Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Peter Tang and Version 1 by João Coelho.
Personal digital devices, emitting high-energy light, namely in the blue wavelength, have raised concerns about possible harmful effects on users’ eyes. Scientific research history has shown a relationship between exposure to blue light and changes in ocular structures.
- cornea
- lens
- refractive development
- light-emitting diode (LED)
- AMD
- digital device
- photoreceptors
- blue light
- retina damage
1. Introduction
Eye exposure to artificial blue radiation has increased with the frequent use of digital devices. COVID-19 and mandatory confinement accelerated this trend, modifying work, studying and socialization habits. Smartphone users have increased worldwide by 70%, while laptop computer users have increased by 40% since the beginning of the pandemic [1]. Bahkir et al. reported that 94% of users increased their average screen time from 4.8 to 8.6 h a day during lockdown [2]. The Global Digital Report 2023 [3] reveals that users spend more time using devices online. Research reveals that internet users spend around 7 h a day on all devices. The time spent online has increased and the daily average has grown by around 4 min per day (+1.0%) compared to 2021.
The duality of blue radiation means that this light can have both a negative and positive impact on human eyes. Thus, according to the UNE EN/IEC 62471, the standard classification for photobiological safety, visible light to the human visual system is between 380 nm and 780 nm [4] and blue radiation is contained between the range of 380–495 nm, depending on the reference documents [5]. This radiation is relevant for adequate vision performance and for some physiological processes’ balance. Human vision and daily biological rhythms evolve with sunlight, the greatest natural blue radiation source with the shortest wavelength and highest frequency within the visible spectrum. When sunlight passes through the atmosphere, it causes an oscillation of the particles that scatter the light, proportionally to the speed of acceleration of these particles. In this interaction called scattering, light is completely absorbed and then re-emitted. The light is scattered by particles much smaller than its wavelength (Rayleigh’s phenomenon). When passing through the atmosphere, the particles oscillate much more towards blue frequencies than towards red frequencies, and this is the reason for the blue perception of the sky [6].
Digital devices that use LED technology, as well as other sources of artificial light, expose their users to digital artificial blue radiation daily. This can be potentially harmful to the human eye due to the proximity of the ultraviolet spectrum, namely to the retina, due to its higher-energy wavelengths and high potential to alter ocular tissues [7]. Light is detected by the human eye, which sends the information received by the brain through the visual pathways. Specialized photoreceptors of the retina cells, cones and rods, responsible for part of the formation of images, respond to different wavelengths. The S, M and L cones contain proteins with photosensitive photopigments (Figure 1) of maximum sensitivity in the blue (S cones), green (M cones) and red (L cones) regions, corresponding to peaks of around 420 nm, 530 nm and 560 nm, respectively [8].
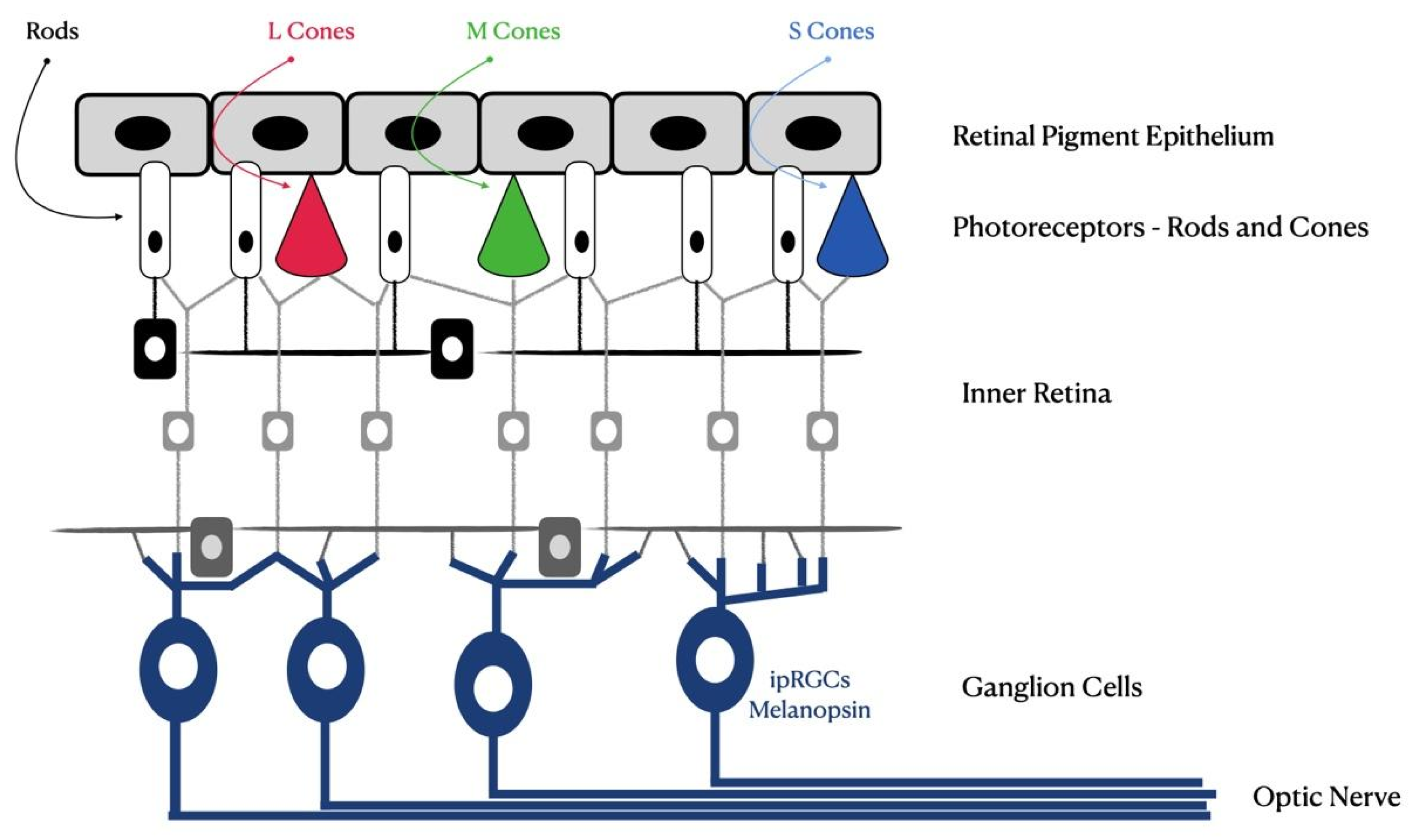
Figure 1.
Schematic of the human retina. Photoreceptors, rods, cones and newly discovered intrinsically photosensitive retinal ganglion cells (ipRGCs) are shown.
