Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Conner Chen and Version 2 by Conner Chen.
Nanocellulosic materials have attracted special attention because of their performance in different advanced applications, biodegradability, availability, and biocompatibility. Nanocellulosic materials can assume three distinct morphologies, including cellulose nanocrystals (CNC), cellulose nanofibers (CNF), and bacterial cellulose (BC).
- cellulose nanocrystals
- cellulose nanofibers
- bacterial cellulose
1. Introduction
Cellulose was discovered by Payen Anselme, a French chemist, in 1838 [1] It is a renewable biopolymer, the most abundant in nature, with estimated natural production of about 1.5 × 1012 tons per year [2]. Besides being abundant, cellulose is nontoxic, biocompatible, and biodegradable. The main sources of cellulose are plants, algae, bacteria, and tunicates (marine invertebrates) [3][4]. Cellulose is a natural macromolecule formed by 300–15,000 units of β-d-glucopyranose, bound by β-1,4-glycoglycodic linkages, and possessing an amorphous–crystalline supramolecular structure [5] Cellulose’s crystalline counterpart is rod-shaped and is found in the structure of elementary fibrils (EFs), where crystalline and amorphous regions alternate [5]. EFs are assembled in microfibrils and macrofibrils, which in turn make up the cell skeleton in plants (Figure 1). Cellulose crystallites can be isolated from native celluloses in different morphologic forms, and these so-called nanocelluloses are nanomaterials. By definition, nanocelluloses have at least one dimension in the nanometric range; i.e., they have a dimension of less than 100 nm [6][7], according to ISO/TS 20477:2017 [8][9]. Due to their unique properties, nanocelluloses can be used in numerous applications in different advanced materials, one of the most exciting being electronic devices and triboelectric nanogenerators (TENGs).
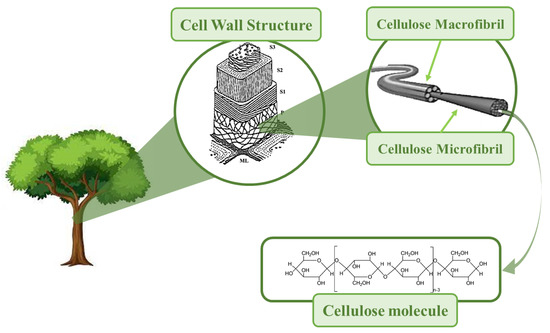
Figure 1. The simplified structural hierarchy of cellulose from a molecule to wood tissue.
The followis reviewng contents focuses on the methods for obtaining nanocellulose and its nanotechnological applications, and is composed of two main parts. In the first part of the reviewcontents, a brief characterization of the three cellulose nanomorphologies is performed, namely cellulose nanocrystals (CNCs), cellulose nanofibers (CNFs), and bacterial cellulose (BC), along with their preparation methods. It should be noted that the terms cellulose nanocrystals, cellulose nanofibers, and bacterial cellulose are strictly established in ISO/TS 20477: 2017 [8] The methods of nanocellulose preparation include such approaches as “bottom-up” or “top-down” [7][10] The mechanical, chemical, and enzymatic methods belong to a “top-down” approach for the production of cellulose nanocrystals (CNCs) and cellulose nanofibers (CNFs), while the “bottom-up” approach is commonly used for BC production. In relation to mechanical methods, methods for preparing CNFs through the disaggregation of cellulose fibers via defibrillation are reviewed. Within the category of mechanical methods, the following approaches are considered: refining, high-pressure homogenization, microfluidization, grinding, ball-milling, cryogenic crushing, steam explosion, ultrasound, extrusion, aqueous counter collision, and electrospinning [11][12]. For these mechanical methods, their functioning is presented, along with some illustrative schemes. In the scope of mechanical methods, there was also the aim to gather a set of investigations whose objective was to evaluate how operational parameters influenced the properties of CNFs. Thus, for each mechanical method and its operational parameters that interfere with the properties of CNF, a set of investigations was gathered in this study whose objective was to evaluate the relationship between the operational parameters and the properties of CNFs.
