tissue via the enhanced permeability and retention (EPR) effect [34–36][34][35][36]. Conjugating a hydrophilic polymer to the surface of liposome reduces opsonization and clearance by the reticuloendothelial system [37]. The surfaces of liposomes are often modified with antibodies or specific receptor ligands to increase the binding of the liposomes to the target cells, which is regarded as a promising strategy for cancer treatment [38–41][38][39][40][41]. Liposome-mediated immunotherapy can be potentially used to mediate efficient delivery to target sites and provoke robust immune responses [42–44][42][43][44]. Since the tumor immunity plays such an important role in tumor development, progression, and metastasis, it offers opportunities for liposomes to improve the efficiency of cancer treatment. The continued development of liposomes is one of the essential aspects of the pursuit of safe and effective cancer immunotherapy.
In spite of improved biodistribution and tumor accumulation, there are also certain issues that need to be addressed to get the maximum benefit from current liposomal platforms. As the complexity of liposomes increases, so do the expenses and difficulties associated with their preparation and quality control. The physicochemical properties of liposomes, including their size, charge, polarity, and any modifications, may also have a negative impact on the ability of the liposomes to reach the tumor via the EPR effect [45,46][45][46]. For example, PEGylated liposomes bigger than 500 nm in diameter are rapidly removed from the blood by optimization. Although liposomes have emerged as a promising approach to overcome the limitations in current cancer treatment and have shown high efficiency in multiple animal models, they might not be sufficient when employed in cancer patients. Unlike in many murine cancer models, a considerable barrier resulting from imperfect or inefficient EPR effect contributes to limit the concentration, penetration, and distribution of liposomes in human tumors [47]. Liposomes should be designed and characterized on the basis of their interactions with complex transport barriers located in the TME [48]. In addition, it is also crucial to improve strategies to achieve strong antitumor effects while minimizing toxicity to normal cells. Herein, we assess the current status based on the current studies in the literature that have focused on liposome-mediated immunotherapy and immunomodulation, and we summarize the recently proposed strategies to overcome the limited immune responses and low efficacy-related issues in this field.
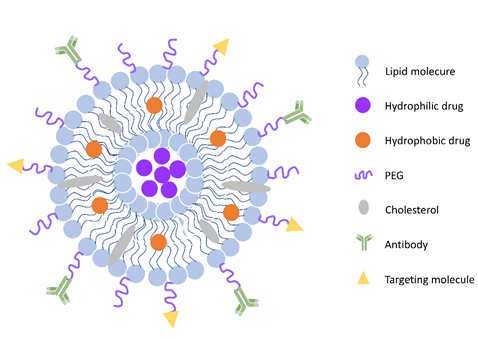
Figure 2. General scheme of liposomes. Liposomes are spherical vesicles with a hydrophilic core formed by a phospholipid and cholesterol bilayer. They can also be modified with polyethylene glycol (PEG) coating for long circulation and various molecules (peptides, antibodies, et al.) for targeting.
Figure 3. Liposome-based treatment used in immunotherapy.
References
- Khalil, D.N.; Smith, E.L.; Brentjens, R.J.; Wolchok, J.D. The future of cancer treatment: Immunomodulation, CARs and combination immunotherapy. Nat. Rev. Clin. Oncol. 2016, 13, 273–290.
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674.
- Steinman, R.M.; Mellman, I. Immunotherapy: Bewitched, bothered, and bewildered no more. Science 2004, 305, 197–200.
- Mellman, I.; Steinman, R.M. Dendritic cells: Specialized and regulated antigen processing machines. Cell 2001, 106, 255–258.
- Goc, J.; Germain, C.; Vo-Bourgais, T.K.D.; Lupo, A.; Klein, C.; Knockaert, S.; De Chaisemartin, L.; Ouakrim, H.; Becht, E.; Alifano, M.; et al. Dendritic cells in tumor-associated tertiary lymphoid structures signal a th1 cytotoxic immune contexture and license the positive prognostic value of infiltrating CD8+ t cells. Cancer Res. 2014, 74, 705–715.
- Ladányi, A.; Kiss, J.; Somlai, B.; Gilde, K.; Fejős, Z.; Mohos, A.; Gaudi, I.; Tímár, J. Density of DC-LAMP + mature dendritic cells in combination with activated T lymphocytes infiltrating primary cutaneous melanoma is a strong independent prognostic factor. Cancer Immunol. Immunother. 2007, 56, 1459–1469.