In the retinal photoexcitation process, the electrical properties of photopigments are altered, triggering a biochemical process that informs the brain about light. At the end of retinal phototransduction, visual sensations occur, which are produced by impulses that reach regions of the primary visual cortex; however, these sensations are secondary to the primary functions of photoreceptors (cones and rods) that carry light information to retinal ganglion cells and the lateral geniculate body of the thalamus [8].
Rods, another type of photoreceptor cell in the retina, are responsible for black-and-white (or monochromatic) vision and are sensitive to light wavelengths of around 500 nm [9]. In the posterior eye segment, light radiation must be refracted by the transparent ocular structures. To reach the retina, blue light can functionally interfere with the cornea and lens, in addition to the ocular surface (tear film), aqueous and vitreous humor [10]. The cornea is located at the anterior chamber, and this is the first surface that light encounters in the human eyes. Corneal epithelial cells have an oxidative increase motivated by light, which triggers inflammation of this structure and may cause cell apoptosis, ocular inflammation and xerophthalmia [11].
The crystalline lens, a suspended structure in the posterior chamber just after the iris, absorbs wavelengths in the visible range of up to 420 nm, and the light reaching the retina is reduced by the pupil. Miosis (pupillary constriction) increases when the eye is exposed to blue light [12,13][12][13]. The crystalline lens has protective action on the retina, but this fact promotes a transparency decrease, changing its appearance and color and inducing cataract formation [14]. Sunlight exposure is considered a risk factor for cataracts due to ultraviolet radiation exposure. Several studies have shown that blue light can also induce the production of oxygen reactive species in lens epithelial cells, which can lead to early cataract development [15].
Light interaction on different ocular surfaces can directly interfere with various tissues, but blue radiation exposure also interferes with the circadian cycle and refractive development. Thus, exposure to environment light, with a high blue light concentration, may also have an advantage against the development and progression of myopia [16]. There is a correlation between myopia low incidence, short-term exposure to near vision and outdoor activities [17]. Regarding digital devices and outdoor activities, Rucker et al. suggested that sunlight is much richer in short-wavelength light than most artificial sources. They add that blue light reduces the eye’s axial length through the mechanism of dopamine release in the retina, which is more favorable for controlling the reactive growth of myopia or astigmatism [18]. The eye’s axial growth and consequent myopia progression are slower during the summer months when children and teenagers spend more time participating in outdoor activities [19]. This information is a theoretical basis for the hypothesized correlation between light exposure and the occurrence and development of myopia. Other experimental works have shown a potential link between light luminance and myopia. Models of myopia experimented in birds found that chicks exposed to high-intensity light (15,000 lx) had greater resistance to myopia development and exhibited slower myopia progression than chicks exposed to low-intensity light (500 lx) and that exposure to bright light can suppress the development of myopia [20,21][20][21].
