Recent scientific developments in understanding the lifespan of the corpus luteum provided new insights into dynamic molecular changes occurring during transition of this fascinating endocrine gland into the organ supporting pregnancy. Processes such as oocyte-sperm interaction, preparation of the uterus for implantation, blastocyst attachment, and successful gestation are mainly driven by progesterone, a steroid hormone produced by the corpus luteum. Inadequate production of progesterone has been described in several mammals, including humans, as luteal phase deficiency, a condition in which endogenous progesterone is insufficient to support pregnancy. Thus, it is essential to extend knowledge about the molecular mechanisms controlling the function of the corpus luteum. Unfortunately, still there is a lack of data explaining the regulation of core molecules responsible for the maintenance of luteal function. Recent studies shed a new light on the molecular mechanisms supporting luteal function, involving microRNAs.
- corpus luteum
- microRNA
- progesterone
- estradiol
- NR4A1
- pregnancy
- corpus luteum regression
- corpus luteum maintenance
1. Introduction
The fundamental role of miRNAs in ovarian function was proved by knockout of DICER, which inhibited follicle growth, reduced the ovulation rate and led to faulty oocyte development [[1][2][3]]. Further studies showed a number of differentially expressed miRNAs in the bovine and ovine corpus luteum (CL) during development, regression, or its rescue during pregnancy [[4][5][6][7][8][9]]. Interestingly, a greater amount of miRNAs was found in the mature than in the developing CL, indicating their potential role in the maintenance of luteal function. Nevertheless, not many studies have provided information on the roles of specific miRNA–mRNA interactions on the function of luteal cells, while only a few have shown changes in the expression of miRNAs occurring in the CL during early pregnancy [[10][7]].
The fundamental role of miRNAs in ovarian function was proved by knockout of DICER, which inhibited follicle growth, reduced the ovulation rate and led to faulty oocyte development [1,2,3]. Further studies showed a number of differentially expressed miRNAs in the bovine and ovine corpus luteum (CL) during development, regression, or its rescue during pregnancy [4,5,6,7,8,9]. Interestingly, a greater amount of miRNAs was found in the mature than in the developing CL, indicating their potential role in the maintenance of luteal function. Nevertheless, not many studies have provided information on the roles of specific miRNA–mRNA interactions on the function of luteal cells, while only a few have shown changes in the expression of miRNAs occurring in the CL during early pregnancy [10,7].Recently, 14 differentially expressed miRNAs were identified in porcine CL collected right after maternal recognition of pregnancy (day 14 of pregnancy) and luteolysis (day 14 of the estrous cycle) [[10]]. Overall, seven miRNAs were upregulated (
Recently, 14 differentially expressed miRNAs were identified in porcine CL collected right after maternal recognition of pregnancy (day 14 of pregnancy) and luteolysis (day 14 of the estrous cycle) [10]. Overall, seven miRNAs were upregulated (e.g.
, miR-21a-3p, miR-345-3p, miR-371-5p), and seven miRNAs were downregulated (e.g.
, miR-181a, miR-532-3p, miR-99b) in the porcine CL on day 14 of the estrous cycle compared to the corresponding day of pregnancy. Interestingly, among miRNA targets upregulated during pregnancy, genes encoding known regulators of luteolysis were found (e.g.
,EDN1
,FOS
,JUN
,PTGS2
,ESR2) [[11][12][13][14]], while miRNAs elevated in the CL during luteolysis could target genes associated with luteal function maintenance (
) [11,12,13,14], while miRNAs elevated in the CL during luteolysis could target genes associated with luteal function maintenance (e.g.
,PGR
,VEGFR1
,CREB
,PTGER2
). Furtherin silico
analysis suggested that miRNAs highly expressed in the CL during early pregnancy can be involved in the cell cycle, cell death and survival, cellular development, growth, and proliferation—events characterizing the time when a decision is made either to regress or maintain the luteal function. Notably, the expression of miRNAs was examined in the whole luteal tissue. Therefore, the aforementioned biological processes can be potentially regulated by differentially expressed miRNAs in various cells forming the porcine CL, such as the endothelial cells, immune cells, or fibroblasts, and not only the luteal cells. Other studies performed on the bovine CL identified 15 miRNAs differentially expressed on day 18 of pregnancyvs. the corresponding day of the estrous cycle, while among predicted targets of these miRNAs, genes involved in immune-related events and apoptosis were found [[7]].
the corresponding day of the estrous cycle, while among predicted targets of these miRNAs, genes involved in immune-related events and apoptosis were found [7].Previously, the high expression of two clusters, miR-183-96-182 and miR-212-132, was noticed in the luteal
Previously, the high expression of two clusters, miR-183-96-182 and miR-212-132, was noticed in the lutealvs. follicular tissue of cows, while miR-96 was found to be an important regulator of steroidogenesis and cell survival [[9]]. In pigs, miRNAs upregulated in the CL of pregnant animals belong to three independent clusters: miR-99b, miR-532, and miR-181a [[10]]. Again, among targets of miRNAs occurring in the same cluster (
follicular tissue of cows, while miR-96 was found to be an important regulator of steroidogenesis and cell survival [9]. In pigs, miRNAs upregulated in the CL of pregnant animals belong to three independent clusters: miR-99b, miR-532, and miR-181a [10]. Again, among targets of miRNAs occurring in the same cluster (i.e.
, miR-532 and miR-99b), crucial regulators of luteolysis were found, including, for example,EDN1
,FOS
,JUN
,NR4A1
,OXTR
, andPTGS2 [[11][12][13][14]]. Interestingly, identified clusters are conserved or broadly conserved among different animal species and humans; thus, it is possible that some of them can support luteal function in other species as well, especially since miRNAs belonging to cluster miR-99b were previously detected among mostly abundant miRNAs in the mature bovine CL [[8]].