The chemical pre-treatment methods commonly applied before mechanical treatments were also reviewed. These are mainly intended for the production of CNFs. Among chemical pretreatments, acid- and alkali-catalyzed organosolvation, TEMPO-mediated oxidation, oxidation with APS and with SPS, ozone pre-treatment, extraction with ionic liquids [12][13][14][15], and two innovative methods for obtaining CNFs were described. One of these new methods consists of simultaneous bleaching and oxidative modification with TEMPO [16] and the other employs sodium persulfate (SPS) with ultraviolet light [17]. Thies reviewe contents also presents the chemical treatments for the production of CNC, which are essentially variations of acid hydrolysis, as it degrades the amorphous part of the cellulose, leaving the crystalline part in the form of CNC [12][15]. Regarding chemical methods of CNC production, it should be noted that the TEMPO system, which has been used for the preparation of CNFs (the so-called TEMPO-CNF), is also suitable for CNC production [18]. ThisSome reviewcontents also covers enzymatic hydrolysis pretreatment, considered by some researchers as the “most ecological route” for CNF production [13][15]..Enzymatic hydrolysis is carried out by cellulases, which more rapidly degrade the disordered cellulose counterpart of fibers. The three types of cellulases (endocellulases, exocellulases, and β-glucosidases), as well as their degradation modes, are specified. The first part of the rconteviewnts also contains a set of approaches in which chemical and enzymatic treatments were successfully combined in the production of nanocelluloses (CNFs and CNCs), and the properties of these nanocelluloses (diameter, length, and crystallinity index) are discussed.
2. Nanocellulose Nanomorphologies
Cellulosic materials can be converted into cellulose nanofibers (CNFs) and nanocrystals (CNCs), depending on the mechanical and chemical treatment applied (Table 1). In addition to these two nanomorphologies, bacterial nanocellulose, BC, also exists. These three types of nanocellulose (CNF, CNC, and BC) have different average sizes (Table 1). However, it should be noted that, for each type of nanocellulose, the values of the average sizes found in the literature present slight differences. These differences are mainly due to the different sources used and the various methods of preparation [12][19].Table 1. Medium size, sources, and preparation of the various cellulose nanomorphologies (adapted from [1][4][11]).
| Approach | Nanomorphologies | Sources | Medium Size | Preparation |
|---|---|---|---|---|
| “Top-down” | Cellulose nanocrystals (CNCs) | Wood, cotton, hemp, linen, straw, tubers, tunicates, algae, bacteria, etc. | Diameter—5–70 nm Length—100 nm to several micrometers | Chemical treatment in the form of acid hydrolysis of cellulose (or enzyme-assisted hydrolysis) |
| “Top-down” | Cellulose nanofibers (CNFs) | Wood, cotton, hemp, beetroot, etc. | Diameter—5–60 nm Length—several micrometers | Defibrillation of wood pulp by mechanical treatment before and/or after chemical (and/or enzymatic) processes |
| “Bottom-up” | Bacterial nanocellulose (BC) | Low-molecular-weight sugars and alcohols | Diameter—20–100 nm Various kinds of nanofiber nets | Synthesized by bacteria |
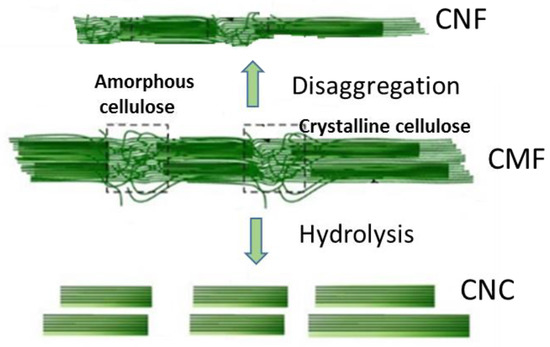
Figure 2. Schematic representation of cellulose nanofibrils (CNFs) production by disaggregation of cellulose microfibrils (CMFs) in the cell wall and the production of cellulose nanocrystals (CNCs) by the hydrolytic degradation of the amorphous part of CMF.
References
- Bhat, A.H.; Khan, I.; Usmani, M.A.; Umapathi, R.; Al-Kindy, S.M.Z. Cellulose an ageless renewable green nanomaterial for medical applications: An overview of ionic liquids in extraction, separation and dissolution of cellulose. Int. J. Biol. Macromol. 2019, 129, 750–777.
- Zhang, C.; Mo, J.; Fu, Q.; Liu, Y.; Wang, S.; Nie, S. Wood-cellulose-fiber-based functional materials for triboelectric nanogenerators. Nano Energy 2021, 81, 105637.
- Mishra, R.K.; Sabu, A.; Tiwari, S.K. Materials chemistry and the futurist eco-friendly applications of nanocellulose: Status and prospect. J. Saudi Chem. Soc. 2018, 22, 949–978.
- Thomas, P.; Duolikun, T.; Rumjit, N.P.; Moosavi, S.; Lai, C.W.; Bin Johan, M.R.; Fen, L.B. Comprehensive review on nanocellulose: Recent developments, challenges and future prospects. J. Mech. Behav. Biomed. Mater. 2020, 110, 103884.
- Wang, J.; Li, X.; Song, J.; Wu, K.; Xue, Y.; Wu, Y.; Wang, S. Direct Preparation of Cellulose Nanofibers from Bamboo by Nitric Acid and Hydrogen Peroxide Enables Fibrillation via a Cooperative Mechanism. Nanomaterials 2020, 10, 943.