2. The Blue Light Emission from Digital Devices Impact
While there is evidence that digital devices can increase eyestrain for long-term users, there is currently not enough evidence to say that blue light from digital devices contributes to the development of eye diseases, such as age-related macular degeneration. On the other hand, there are no publications on the effect of long-term exposure to blue light and consequent eye diseases in humans. There are only data on the effects of visible blue light irradiation on rat and monkey retinas. Based on the available evidence, the Association of Optometrists (AOP) concludes that there is insufficient evidence to support the claim that exposure to visible blue light from digital devices leads to eye pathologies and damage to eye health [22]. However, blue light is not all harmful. It can be essentially divided into two ranges: blue-violet light (380–455 nm) and turquoise light (455–495 nm), and these can affect the ocular tissues quite differently [23]. Turquoise light is essential for synchronizing our biological rhythms (the circadian cycle). It helps to maintain and regulate memory, cognition, mood and hormonal balance [24]. It urges a scientific commitment to develop solutions to the potential risk of blue light exposure and it is necessary to know which wavelengths help guide technological decisions and research. Retinal damage, changes in the lens and cornea, dry-eye syndrome, digital eye fatigue, aging, sleep disorders and circadian rhythm are the most investigated topics. The present revisewarch has blue light as its central object. Recent concerns about this radiation impact on visual health and environment due to increased exposure to artificial sources of blue light (mainly from digital devices) are also relevant.2.1. Retinal Impact
Animal models have shown that phototoxicity results in human degenerative pathologies, such as age-related macular degeneration (AMD). A recent model of blue LED-induced phototoxicity in rats was developed, causing damage to the outer layers of the retina [26][25]. Progressive reduction in retinal thickness was noted in these in vivo studies. The photoreceptor layer was the most affected, and in electroretinogram evaluation, a transient reduction in the amplitude of waves a and b was observed [27][26]. The progressive reduction in the cones and the involvement of retinal pigment epithelium cells around the injury perfectly circumscribes the damage to the outer layer of the retina [28][27]. Many models of phototoxicity use white light due to its similarity to sunlight when studying the effect of blue light on the retina and retinal pigment epithelium (RPE) [27,29,30,31,32][26][28][29][30][31]. Knowing that lipofuscin accumulation intensifies aging in RPE, the relationship between phototoxicity and AMD supports the hypothesis of the potential risk of retinal phototoxicity in the elderly [33,34][32][33]. However, six of the eight most significant epidemiological studies found no correlation between AMD and light exposure over the users’ lifetime [35,36,37,38,39,40,41,42][34][35][36][37][38][39][40][41]. Figure 2 shows the blue light intensities’ radiance used in each referenced laboratory research study [43,44,45,46][42][43][44][45]. Only studies that used comparable units were included. Values represent the intensity used in each research study, which one can compare with the intensities produced by personal digital devices, such as those that can be found in the study by Gringas et al. (iPhone 5s, Kindle Paperwhite and iPad Air) [47][46]. Naturally, due to the fast evolution of these devices, future tests will always require updates on their emission spectra and intensity. Furthermore, the values can be useful for further studies about damage induced by blue light, and they are easily measured. Blue light irradiance (W/cm2) can be compared with peak spectral radiance (nm) or the ability of each source to stimulate different photopigments of the retina of a human eye, i.e., the capacity of each source to stimulate different photopigments to the S, M and L cones, rods and intrinsically photosensitive retinal ganglion cells [47][46]. No such comparison was found in the literature search considered in this articlere.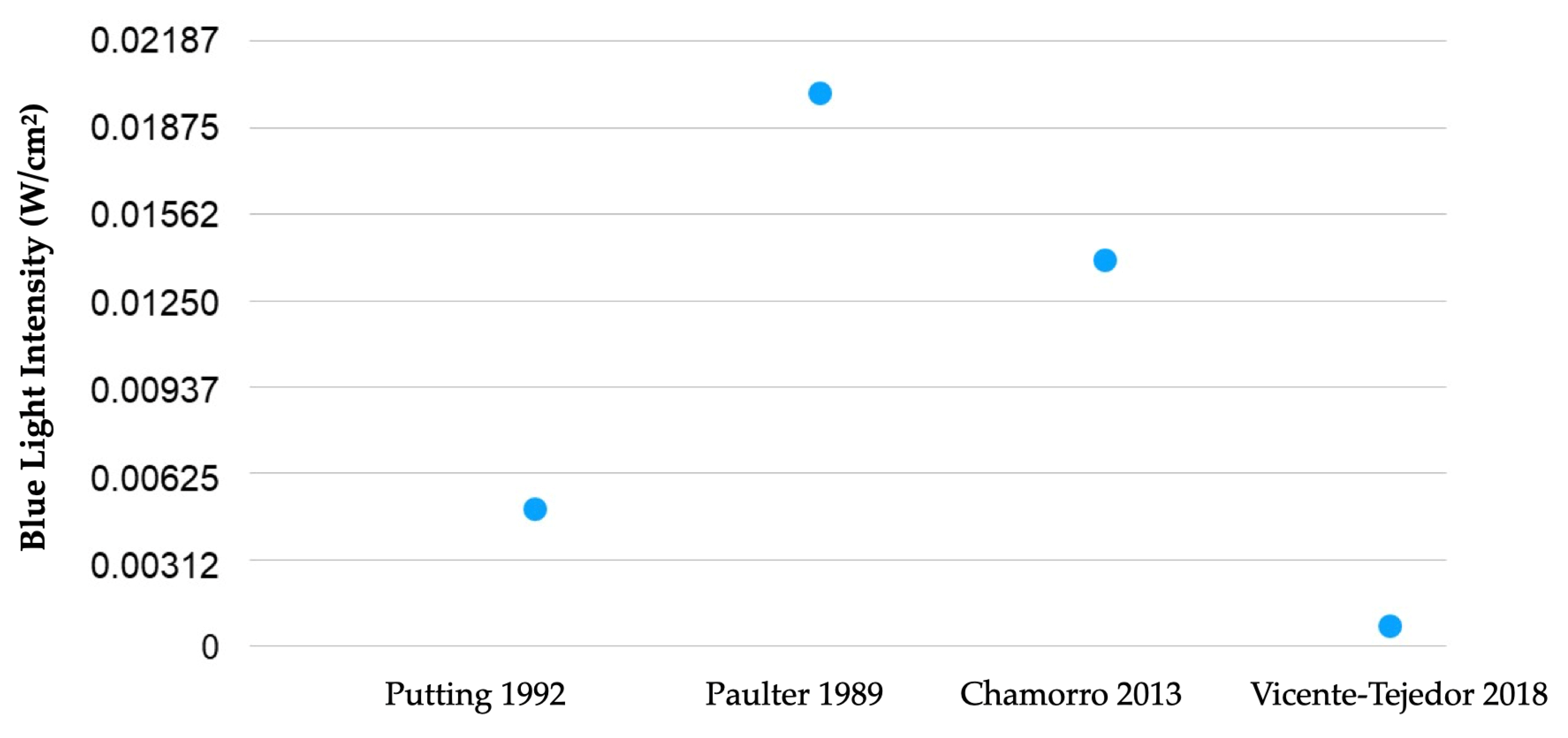
2.2. Impact on Crystalline Lens
