Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Beatrix Zheng and Version 2 by Beatrix Zheng.
Unlike with chemotherapy, which imparts a relatively short duration of selective pressure on acute myeloid leukemia clonal architecture, the immunological effect related to allogeneic hematopoietic stem cell transplant is prolonged over time and must be overcome for relapse to occur. This means that not all molecular abnormalities detected after transplant always imply inevitable relapse. Therefore, transplant represents a critical setting where a measurable residual disease-based strategy, performed during post-transplant follow-up by highly sensitive methods such as next-generation sequencing, could optimize and improve treatment outcome.
- acute myeloid leukemia
- measurable residual disease
- clonal evolution
1. Introduction
Acute myeloid leukemias (AMLs) are a heterogeneous group of clonal disorders occurring after chromosomal and or genomic alterations in hematopoietic stem cells or progenitor cells [1][2][3][4][5].
Although 70% of patients with AMLs attain morphologic complete remission (mCR) with intensive induction chemotherapy (IIC), approximately 50% of these patients experience relapse [6][7]. Moreover, disease recurrence represents the primary reason for treatment failure and death also after allogeneic hematopoietic stem cell transplant (alloHSCT) [8].
Therefore, a significant effort is needed to translate the advances in understanding the genetic complexity of AMLs into routine clinical practice.
Unlike other disease that are characterized by a single somatic mutation (as in chronic myeloid leukemia with Abelson murine leukemia gene translocation onto the breakpoint cluster region (BCR/ABL translocation) or in acute lymphoblastic leukemia with T-cell receptor (TCR) rearrangements), individual AMLs are potentially composed of clones and subclones that harbor multiple mutations, making each patient genetically unique [9][10][11][12][13].
Traditionally, in AMLs, molecular and cytogenetic risk classification has been used to predict disease progression and then survival, allowing patient selection for more intensive treatments, including transplant [14][15][16][17]. However, conventional cytogenetic analysis is not enough for understanding the underlying disease clonal architecture and the genotypic/phenotypic drifts that can occur during the treatment course [12].
One of the most important advances in this setting was the introduction of next-generation sequencing (NGS), which enables reliable detection of patient-specific mutations (and other alterations) including complete gene assessment at diagnosis, complete remission (CR), and relapse [18].
Despite the fact that the relevance of NGS at diagnosis has now been ascertained, its role in other phase of the disease—during treatment, at relapse, before and after transplant—is less clear.
2. Clonal Evolution Detected by Next-Generation Sequencing: From Diagnosis to Post-Transplant Relapse
It is well known that AML patients at relapse can present the same genetic lesion observed at diagnosis or can have higher clonal complexity through the acquisition of new mutations, or lose some mutation, or both.
From a pathophysiological point of view, the prevailing hypothesis is that a previously present minor subclone may exert a survival advantage under the selective pressure of chemotherapy [19].
To study the mechanisms of therapy resistance and disease progression, Greif et al. [20] compared the coding mutational patterns of matched diagnosis, remission, and relapse samples from 50 AML patients submitted to IIC. Both molecular devolution and evolution were widely detected at relapse, with a predominance of the latter. Moreover, they observed that alterations of epigenetic regulators (for example, KDM6A (Lysine Demethylase 6A) mutation associated to cytarabine resistance) were frequently gained at relapse; low KDM6A expression correlated with adverse clinical outcome.
This and other studies provide examples of clonal stability and clonal devolution all occurring either alone or in all possible combination at relapse [19][21][22].
Another pathophysiologic mechanism of clonal evolution during relapse is that the clone at relapse could be therapy-related and thus independent of the previous clone. In a landmark study, Ding et al. [23] analyzed (at diagnosis and at relapse) the mutational patterns of eight AML patients.
They found two major clonal evolution patterns during relapse: in one, the clone present at diagnosis acquired mutations and evolved into the relapsed one; in the other, a subclone of the founding clone survived during treatment, acquired other mutations, and expanded at relapse.
In any cases, chemotherapy failed to eradicate the founding clone. The analysis of relapse-specific clones versus primary clone mutations in all eight AML patients revealed an increase in transversions, maybe due to DNA damage caused by treatment.
All these studies provided insights into the AML clonal structure, revealing a clonal dynamic throughout the course of treatment and a refined risk stratification in ICC-treated patients, switching from a static risk classification (diagnosis) to a dynamic one (during treatment).