- Palucka, K.; Banchereau, J.; Mellman, I. Designing vaccines based on biology of human dendritic cell subsets. Immunity 2010, 33, 464–478.
- Keir, M.E.; Butte, M.J.; Freeman, G.J.; Sharpe, A.H. PD-1 and Its Ligands in Tolerance and Immunity. Annu. Rev. Immunol. 2008, 26, 677–704.
- Hodi, F.S.; Butler, M.; Oble, D.A.; Seiden, M.V.; Haluska, F.G.; Kruse, A.; MacRae, S.; Nelson, M.; Canning, C.; Lowy, I.; et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc. Natl. Acad. Sci. USA 2008, 105, 3005–3010.
- Li, B.; Vanroey, M.; Wang, C.; Chen, T.H.T.; Korman, A.; Jooss, K. Anti-programmed death-1 synergizes with granulocyte macrophage colony-stimulating factor-secreting tumor cell immunotherapy providing therapeutic benefit to mice with established tumors. Clin. Cancer Res. 2009, 15, 1623–1634.
- Tripathi, M.; Billet, S.; Bhowmick, N.A. Understanding the role of stromal fibroblasts in cancer progression. Cell Adhes. Migr. 2012, 6, 231–235.
- Watnick, R.S. The role of the tumor microenvironment in regulating angiogenesis. In Biomarkers of the Tumor Microenvironment; Springer: Cham, Switzerland, 2017; pp. 3–23.
- Gajewski, T.F.; Schreiber, H.; Fu, Y.X. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 2013, 14, 1014–1022.
- Furuta, S.; Jeng, Y.M.; Zhou, L.; Huang, L.; Kuhn, I.; Bissell, M.J.; Lee, W.H. IL-25 causes apoptosis of IL-25R-expressing breast cancer cells without toxicity to nonmalignant cells. Sci. Transl. Med. 2011, 3, 78ra31.
- Shin, J.Y.; Yoon, I.H.; Kim, J.S.; Kim, B.; Park, C.G. Vascular endothelial growth factor-induced chemotaxis and IL-10 from T cells. Cell. Immunol. 2009, 256, 72–78.
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009, 9, 162–174.
- Burugu, S.; Dancsok, A.R.; Nielsen, T.O. Emerging targets in cancer immunotherapy. Semin. Cancer Biol. 2018, 52, 39–52.
- Gül, N.; Van Egmond, M. Antibody-dependent phagocytosis of tumor cells by Macrophages: A Potent effector mechanism of monoclonal antibody therapy of cancer. Cancer Res. 2015, 75, 5008–5013.
- Sanmamed, M.F.; Chen, L. A Paradigm Shift in Cancer Immunotherapy: From Enhancement to Normalization. Cell 2018, 175, 313–326.
- Chen, L.; Han, X. Anti–PD-1 / PD-L1 therapy of human cancer : Past , present , and future Find the latest version : Anti–PD-1/PD-L1 therapy of human cancer: Past , present , and future. J. Clin. Invest. 2015, 125, 3384–3391.
- Tarhini, A.A.; Kirkwood, J.M.; Gooding, W.E.; Cai, C.; Agarwala, S.S. Durable complete responses with high-dose bolus interleukin-2 in patients with metastatic melanoma who have experienced progression after biochemotherapy. J. Clin. Oncol. 2007, 25, 3802–3807.
- Shi, J.; Votruba, A.R.; Farokhzad, O.C.; Langer, R. Nanotechnology in drug delivery and tissue engineering: From discovery to applications. Nano Lett. 2010, 10, 3223–3230.
- Hu, Q.; Sun, W.; Wang, C.; Gu, Z. Recent advances of cocktail chemotherapy by combination drug delivery systems. Adv. Drug Deliv. Rev. 2016, 98, 19–34.
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37.
- Gu, Z.; Chang, M.; Fan, Y.; Shi, Y.; Lin, G. NGR-modified pH-sensitive liposomes for controlled release and tumor target delivery of docetaxel. Colloids Surf. B Biointerfaces 2017, 160, 395–405.
- Linton, S.S.; Sherwood, S.G.; Drews, K.C.; Kester, M. Targeting cancer cells in the tumor microenvironment: Opportunities and challenges in combinatorial nanomedicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 208–222.
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal formulations in clinical use: An updated review. Pharmaceutics 2017, 9, 1–33.