The lens is composed of structural proteins, enzymes and metabolites that absorb the most energetic visible light. These substances produce yellow pigments that gradually cause the crystalline lens to opacify and turn yellow. This radiation absorption by the lens blocks and protects the retina from potential damage from blue light exposure [37][36]. Some studies have shown that blue light can induce the production of reactive oxygen species (ROS) in the mitochondria of lens epithelial cells, which can anticipate cataract occurrence [13,15][13][15]. The lens blocks most UV radiation between 300 nm and 420 nm and light transmission decreases with aging, with medical–surgical intervention being the only effective solution to treat cataracts, which is one of the main causes of blindness in the world [14,58,59,60][14][57][58][59]. In around 1980, specialists realized that the intraocular lens (IOL) could not only provide major optical power (in diopters), but it could also filter short light waves, reducing the risks of retinal damage. Consequently, most IOLs used in cataract surgery have incorporated UV-blocking filters since 1986 [61][60]. Blue light loss is permanent for pseudophakes with blue-blocking IOLs, and despite the lack of evidence, blue light hazard is often discussed [62][61]. The hypothesis of phototoxicity by exposure to light has increased concern and interest in blocking, in addition to ultraviolet radiation, part of visible light, by causing or accelerating age-related macular degeneration (AMD) [63,64,65,66,67,68,69,70,71,72][62][63][64][65][66][67][68][69][70][71]. Carotenoids found in the lens, such as lutein and zeaxanthin, are effective in absorbing blue light due to their antioxidant characteristics [73][72]. In the oxidative stress of the lens, antioxidants provide greater protection [74][73].2.3. Impact on the Cornea and Ocular Surface
Some articles have shown that after exposure to blue radiation, the survival rate of corneal epithelial cells decreases [75][74]. The oxidative increase in corneal epithelial cells triggers inflammation and causes oxidative damage and, consequently, cell apoptosis and formation of xerophthalmia [11]. The cornea, aqueous humor and vitreous are the ocular refractive media, permeable to wavelengths between 300 nm and 400 nm. UVA radiation causes damage to the basal layer of keratinocytes, which is responsible for the occurrence of most skin tumors. Sunburn, photokeratitis, cataracts and retinal damage are common consequences of UV-B exposure rays (290 nm to 320 nm) [76][75]. Digital device users with digital eye strain (DES) experience symptoms such as eye irritation, burning, tiredness and redness, dryness, blurred vision and double vision. During digital devices’ prolonged use, a significant proportion of users (40–60%) experience visual or ocular symptoms. Most blue-light-blocking lenses used to reduce DES symptoms have not shown to be effective in reducing visual symptomatology, such as reading a task on a computer for 30 min [77][76]. Other studies have investigated the relationship between the use of digital monitors and changes in the ocular surface. Tear film break-up time (BUT), tear film volume (tear film meniscus and Schirmer’s test) and the lipid layer of the tear film state were quantified. Cardona et al. verified several changes in the tear film. Twenty-five healthy young adults were exposed to 20 min of video games. The lacrimal meniscus decreased, the time and area of tear rupture increased, and the interference patterns of the lipid layer were also altered after the game [78][77]. Another article studied changes in tear film over an 8-h workday in computer users [79][78]. The results did not demonstrate significant changes in Schirmer’s test, but BUT (tear break-up time test) decreased after screen exposure [80][79]. Reading on a computer, the conjunctival surface presents greater hyperemia compared to reading on a smartphone. When using a smartphone, a smaller extent of the exposed ocular surface was identified, due to convergence, in comparison with a computer [81,82][80][81]. Most results suggest that there is a relationship between deterioration in tear film quality and the use of digital devices.References
- Watson, A. In-Home Media Consumption Due to the Coronavirus Outbreak among Internet Users Worldwide as of March 2020, by Country. Available online: https://www.statista.com/statistics/1106498/home-media-consumption-coronavirus-worldwide-by-country/ (accessed on 31 January 2023).
