Cancer is one of the leading causes of death, and latest predictions indicate that cancer-related deaths will increase over the next few decades. Despite significant advances in conventional therapies, treatments are still far from ideal due to limitations such as lack of selectivity, non-specific distribution, and multidrug resistance. SomCurrent researches are is focusing on the development of several strategies to improve the efficiency of chemotherapeutic agents and, as a result, overcome the challenges associated with conventional therapies. In this regard, combined therapy with natural compounds and other therapeutic agents, such as chemotherapeutics or nucleic acids, has recently emerged as a new strategy for tackling the drawbacks of conventional therapies.
- cancer
- conventional therapy
- combined therapy
- natural compounds
1. Introduction
2. Natural Compounds: Advantages of Combination Therapy in Cancer
Conventional therapy has evident benefits in cancer treatment; however, despite the continuous emergence of new anticancer agents, the majority of chemotherapy-based treatment continues to remain ineffective due to an array of factors, which include chemotherapy-induced toxicity and adverse reactions, insufficient target specificity, and, most importantly, drug resistance during cancer progression (Figure 1) [9].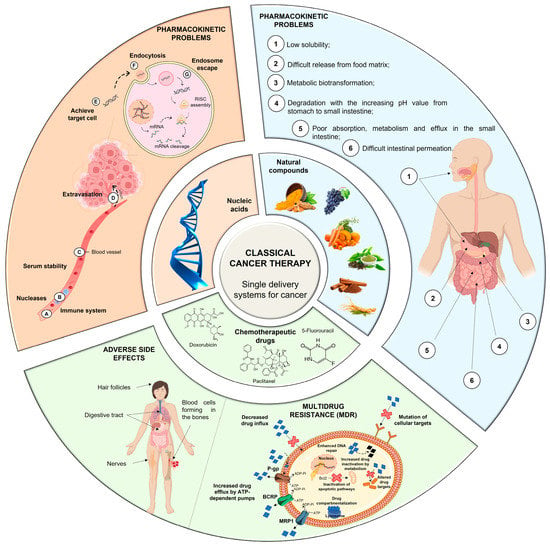
2.1. Overcoming Multidrug Resistance
MDR is a mechanism that emerges after cells’ exposure to chemotherapeutic agents and refers to the capacity of cancer cells to become resistant to the agents’ effect and can result in the development of malignant cell metastases [36][37][40,41]. The cellular mechanisms of MDR can be divided into two general classes: (i) those that block the delivery of chemotherapeutic agents to their target sites, and include the abnormal vasculature which results in low oral chemotherapeutic absorption, early renal clearance, poor bioavailability, and lower tumor site accumulation; or (ii) those that emerge in cancer cells primarily as a result of genetic and epigenetic alterations and directly affect the efficacy of chemotherapeutic agents, and include apoptosis deregulation, increased repair of drug-induced DNA damage, and, enhanced efflux of chemotherapeutic agents [36][37][40,41]. Although a wide range of different factors can contribute to MDR, drug efflux changes are considered the major cause of classical MDR [38][42]. Drug efflux is enhanced by the overexpression of human ATP-binding cassette (ABC) membrane transporters. These transporters are accountable for removing chemotherapeutic agents from cancer cells. Among the ABC transporters, the multidrug resistance protein (MRP) P-glycoprotein (P-gp) is an ATP-dependent drug efflux pump also referred to as multidrug resistance protein 1 (MRP1) (Figure 1). P-gp, the best-studied drug efflux pump, is a significant contributor to chemotherapy failure [38][39][42,43]. Furthermore, it has been reported that resistant cells have significantly greater levels of P-gp, and their overexpression is linked to a poor prognosis in a variety of cancers [40][44]. P-gp-mediated MDR affects several classes of chemotherapeutic agents, such as anthracyclines (e.g., daunorubicin and doxorubicin (DOX)), taxanes (e.g., paclitaxel (PTX) and docetaxel (DTX)), epipodophyllotoxins (e.g., etoposide), and camptothecins (e.g., topotecan and methotrexate (MTX)). As a result, strategies to reverse P-gp-mediated MDR have been extensively researched since the early 1980s, and three generations of P-gp inhibitors are currently classified [36][37][41][40,41,45]. Despite promising in vitro results, there is not, unfortunately, an irrefutable proof of efficacy for the currently available inhibitors, since various clinical trials have been performed to evaluate their anticancer effect, but no significant improvements have been found [21][36][21,40]. The development of an ideal inhibitor is commonly associated with the difficulty of finding compounds with high potency and specificity, and with low intrinsic toxicity. Furthermore, it is difficult to achieve specificity of the inhibitors to the ABC transporters, as well as interactions between chemotherapeutic agents and inhibitors [21]. Consequently, in order to overcome such limitations, researchers have shifted their attention