Given the distinct kinetic and curative mechanisms in alloHSCT, compared to conventional chemotherapy, it is plausible that different disease-related AML-specific mutations may be detected and targeted at post-transplant relapse.
In detail, chemotherapy could impart a selective pressure on AML clonal architecture of a relatively short duration, while the immunological effect of the donor immune system is prolonged overtime and should be overcome for relapse to occur [24]. Moreover, it could also be assumed that the donor immune system itself could modulate pressure on leukemia clonal architecture.
This poses additional challenges for highly sensitive methods of MRD assessment, as the persistence of molecular abnormality post-transplant does not always imply imminent relapse [9][24] (Figure 31).
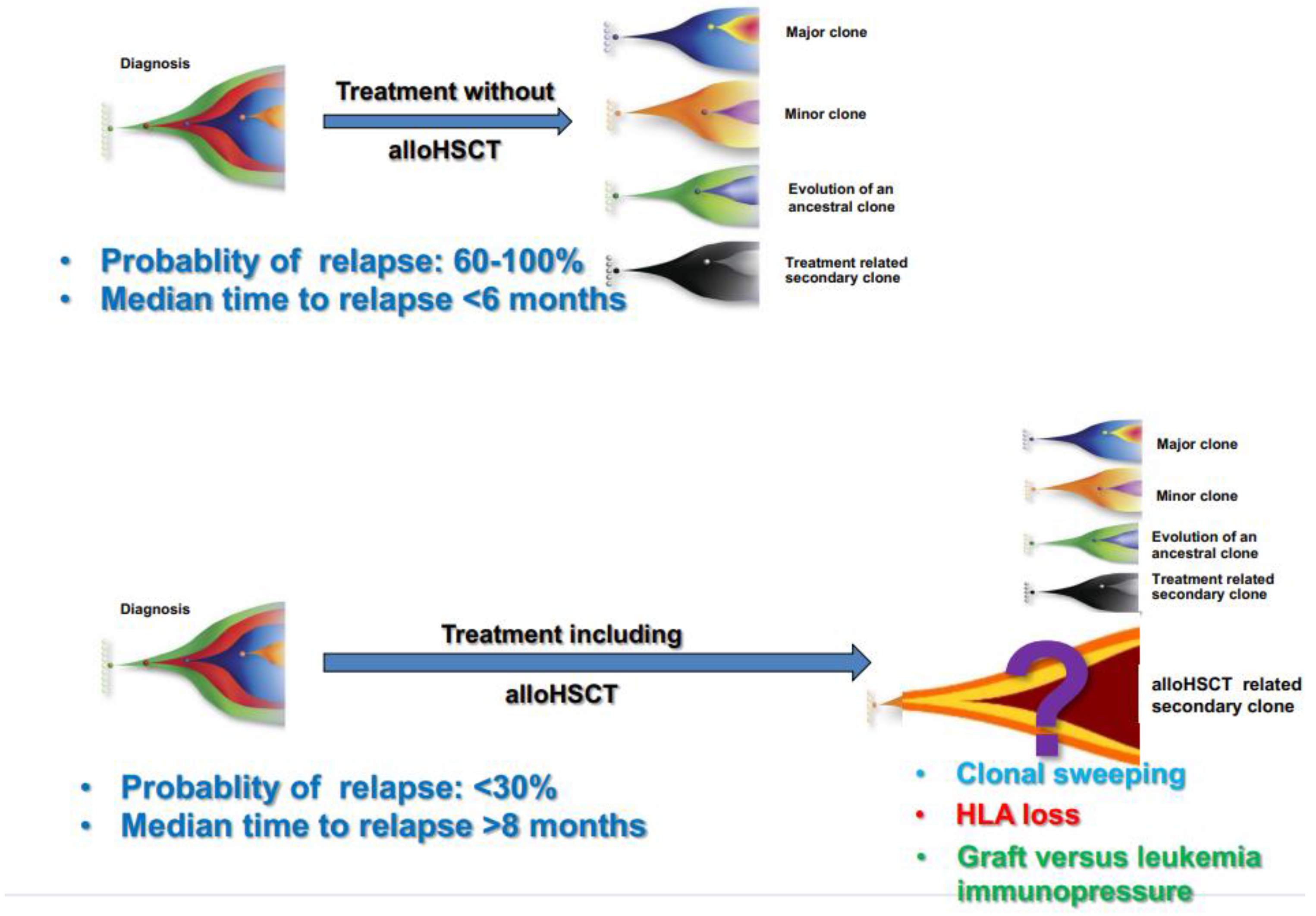
Figure 31. Clonal evolution and probability of relapse after conventional chemotherapy without transplant and after a program including transplant.