- Bahkir, F.A.; Grandee, S.S. Impact of the COVID-19 lockdown on digital device-related ocular health. Indian J. Ophthalmol. 2020, 68, 2378–2383.
- Digital 2023: Global Overview Report. Available online: https://datareportal.com/reports/digital-2023-global-overview-report (accessed on 5 April 2023).
- CIE 2019. CIE Position Statement on the Blue Light Hazard. 2019. Available online: http://cie.co.at/publications/position-statement-blue-light-hazard-april-23-2019 (accessed on 5 April 2023).
- Renard, G.; Leid, J. Les dangers de la lumière bleue: La vérité! . J. Fr. Ophtalmol. 2016, 39, 483–488.
- Nasrallah, M. Why is the sky blue? Sci. Am. 2003, 289, 103.
- Sanchez-Ramos, C.; Bonnin-Arias, C.; Blázquez-Sánchez, V.; Aguirre-Vilacoro, V.; Cobo, T.; García-Suarez, O.; Perez-Carrasco, M.J.; Alvarez-Peregrina, C.; Vega, J.A. Retinal Protection from LED-Backlit Screen Lights by Short Wavelength Absorption Filters. Cells 2021, 10, 3248.
- Jaadane, I.; Villalpando Rodriguez, G.E.; Boulenguez, P.; Chahory, S.; Carré, S.; Savoldelli, M.; Jonet, L.; Behar-Cohen, F.; Martinsons, C.; Torriglia, A. Effects of white light-emitting diode (LED) exposure on retinal pigment epithelium in vivo. J. Cell Mol. Med. 2017, 21, 3453–3466.
- Jameson, D.; Hurvich, L.M. Handbook of Sensory Physiology: Visual Psychophysics; Springer: Berlin/Heidelberg, Germany, 1973; Volume 7/4.
- Widomska, J.; Witold, K.S. Why has Nature Chosen Lutein and Zeaxanthin to Protect the Retina? J. Clin. Exp. Ophthalmol. 2014, 5, 326.
- Zheng, Q.; Ren, Y.; Reinach, P.S.; Xiao, B.; Lu, H.; Zhu, Y.; Qu, J.; Chen, W. Reactive oxygen species activated NLRP3 inflammasomes initiate inflammation in hyperosmolarity stressed human corneal epithelial cells and environment-induced dry eye patients. Exp. Eye Res. 2015, 134, 133–140.
- Boettner, E.A.; Wolter, J.R. Transmission of the Ocular Media. Investig. Ophthalmol. Vis. Sci. 1962, 1, 776–783.
- Norren, D.V.; Vos, J.J. Spectral transmission of the human ocular media. Vis. Res. 1974, 14, 1237–1244.
- Mellerio, J. Yellowing of the human lens: Nuclear and cortical contributions. Vis. Res. 1987, 27, 1581–1587.
- Babizhayev, M.A. Mitochondria induce oxidative stress, generation of reactive oxygen species and redox state unbalance of the eye lens leading to human cataract formation: Disruption of redox lens organization by phospholipid hydroperoxides as a common basis for cataract disease. Cell Biochem. Funct. 2011, 29, 183–206.
- Norton, T.T.; Siegwart, J.T., Jr. Light levels, refractive development, and myopia–a speculative review. Exp. Eye Res. 2013, 114, 48–57.
- Ramamurthy, D.; Chua, S.Y.L.; Saw, S. A review of environmental risk factors for myopia during early life, childhood and adolescence. Clin. Exp. Optom. 2015, 98, 497–506.
- Rucker, F.J.; Britton, S.; Spatcher, M.; Hanowsky, S. Blue Light Protects Against Temporal Frequency Sensitive Refractive Changes. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6121–6131.
- Fulk, G.W.; Cyert, L.A.; Parker, D.A. Seasonal Variation in Myopia Progression and Ocular Elongation. Optom. Vis. Sci. 2002, 79, 46–51.
- Ashby, R.; Ohlendorf, A.; Schaeffel, F. The Effect of Ambient Illuminance on the Development of Deprivation Myopia in Chicks. Investig. Ophthalmol. Vis. Sci. 2009, 50, 5348–5354.
- Stone, R.A.; Cohen, Y.; McGlinn, A.M.; Davison, S.; Casavant, S.; Shaffer, J.; Khurana, T.S.; Pardue, M.T.; Iuvone, P.M. Development of Experimental Myopia in Chicks in a Natural Environment. Investig. Ophthalmol. Vis. Sci. 2016, 57, 4779–4789.