[11,12,14]. Interestingly, identified clusters are conserved or broadly conserved among different animal species and humans; thus, it is possible that some of them can support luteal function in other species as well, especially since miRNAs belonging to cluster miR-99b were previously detected among mostly abundant miRNAs in the mature bovine CL [8].2. Influence and application
Luteotrophic and/or luteolytic factors were suggested to be effective modulators of miRNA expression [[9][15]]. In pigs, incubation of luteal tissue slices with estradiol-17beta (E2) stimulated the expression of miRNAs belonging to the miR-99b cluster [[10]]. Further, bioinformatics analysis showed multiple SP1 and estrogen receptor 1 (ESR1) binding sites in the promoter of the miR-99b cluster. SP1 is a zinc finger transcription factor that acts synergistically with ESR1, one of the genomic receptors of estradiol, in the regulation of gene transcription. Therefore, we suggest that E2 increases the expression of miR-99b by the ESR1-SP1 pathway in the CL during early pregnancy. Remarkably, the aforementioned effects of E2 on the expression of the miRNA-99b cluster were concomitant with the inhibitory effect of E2 on the expression of
Luteotrophic and/or luteolytic factors were suggested to be effective modulators of miRNA expression [9,15]. In pigs, incubation of luteal tissue slices with estradiol-17beta (E2) stimulated the expression of miRNAs belonging to the miR-99b cluster [10]. Further, bioinformatics analysis showed multiple SP1 and estrogen receptor 1 (ESR1) binding sites in the promoter of the miR-99b cluster. SP1 is a zinc finger transcription factor that acts synergistically with ESR1, one of the genomic receptors of estradiol, in the regulation of gene transcription. Therefore, we suggest that E2 increases the expression of miR-99b by the ESR1-SP1 pathway in the CL during early pregnancy. Remarkably, the aforementioned effects of E2 on the expression of the miRNA-99b cluster were concomitant with the inhibitory effect of E2 on the expression ofNR4A1
andAKR1C1 genes in luteal tissue slices [[10]]. NR4A1 is encoded by immediate-early response genes, which can induce apoptosis or elevate transcription of AKR1C1, an enzyme responsible for the metabolism of progesterone [[16][17]]. In addition, NR4A1 was identified as a marker of luteolysis in the CL of cows and rats [[16][17][18]]. Our further studies showed that the transfection of luteal tissue slices with mimics of miRNAs belonging to the miR-99b cluster decreases the expression of
genes in luteal tissue slices [10]. NR4A1 is encoded by immediate-early response genes, which can induce apoptosis or elevate transcription of AKR1C1, an enzyme responsible for the metabolism of progesterone [16,17]. In addition, NR4A1 was identified as a marker of luteolysis in the CL of cows and rats [16,17,18]. Our further studies showed that the transfection of luteal tissue slices with mimics of miRNAs belonging to the miR-99b cluster decreases the expression ofNR4A1
andAKR1C1 genes, and increases the production of progesterone by luteal tissue slices of pigs [[10]]. Therefore, it seems likely that E2 can induce the expression of the miR-99b cluster in porcine CL during the peri-implantation period, leading to decreased expression of genes involved in luteolysis (Figure 1).
genes, and increases the production of progesterone by luteal tissue slices of pigs [10]. Therefore, it seems likely that E2 can induce the expression of the miR-99b cluster in porcine CL during the peri-implantation period, leading to decreased expression of genes involved in luteolysis (Figure 1).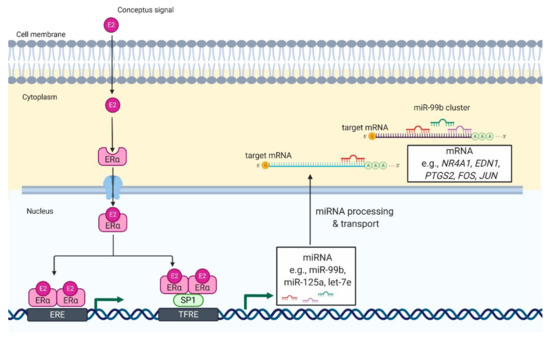
Figure 1.
Proposed model of E2-mediated regulation of miRNA expression in luteal tissue during early pregnancy in pigs. E2 enters the luteal cell and binds with its genomic receptor, ERα
, located in the cytoplasm. It leads to formation of ERα homodimers and their translocation to the nucleus. In the nucleus, ERα homodimers bind to (1) an estrogen-responsive element (ERE) or (2) transcription factor SP1, which binds to transcriptional factor responsive elements (TFRE) in the promoter region of miR-99b cluster. As a result, there is an increase in the expression of miRNAs belonging to miR-99b cluster (miR-99b, miR-125a, and let-7e), which can target mRNA involved in luteolysis including NR4A1, nuclear receptor subfamily 4 group A member 1; EDN1, endothelin 1; FOS, fos proto-oncogene; JUN, jun proto-oncogene. That mechanisms support the function of luteal cells and allow continuation of progesterone production required for pregnancy establishment and maintenance.
homodimers and their translocation to the nucleus. In the nucleus, ERα homodimers bind to (1) an estrogen-responsive element (ERE) or (2) transcription factor SP1, which binds to transcriptional factor responsive elements (TFRE) in the promoter region of miR-99b cluster. As a result, there is an increase in the expression of miRNAs belonging to miR-99b cluster (miR-99b, miR-125a, and let-7e), which can target mRNA involved in luteolysis including NR4A1, nuclear receptor subfamily 4 group A member 1; EDN1, endothelin 1; FOS, fos proto-oncogene; JUN, jun proto-oncogene. That mechanisms support the function of luteal cells and allow continuation of progesterone production required for pregnancy establishment and maintenance.