Post-transplant administration of either cellular or pharmacological therapy represents a key approach to reducing disease relapse. Such therapies can augment the immunological effect of transplant (for example, by donor lymphocyte infusions and/or checkpoint inhibitors), or they can modulate disease relapse kinetics (for example by FLT3 inhibitors and/or hypomethylating agents). Obviously, rational employment of post-transplant treatment would be aided by a deeper understanding of actionable leukemia mutations in recurrent disease (Figure 2).
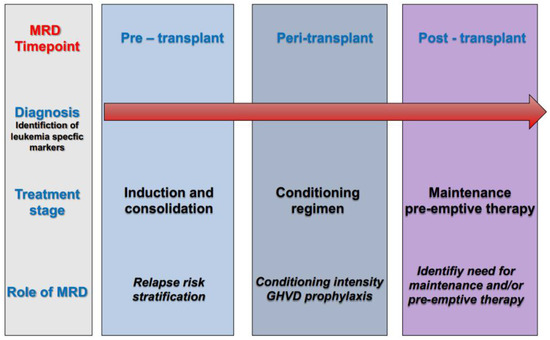
Figure 2. Role of measurable residual disease (MRD) at different timepoints of the treatment pathway in acute myeloid leukemia (AML).
Kim et al. [24] presented an NGS-MRD analysis of a single case at 12 timepoints, from the initial diagnosis until a fourth relapse. The analysis tracked the hierarchy of genetic acquisition, and subsequently described the evolution of somatic variants to infer clonal structure.
New subclones appeared after the first mCR; the presence or absence of different subclones during remission and relapse implies differing drug responses among subclones. They concluded that NGS analysis-compared remission and relapse provides a comprehensive view of clonal structure and evolution.
Quek et al. [21] studied changes in clonal structure-compared mutational profiles in bone marrow samples at diagnosis and at relapse in 19 AML patients. In 13 patients, mutational profiles were altered at relapse, and in 9 patients, mutations at relapse were not presented at diagnosis. In 15 patients, additional samples, available pre-transplant, were analyzed; in two patients, mutations identified post-transplant but not at diagnosis were detectable immediately prior to transplant.
Kim et al. [25] analyzed clonal dynamics evolution after transplant in their series; bone marrow samples at relapse were available in 17 patients. Sixteen patients (94.0%) had, at post-transplant relapse, some or all of the same mutations detected at diagnosis. In three patients, longitudinal tracking revealed detectable mutations at 2 or 3 months before relapse. In addition, one patient with a Kirsten Rat Sarcoma virus (KRAS) mutation at initial diagnosis showed a DNMT3A mutation post-transplant, which was thought to be of donor origin. Furthermore, three clonal mutations of the FLT3, Neuroblastoma RAS (NRAS), and Protein Tyrosine Phosphatase (PTPN) genes had evolved at 29 months post-transplant.
In the analysis of Kim et al. [26], clonal evolution of allelic burden, detected at diagnosis and at post-transplant, showed a significant reduction of allelic burden, both after ICC and after transplant. Among the 256 mutations with detected VAF over 2% at initial diagnosis, only 42 mutations (16.4%) remained at a VAF over 2% pre-transplant, whereas 114 of the initial mutations were eradicated. Twenty-three out of 104 patients (22.1%) relapsed post-transplant, and samples at relapse were available for 20 patients; these samples were sequenced for tracing clonal evolution from diagnosis to relapse. VAFs of mutations detected in the initial clone and in the post-transplant relapse were comparable (28.2% vs. 28.4%). Among the 61 mutations detected from longitudinal monitoring in relapsed patients, 37 were stable (60.6%), 9 were cleared (14.8%), and 15 were acquired/selected at relapse (24.6%). At least one mutation shared between the initial clone and the post-transplant relapse clone was detected in 17 (85%) patients. In the remaining three patients, NGS analysis revealed that the post-transplant relapse clone shared at least one mutation with the initial leukemia clone in two patients. Altogether, in 19 out of 20 relapsed patients (95%), the clone at relapse shared at least one mutation with the clone at diagnosis.