- Allan, J.; Belz, S.; Hoeveler, A.; Hugas, M.; Okuda, H.; Patri, A.; Rauscher, H.; Silva, P.; Slikker, W.; Sokull-Kluettgen, B.; et al. Regulatory landscape of nanotechnology and nanoplastics from a global perspective. Regul. Toxicol. Pharmacol. 2021, 122, 104885.
- Mazela, B.; Perdoch, W.; Peplińska, B.; Zieliński, M. Influence of Chemical Pre-Treatments and Ultrasonication on the Dimensions and Appearance of Cellulose Fibers. Materials 2020, 13, 5274.
- ISO/TS 20477:2017; Nanotechnologies—Standard Terms and Their Definition for Cellulose Nanomaterial. International Organization for Standardization: London, UK, 2017. Available online: https://www.iso.org/obp/ui/#iso:std:iso:ts:20477:ed-1:v1:en (accessed on 3 April 2021).
- Charreau, H.; Cavallo, E.; Foresti, M.L. Patents involving nanocellulose: Analysis of their evolution since 2010. Carbohydr. Polym. 2020, 237, 116039.
- Filipova, I.; Serra, F.; Tarrés, Q.; Mutjé, P.; Delgado-Aguilar, M. Oxidative treatments for cellulose nanofibers production: A comparative study between TEMPO-mediated and ammonium persulfate oxidation. Cellulose 2020, 27, 10671–10688.
- Wang, D. A critical review of cellulose-based nanomaterials for water purification in industrial processes. Cellulose 2019, 26, 687–701.
- Yadav, C.; Saini, A.; Zhang, W.; You, X.; Chauhan, I.; Mohanty, P.; Li, X. Plant-based nanocellulose: A review of routine and recent preparation methods with current progress in its applications as rheology modifier and 3D bioprinting. Int. J. Biol. Macromol. 2021, 166, 1586–1616.
- Kargarzadeh, H.; Ioelovich, M.; Ahmad, I.; Thomas, S.; Dufresne, A. Methods for Extraction of Nanocellulose from Various Sources. In Handbook of Nanocellulose and Cellulose Nanocomposites; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 1–49. ISBN 978-3-527-68997-2.
- Thakur, V.; Guleria, A.; Kumar, S.; Sharma, S.; Singh, K. Recent advances in nanocellulose processing, functionalization and applications: A review. Mater. Adv. 2021, 2, 1872–1895.
- Xie, H.; Du, H.; Yang, X.; Si, C. Recent Strategies in Preparation of Cellulose Nanocrystals and Cellulose Nanofibrils Derived from Raw Cellulose Materials. Int. J. Polym. Sci. 2018, 2018, e7923068.
- Kaffashsaie, E.; Yousefi, H.; Nishino, T.; Matsumoto, T.; Mashkour, M.; Madhoushi, M.; Kawaguchi, H. Direct conversion of raw wood to TEMPO-oxidized cellulose nanofibers. Carbohydr. Polym. 2021, 262, 117938.
- Wang, C.; Yuan, Z.; Wang, A.; Qu, J.; Fang, Z.; Wen, Y. Ultraviolet light enhanced sodium persulfate oxidation of cellulose to facilitate the preparation of cellulose nanofibers. Cellulose 2020, 27, 2041–2051.
- Isogai, A.; Zhou, Y. Diverse nanocelluloses prepared from TEMPO-oxidized wood cellulose fibers: Nanonetworks, nanofibers, and nanocrystals. Curr. Opin. Solid State Mater. Sci. 2019, 23, 101–106.
- Guo, R.; Zhang, L.; Lu, Y.; Zhang, X.; Yang, D. Research progress of nanocellulose for electrochemical energy storage: A review. J. Energy Chem. 2020, 51, 342–361.
- Lin, N.; Dufresne, A. Nanocellulose in biomedicine: Current status and future prospect. Eur. Polym. J. 2014, 59, 302–325.
- Golmohammadi, H.; Morales-Narváez, E.; Naghdi, T.; Merkoçi, A. Nanocellulose in Sensing and Biosensing. Chem. Mater. 2017, 29, 5426–5446.
- Araldi da Silva, B.; de Sousa Cunha, R.; Valério, A.; De Noni Junior, A.; Hotza, D.; Gómez González, S.Y. Electrospinning of cellulose using ionic liquids: An overview on processing and applications. Eur. Polym. J. 2021, 147, 110283.
- Phanthong, P.; Reubroycharoen, P.; Hao, X.; Xu, G.; Abudula, A.; Guan, G. Nanocellulose: Extraction and application. Carbon Resour. Convers. 2018, 1, 32–43.
- Cazón, P.; Vázquez, M. Bacterial cellulose as a biodegradable food packaging material: A review. Food Hydrocoll. 2021, 113, 106530.
More