- McClean, A.; Derbyshire, L. Understanding Blue Light. Available online: https://www.aop.org.uk/our-voice/policy/position-statements/2023/01/03/visible-blue-light (accessed on 25 March 2023).
- Arnault, E.; Barrau, C.; Nanteau, C.; Gondouin, P.; Bigot, K.; Viénot, F.; Gutman, E.; Fontaine, V.; Villette, T.; Cohen-Tannoudji, D.; et al. Phototoxic Action Spectrum on a Retinal Pigment Epithelium Model of Age-Related Macular Degeneration Exposed to Sunlight Normalized Conditions. PLoS ONE 2013, 8, e71398.
- Wahl, S.; Engelhardt, M.; Schaupp, P.; Lappe, C.; Ivanov, I.V. The inner clock—Blue light sets the human rhythm. J. Biophotonics 2019, 12, e201900102.
- Youssef, P.N.; Sheibani, N.; Albert, D.M. Retinal light toxicity. Eye 2011, 25, 1–14.
- Behar-Cohen, F.; Martinsons, C.; Viénot, F.; Zissis, G.; Barlier-Salsi, A.; Cesarini, J.; Enouf, O.; Garcia, M.; Picaud, S.; Attia, D. Light-emitting diodes (LED) for domestic lighting: Any risks for the eye? Prog. Retin. Eye Res. 2011, 30, 239–257.
- Valiente-Soriano, F.J.; Ortín-Martínez, A.; Di Pierdomenico, J.; García-Ayuso, D.; Gallego-Ortega, A.; de Imperial-Ollero, J.A.M.; Jiménez-López, M.; Villegas-Pérez, M.P.; Wheeler, L.A.; Vidal-Sanz, M. Topical Brimonidine or Intravitreal BDNF, CNTF, or bFGF Protect Cones Against Phototoxicity. Transl. Vis. Sci. Technol. 2019, 8, 36.
- Jaadane, I.; Boulenguez, P.; Chahory, S.; Carré, S.; Savoldelli, M.; Jonet, L.; Behar-Cohen, F.; Martinsons, C.; Torriglia, A. Retinal damage induced by commercial light emitting diodes (LEDs). Free Radic. Biol. Med. 2015, 84, 373–384.
- Lin, C.-H.; Wu, M.-R.; Huang, W.-J.; Chow, D.S.-L.; Hsiao, G.; Cheng, Y.-W. Low-Luminance Blue Light-Enhanced Phototoxicity in A2E-Laden RPE Cell Cultures and Rats. Int. J. Mol. Sci. 2019, 20, 1799.
- Krigel, A.; Berdugo, M.; Picard, E.; Levy-Boukris, R.; Jaadane, I.; Jonet, L.; Dernigoghossian, M.; Andrieu-Soler, C.; Torriglia, A.; Behar-Cohen, F. Light-induced retinal damage using different light sources, protocols and rat strains reveals LED phototoxicity. Neuroscience 2016, 339, 296–307.
- Xia, H.; Hu, Q.; Li, L.; Tang, X.; Zou, J.; Huang, L.; Li, X. Protective effects of autophagy against blue light-induced retinal degeneration in aged mice. Sci. China Life Sci. 2019, 62, 244–256.
- Nakamura, M.; Yako, T.; Kuse, Y.; Inoue, Y.; Nishinaka, A.; Nakamura, S.; Shimazawa, M.; Hara, H. Exposure to excessive blue LED light damages retinal pigment epithelium and photoreceptors of pigmented mice. Exp. Eye Res. 2018, 177, 1–11.
- Feeney-Burns, L.; Berman, E.R.; Rothman, H. Lipofuscin of Human Retinal Pigment Epithelium. Am. J. Ophthalmol. 1980, 90, 783–791.
- Weiter, J.J.; Delori, F.C.; Wing, G.L.; Fitch, K.A. Retinal pigment epithelial lipofuscin and melanin and choroidal melanin in human eyes. Investig. Ophthalmol. Vis. Sci. 1986, 27, 145–152.
- Feeney-Burns, L.; Hilderbrand, E.S.; Eldridge, S. Aging human RPE: Morphometric analysis of macular, equatorial, and peripheral cells. Investig. Ophthalmol. Vis. Sci. 1984, 25, 195–200.
- Taylor, H.R.; West, S.; Muñoz, B.; Rosenthal, F.S.; Bressler, S.B.; Bressler, N.M. The Long-term Effects of Visible Light on the Eye. Arch. Ophthalmol. 1992, 110, 99–104.
- The Eye Disease Case-Control Study Group. Risk Factors for Neovascular Age-Related Macular Degeneration. Arch. Ophthalmol. 1992, 110, 1701–1708.
- Hirvelä, H.; Luukinen, H.; Läärä, E.; Laatikainen, L. Risk Factors of Age-related Maculopathy in a Population 70 Years of Age or Older. Ophthalmology 1996, 103, 871–877.