to novel approaches for MDR prevention in cancer. In this regard, natural compounds have emerged as an appealing solution, primarily due to their chemosensitizing capacity [42][46]. Chemosensitizers are small molecules that can increase the sensitivity of cancer cells to chemotherapeutic agents, and those that act as ABC membrane transporter inhibitors are particularly effective. The main example is inhibitors obtained from natural sources, also known as fourth-generation inhibitors, which can interact with ATP binding sites or act directly at MRP binding sites. Natural inhibitors have the potential to be considerably more successful since they offer the most diverse and innovative chemical scaffolds [21]. Moreover, natural compounds with anticancer properties are widely available, as evidenced by the Naturally Occurring Plant-based Anti-Cancer Compound-Activity-Target Database (NPACT) [43][47]. The main natural compounds evaluated as chemosensitizing agents are highlighted in Figure 2.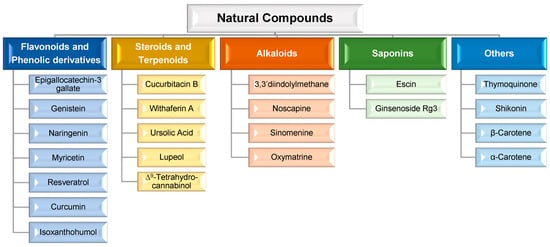
2.2. Synergistic, Additive, and Potentiation Effects
The combination of therapeutic agents can result in the following complementary effects [35][44][35,48]: (i) synergistic, when the final effect is greater than the sum of individual agents’ effects, resulting in cooperative targeting of activity regulation but with each agent targeting different sites; (ii) additive, that promotes greater or equal effect to the sum of individual agents’ effect; however, both agents act on the same target or pathway; and, (iii) potentiation, in which one agent can enhance the effect of the other or minimize its side effects by regulating pharmacokinetics and/or pharmacodynamics. Furthermore, when both agents in a combination therapy act on the same pathway or target, an undesirable antagonist effect may occur (i.e., when the resultant therapeutic effect is less than the sum of effects of each agent delivered).2.3. Reducing the Side Effects
Combination therapy may also avoid the toxic side effects that normally affect healthy cells. This could happen if one of the co-delivered agents is antagonistic to the other in terms of cytotoxicity. For example, antioxidant supplementation during anticancer treatment may decrease adverse reactions, primarily due to the prevention of reactive oxygen species (ROS)-mediated injury, without compromising anticancer activity [43][47].2.4. Decreasing the Effective Chemotherapy Dose
One significant drawback of chemotherapy is the high dose of cytotoxic drugs required to achieve a therapeutic effect, which causes serious side effects. In this context, combination therapy appears to be a promising alternative, since the combination of a natural compound and a chemotherapeutic drug may promote an increase in the cytotoxic effect (due to previously described synergistic, additive, or potentiation effects), improve chemotherapeutic performance, and reduce the effective dose required to achieve the necessary therapeutic outcomes [43][47].3. Conclusions
Conventional cancer therapies are still unable to achieve the desired outcomes due to current limitations related with inefficiency and selectivity. As a result, the development of novel therapeutic strategies has become critical. Combination therapy has been extensively explored in this context, since co-delivery of natural compounds and chemotherapeutic agents or nucleic acids can achieve stronger anticancer effects via synergistic/additive/potentiation mechanisms, or by improving selectivity, and overcoming MDR.
However, while this strategy provides new therapeutic results, it also introduces several new challenges, such as the need to clearly identify the mechanism behind the enhanced anticancer activity. It is also required to better define the concentration-dependent effect of natural compounds, as well as to evaluate the improvement of their pharmacokinetic parameters when delivered by lipid-based nanocarriers. Moreover, as far as we know, no clinical trials with nanocarriers co-delivering natural compounds or other therapeutic agents have been performed.
Despite the critical points that remain unresolved, the co-delivery strategy of natural compounds and chemotherapeutic agents/nucleic acids is undeniably very promising, especially by further exploring versatile nanocarriers such as LLCNs.