References
- Welch, J.S.; Ley, T.J.; Link, D.C.; Miller, C.A.; Larson, D.E.; Koboldt, D.C.; Wartman, L.D.; Lamprecht, T.L.; Liu, F.; Xia, J.; et al. The Origin and Evolution of Mutations in Acute Myeloid Leukemia. Cell 2012, 150, 264–278.
- Shlush, L.I.; Zandi, S.; Mitchell, A.; Chen, W.C.; Brandwein, J.M.; Gupta, V.; Kennedy, J.A.; Schimmer, A.D.; Schuh, A.C.; Yee, K.W.; et al. Identification of pre-leukaemic haematopoietic stem cells in acute leukaemia. Nature 2014, 20, 328–333.
- Hirsch, P.; Zhang, Y.; Tang, R.; Joulin, V.; Boutroux, H.; Pronier, E.; Moatti, H.; Flandrin, P.; Marzac, C.; Bories, D.; et al. Genetic Hierarchy and temporal variegation in the clonal history of acute myeloid leukaemia. Nat. Commun. 2016, 7, 12475.
- Corces-Zimmerman, M.R.; Hong, W.-J.; Weissman, I.L.; Medeiros, B.C.; Majeti, R. Preleukemic mutations in human acute myeloid leukemia affect epigenetic regulators and persist in remission. Proc. Natl. Acad. Sci. USA 2014, 111, 2548–2553.
- Jan, M.; Snyder, T.M.; Corces-Zimmerman, M.R.; Vyas, P.; Weissman, I.L.; Quake, S.R.; Majeti, R. Clonal evolution of preleukemic hematopoietic stem cells precedes human acute myeloid leukemia. Sci. Transl. Med. 2012, 4, 149ra118.
- Mandelli, F.; Vignetti, M.; Suciu, S.; Stasi, R.; Petti, M.-C.; Meloni, G.; Muus, P.; Marmont, F.; Marie, J.-P.; LaBar, B.; et al. Daunorubicin Versus Mitoxantrone Versus Idarubicin as Induction and Consolidation Chemotherapy for Adults with Acute Myeloid Leukemia: The EORTC and GIMEMA Groups Study AML-10. J. Clin. Oncol. 2009, 27, 5397–5403.
- Walter, R.B.; Othus, M.; Burnett, A.K.; Lowenberg, B.; Kantarjian, H.M.; Ossenkoppele, G.J.; Hills, R.K.; Ravandi, F.; Pabst, T.; Evans, A.; et al. Resistance prediction in AML: Analysis of 4601 patients from MRC/NCRI, HOVON/SAKK, SWOG and MD Anderson Cancer Center. Leukemia 2015, 29, 312–320.
- Lowenberg, B.; Downing, J.R.; Burnett, A. Acute myeloid leukemia. N. Engl. J. Med. 1999, 341, 1051–1062.
- Toren, A.; Rechavi, G.; Nagler, A. Minimal Residual Disease Post-Bone Marrow Transplantation for Hemato-Oncological Diseases. Stem Cells 1996, 14, 300–311.
- Logan, A.C.; Vashi, N.; Faham, M.; Carlton, V.; Kong, K.; Buño, I.; Zheng, J.; Moorhead, M.; Klinger, M.; Zhang, B.; et al. Immunoglobulin and T Cell Receptor Gene High-Throughput Sequencing Quantifies Minimal Residual Disease in Acute Lymphoblastic Leukemia and Predicts Post-Transplantation Relapse and Survival. Biol. Blood Marrow Transplant. 2014, 20, 1307–1313.
- Duncavage, E.J.; Tandon, B. The utility of next-generation sequencing in diagnosis and monitoring of acute myeloid leukemia and myelodysplastic syndromes. Int. J. Lab. Hematol. 2015, 37, 115–121.
- Grimwade, D.; Freeman, S.D. Defining minimal residual disease in acute myeloid leukemia: Which platforms are ready for “prime time”? Blood 2014, 124, 3345–3355.
- Salipante, S.J.; Fromm, J.R.; Shendure, J.; Wood, B.L.; Wu, D. Detection of minimal residual disease in NPM1-mutated acute myeloid leukemia by next-generation sequencing. Mod. Pathol. 2014, 27, 1438–1446.