- Gao, A.; Hu, X.L.; Saeed, M.; Chen, B.F.; Li, Y.P.; Yu, H.J. Overview of recent advances in liposomal nanoparticle-based cancer immunotherapy. Acta Pharmacol. Sin. 2019, 40, 1129–1137.
- Zununi Vahed, S.; Salehi, R.; Davaran, S.; Sharifi, S. Liposome-based drug co-delivery systems in cancer cells. Mater. Sci. Eng. C 2017, 71, 1327–1341.
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48.
- Allen, T.M.; Cullis, P.R. Drug Delivery Systems: Entering the Mainstream. Science 2004, 303, 1818–1822.
- Maruyama, K. Intracellular targeting delivery of liposomal drugs to solid tumors based on EPR effects. Adv. Drug Deliv. Rev. 2011, 63, 161–169.
- Fang, J.; Nakamura, H.; Maeda, H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv. Drug Deliv. Rev. 2011, 63, 136–151.
- Ozcelikkale, A.; Ghosh, S.; Han, B. Multifaceted transport characteristics of nanomedicine: Needs for characterization in dynamic environment. Mol. Pharm. 2013, 10, 2111–2126.
- Otsuka, H.; Nagasaki, Y.; Kataoka, K. PEGylated nanoparticles for biological and pharmaceutical applications. Adv. Drug Deliv. Rev. 2003, 55, 403–419.
- Iyer, A.K.; Su, Y.; Feng, J.; Lan, X.; Zhu, X.; Liu, Y.; Gao, D.; Seo, Y.; Vanbrocklin, H.F.; Broaddus, V.C.; et al. Biomaterials The effect of internalizing human single chain antibody fragment on liposome targeting to epithelioid and sarcomatoid mesothelioma. Biomaterials 2011, 32, 2605–2613.
- Guo, C.; Chen, Y.; Gao, W.; Chang, A.; Ye, Y.; Shen, W.; Luo, Y. Liposomal Nanoparticles Carrying anti-IL6R Antibody to the Tumour Microenvironment Inhibit Metastasis in Two Molecular Subtypes of Breast Cancer Mouse Models. Theranostics 2017, 7, 775.
- Feng, B.; Tomizawa, K.; Michiue, H.; Han, X.; Miyatake, S. Development of a bifunctional immunoliposome system for combined drug delivery and imaging in vivo. Biomaterials 2010, 31, 4139–4145.
- Moles, E.; Urbán, P.; Jiménez-díaz, M.B.; Viera-morilla, S.; Angulo-barturen, I. Immunoliposome-mediated drug delivery to Plasmodium-infected and non-infected red blood cells as a dual therapeutic/prophylactic antimalarial strategy. J. Control. Release 2015, 210, 217–229.
- Schmidt, S.T.; Foged, C.; Korsholm, K.S.; Rades, T.; Christensen, D. Liposome-based adjuvants for subunit vaccines: Formulation strategies for subunit antigens and immunostimulators. Pharmaceutics 2016, 8, 1–22.
- Watson, D.S.; Endsley, A.N.; Huang, L. Design considerations for liposomal vaccines: Influence of formulation parameters on antibody and cell-mediated immune responses to liposome associated antigens. Vaccine 2012, 30, 2256–2272.
- Landesman-Milo, D.; Peer, D. Altering the immune response with lipid-based nanoparticles. J. Control. Release 2012, 161, 600–608.
- Maeda, H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv. Drug Deliv. Rev. 2015, 91, 3–6.
- Ojha, T.; Pathak, V.; Shi, Y.; Hennink, W.E.; Moonen, C.T.W.; Storm, G.; Kiessling, F.; Lammers, T. Pharmacological and physical vessel modulation strategies to improve EPR-mediated drug targeting to tumors. Adv. Drug Deliv. Rev. 2017, 119, 44–60.
- Chauhan, V.P.; Jain, R.K. Strategies for advancing cancer nanomedicine. Nat. Mater. 2013, 12, 958–962.
- Danhier, F. To exploit the tumor microenvironment: Since the EPR effect fails in the clinic, what is the future of nanomedicine? J. Control. Release 2016, 244, 108–121.
- Gu, Z.; Shaikh, A.S.; Lin, G. Novel “Stereoscopic Response” Strategy Can Be Used in Combination Therapy. Crit. Rev. Ther. Drug Carr. Syst. 2018, 35, 369–390.
- Blattman, J.N.; Greenberg, P.D. Cancer immunotherapy: A treatment for the masses. Science 2004, 305, 200–205.