- Darzins, P.; Mitchell, P.; Heller, R. Sun Exposure and Age-related Macular Degeneration. An Australian case-control study. Ophthalmology 1997, 104, 770–776.
- McCarty, C.A.; Mukesh, B.N.; Fu, C.L.; Mitchell, P.; Wang, J.J.; Taylor, H.R. Risk Factors for Age-Related Maculopathy: The Visual Impairment Project. Arch. Ophthalmol. 2001, 119, 1455–1462.
- Delcourt, C.; Carrière, I.; Ponton-Sanchez, A.; Fourrey, S.; Lacroux, A.; Papoz, L.; POLA Study Group. Light Exposure and the Risk of Age-Related Macular Degeneration: The Pathologies Oculaires Liées à l’Age (POLA) study. Arch. Ophthalmol. 2001, 119, 1463–1468.
- Putting, B.J.; Zweypfenning, R.C.; Vrensen, G.F.; Oosterhuis, J.A.; Van Best, J.A. Blood-retinal barrier dysfunction at the pigment epithelium induced by blue light. Investig. Ophthalmol. Vis. Sci. 1992, 33, 3385–3393.
- Pautler, E.L.; Morita, M.; Beezley, D. Reversible and irreversible blue light damage to the isolated, mammalian pigment epithelium. Prog. Clin. Biol. Res. 1989, 314, 555–567.
- Chamorro, E.; Bonnin-Arias, C.; Pérez-Carrasco, M.J.; de Luna, J.M.; Vázquez, D.; Sánchez-Ramos, C. Effects of Light-emitting Diode Radiations on Human Retinal Pigment Epithelial Cells In Vitro. Photochem. Photobiol. 2013, 89, 468–473.
- Vicente-Tejedor, J.; Marchena, M.; Ramírez, L.; García-Ayuso, D.; Gómez-Vicente, V.; Sánchez-Ramos, C.; De La Villa, P.; Germain, F. Removal of the blue component of light significantly decreases retinal damage after high intensity exposure. PLoS ONE 2018, 13, e0194218.
- Egringras, P.; Emiddleton, B.; Skene, D.J.; Revell, V.L. Bigger, Brighter, Bluer-Better? Current Light-Emitting Devices—Adverse Sleep Properties and Preventative Strategies. Front. Public Health 2015, 3, 233.
- Pazikadin, A.R.; Rifai, D.; Ali, K.; Mamat, N.H.; Khamsah, N. Design and Implementation of Fuzzy Compensation Scheme for Temperature and Solar Irradiance Wireless Sensor Network (WSN) on Solar Photovoltaic (PV) System. Sensors 2020, 20, 6744.
- Udovicic, L.; Janßen, M. Photobiological Safety of Common Office Light Sources. In Proceedings of the 29th CIE Session, Washington, DC, USA, 14–22 June 2019; CIE: Vienna, Austria, 2019; pp. 1256–1261.
- Tomany, S.C.; Cruickshanks, K.J.; Klein, R.; Klein, B.E.K.; Knudtson, M.D. Sunlight and the 10-Year Incidence of Age-Related Maculopathy: The Beaver Dam Eye Study. Arch. Ophthalmol. 2004, 122, 750–757, Erratum in Arch. Ophthalmol. 2005, 123, 362.
- Rozanowska, M.B. Light-Induced Damage to the Retina: Current Understanding of the Mechanisms and Unresolved Questions: A Symposium-in-Print. Photochem. Photobiol. 2012, 88, 1303–1308.
- Sciences Online: American Society for Photobiology. Available online: http://photobiology.info/Rozanowska.html (accessed on 20 March 2022).
- Noell, W.K.; Walker, V.S.; Kang, B.S.; Berman, S. Retinal damage by light in rats. Investig. Ophthalmol. 1966, 5, 450–473.
- O’Hagan, J.B.; Khazova, M.; Price, L.L.A. Low-energy light bulbs, computers, tablets and the blue light hazard. Eye 2016, 30, 230–233.
- Tosini, G.; Ferguson, I.; Tsubota, K. Effects of blue light on the circadian system and eye physiology. Mol. Vis. 2016, 22, 61–72.
- Nash, T.R.; Chow, E.S.; Law, A.D.; Fu, S.D.; Fuszara, E.; Bilska, A.; Bebas, P.; Kretzschmar, D.; Giebultowicz, J.M. Daily blue-light exposure shortens lifespan and causes brain neurodegeneration in Drosophila. NPJ Aging Mech. Dis. 2019, 5, 8.
- Xie, C.; Li, X.; Tong, J.; Gu, Y.; Shen, Y. Effects of white light-emitting diode (LED) light exposure with different Correlated Color Temperatures (CCTs) on human lens epithelial cells in culture. Photochem. Photobiol. 2014, 90, 853–859.
- Weale, R.A. Age and the transmittance of the human crystalline lens. J. Physiol. 1988, 395, 577–587.
- Xu, J.; Pokorny, J.; Smith, V.C. Optical density of the human lens. J. Opt. Soc. Am. A 1997, 14, 953–960.