- Byrd, J.C.; Mrozek, K.; Dodge, R.K.; Carroll, A.J.; Edwards, C.G.; Arthur, D.C.; Pettenati, M.J.; Patil, S.R.; Rao, K.W.; Watson, M.S.; et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: Results from Cancer and Leukemia Group B (CALGB 8461). Blood 2002, 100, 4325–4336.
- Slovak, M.L.; Kopecky, K.J.; Cassileth, P.A.; Harrington, D.H.; Theil, K.S.; Mohamed, A.; Paietta, E.; Willman, C.L.; Head, D.R.; Rowe, J.M.; et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: A Southwest Oncology Group/Eastern Cooperative Oncology Group study. Blood 2000, 96, 4075–4083.
- Greenberg, P.; Cox, C.; LeBeau, M.M.; Fenaux, P.; Morel, P.; Sanz, G.; Sanz, M.; Vallespi, T.; Hamblin, T.; Oscier, D.; et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood 1997, 89, 2079–2088.
- Greenberg, P.L.; Tuechler, H.; Schanz, J.; Sanz, G.; Garcia-Manero, G.; Solè, F.; Bennett, J.M.; Bowen, D.; Fenaux, P.; Dreyfus, F.; et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood 2012, 120, 2454–2465.
- Leisch, M.; Jansko, B.; Zaborsky, N.; Greil, P.; Pleyer, L. Next Generation Sequencing in AML-on the way to becoming a new standard for treatment initiation and/or modulation? Cancer 2019, 11, 252.
- Wong, T.N.; Ramsingh, G.; Young, A.L.; Miller, C.A.; Touma, W.; Welch, J.S.; Lamprecht, T.L.; Shen, D.; Hundal, J.; Fulton, R.S.; et al. Role of TP53 mutations in the origin and evolution of therapy-related acute myeloid leukaemia. Nature 2015, 518, 552–555.
- Greif, P.A.; Hartmann, L.; Vosberg, S.; Stief, S.M.; Mattes, R.; Hellmann, I.; Metzeler, K.H.; Herold, T.; Bamopoulos, S.A.; Kerbs, P.; et al. Evolution of cytogenetically normal acute myeloid leukemia during therapy and relapse: An exome sequencing study of 50 patients. Clin. Cancer Res. 2018, 24, 1716–1726.
- Quek, L.; Ferguson, P.; Metzner, M.; Ahmed, I.; Kennedy, A.; Garnett, C.; Jeffries, S.; Walter, C.; Piechocki, K.; Timbs, A.; et al. Mutational analysis of disease relapse in patients allografted for acute myeloid leukemia. Blood Adv. 2016, 1, 193–204.
- Kim, T.; Yoshida, K.; Kim, Y.K.; Tyndel, M.S.; Park, H.J.; Choi, S.H.; Ahn, J.-S.; Jung, S.-H.; Yang, D.-H.; Lee, J.-J.; et al. Clonal dynamics in a single AML case tracked for 9 years reveals the complexity of leukemia progression. Leukemia 2016, 30, 295–302.
- Ding, L.; Ley, T.J.; Larson, D.E.; Miller, C.A.; Koboldt, D.C.; Welch, J.S.; Ritchey, J.K.; Young, M.A.; Lamprecht, T.; McLellan, M.D.; et al. Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature 2012, 481, 506–510.
- Ommen, H.B. Monitoring minimal residual disease in acute myeloid leukaemia: A review of the current evolving strategies. Ther. Adv. Hematol. 2016, 7, 3–16.
- Kim, H.-J.; Kim, Y.; Kang, D.; Kim, H.S.; Lee, J.-M.; Kim, M.; Cho, B.-S. Prognostic value of measurable residual disease monitoring by next-generation sequencing before and after allogeneic hematopoietic cell transplantation in acute myeloid leukemia. Blood Cancer J. 2021, 11, 109.
- Kim, T.; Moon, J.H.; Ahn, J.-S.; Kim, Y.-K.; Lee, S.-S.; Ahn, S.-Y.; Jung, S.-H.; Yang, D.-H.; Lee, J.-J.; Choi, S.H.; et al. Next-generation sequencing–based posttransplant monitoring of acute myeloid leukemia identifies patients at high risk of relapse. Blood 2018, 132, 1604–1613.
More