- Resnikoff, S.; Pascolini, D.; Etya’Ale, D.; Kocur, I.; Pararajasegaram, R.; Pokharel, G.P.; Mariotti, S.P. Global data on visual impairment in the year 2002. Bull. World Health Organ. 2004, 82, 844–851.
- Mainster, M.A. The Spectra, Classification, and Rationale of Ultraviolet-Protective Intraocular Lenses. Am. J. Ophthalmol. 1986, 102, 727–732.
- Van Der Hoeve, J. Eye Lesions Produced by Light Rich in Ultraviolet Rays. Senile Cataract, Senile Degeneration of Macula. Am. J. Ophthalmol. 1920, 3, 178–194.
- Tso, M.O.; La Piana, F.G. The human fovea after sungazing. Trans. Sect. Ophthalmol. Am. Acad. Ophthalmol. Otolaryngol. 1975, 79, OP788-95.
- Mainster, M.A.; Findl, O.; Dick, H.B.; Desmettre, T.; Ledesma-Gil, G.; Curcio, C.A.; Turner, P.L. The Blue Light Hazard Versus Blue Light Hype. Am. J. Ophthalmol. 2022, 240, 51–57.
- Tso, M.O. Pathogenetic Factors of Aging Macular Degeneration. Ophthalmology 1985, 92, 628–635.
- Marshall, J. Radiation and the ageing eye. Ophthalmic Physiol. Opt. 1985, 5, 241–263.
- Mainster, M.A. Light and macular degeneration: A biophysical and clinical perspective. Eye 1987, 1, 304–310.
- Mainster, M.A. Violet and blue light blocking intraocular lenses: Photoprotection versus photoreception. Br. J. Ophthalmol. 2006, 90, 784–792.
- Marshall, J.D. The ageing retina: Physiology or pathology. Eye 1987, 1, 282–295.
- Remé, C.; Reinboth, J.; Clausen, M.; Hafezi, F. Light damage revisited: Converging evidence, diverging views? Graefes Arch. Clin. Exp. Ophthalmol. 1996, 234, 2–11.
- Boulton, M.; Różanowska, M.; Różanowski, B. Retinal photodamage. J. Photochem. Photobiol. B Biol. 2001, 64, 144–161.
- Margrain, T.H.; Boulton, M.; Marshall, J.; Sliney, D.H. Do blue light filters confer protection against age-related macular degeneration? Prog. Retin. Eye Res. 2004, 23, 523–531.
- Bernstein, P.S.; Khachik, F.; Carvalho, L.S.; Muir, G.J.; Zhao, D.-Y.; Katz, N.B. Identification and Quantitation of Carotenoids and their Metabolites in the Tissues of the Human Eye. Exp. Eye Res. 2001, 72, 215–223.
- Gao, S.; Qin, T.; Liu, Z.; Caceres, M.A.; Ronchi, C.F.; Chen, C.-Y.O.; Yeum, K.-J.; Taylor, A.; Blumberg, J.B.; Liu, Y.; et al. Lutein and zeaxanthin supplementation reduces H2O2-induced oxidative damage in human lens epithelial cells. Mol. Vis. 2011, 17, 3180–3190.
- Lee, H.S.; Cui, L.; Li, Y.; Choi, J.S.; Choi, J.-H.; Li, Z.; Kim, G.E.; Choi, W.; Yoon, K.C. Correction: Influence of Light Emitting Diode-Derived Blue Light Overexposure on Mouse Ocular Surface. PLoS ONE 2016, 11, e0167671.
- Yam, J.C.S.; Kwok, A.K.H. Ultraviolet light and ocular diseases. Int. Ophthalmol. 2014, 34, 383–400.
- Vera, J.; Redondo, B.; Ortega-Sanchez, A.; Molina-Molina, A.; Molina, R.; Rosenfield, M.; Jiménez, R. Blue-blocking filters do not alleviate signs and symptoms of digital eye strain. Clin. Exp. Optom. 2023, 106, 85–90.
- Cardona, G.; García, C.; Serés, C.; Vilaseca, M.; Gispets, J. Blink Rate, Blink Amplitude, and Tear Film Integrity during Dynamic Visual Display Terminal Tasks. Curr. Eye Res. 2011, 36, 190–197.
- Portello, J.K.; Rosenfield, M.; Bababekova, Y.; Estrada, J.M.; Leon, A. Computer-related visual symptoms in office workers. Ophthalmic Physiol. Opt. 2012, 32, 375–382.
- Akkaya, S.; Atakan, T.; Acikalin, B.; Aksoy, S.; Ozkurt, Y. Effects of long-term computer use on eye dryness. North. Clin. Istanb. 2018, 5, 319–322.
- Rosenfield, M. Computer vision syndrome: A review of ocular causes and potential treatments. Ophthalmic Physiol. Opt. 2011, 31, 502–515.
- Talens-Estarelles, C.; Sanchis-Jurado, V.; Esteve-Taboada, J.J.; Pons, M.; García-Lázaro, S. How Do Different Digital Displays Affect the Ocular Surface? Optom. Vis. Sci. 2020, 97, 1070–1079.
More
