Being immune privileged, the central nervous system (CNS) is populated by unique parenchymal and non-parenchymal tissue-resident macrophages, namely, microglia and border-associated macrophages (BAMs), respectively. BAMs are found in the choroid plexus, meningeal and perivascular spaces, playing critical roles in maintaining CNS homeostasis while being phenotypically and functionally distinct from microglial cells. Although the ontogeny of microglia has been largely determined, BAMs need comparable scrutiny as they have been recently discovered and have not been thoroughly explored. Shedding light on the molecular cues and drivers orchestrating BAM generation is essential for delineating their cellular identity. BAMs are receiving more attention since they are gradually incorporated into neurodegenerative and neuroinflammatory disease evaluations. Understanding the ontogeny of BAMs and their involvement in CNS diseases paves the way for targeted therapeutic strategies and precision medicine.
- CNS border-associated macrophages
- tissue-resident macrophages
- origin
- yolk sac
- molecular cues
- development
- disease
1. Introduction
2. Origin of BAMs during Embryogenesis and Adulthood
The BAM embryonic origin was first investigated in rodents using bone marrow chimeras and whole-body irradiation, proposing that BAMs are bone marrow-derived [37][38][37,38]. In 2016, Goldmann et al., performing fate mapping analysis, observed that BAMs originate from the mouse yolk sac’s early erythro-myeloid progenitors (EMPs) during embryogenesis [8]. A tamoxifen-inducible Runx1CreERR26YFPfate-mapping mouse model confirmed that BAMs originate from early EMPs in the yolk sac, which gave rise to two different macrophage populations, namely, CD206+ (BAM progenitors) and CD206− (microglial progenitors) without the contribution of fetal liver or definitive hematopoiesis [20]. Interestingly, the mannose receptor C-type 1 (MRC1 or CD206) is a unique marker for BAMs [8][12][14][8,12,14]. The expression of Mrc1 is upregulated from E8.5 when the primitive macrophages, originating from EMPs, prepare to invade the embryonic tissues [39]. Recently, Masuda et al. investigated the progenitors of BAMs utilizing single-cell RNA sequencing and fate mapping analysis in the Mrc1CreERT2 mouse model. Although flow cytometry confirmed the presence of a CD206+ subpopulation within the A2 cells (CD45+ c-kit− CX3CR1+ cells), meningeal macrophages and microglia were found to originate from common CD206+ A2 progenitors in contrast with previous results [20][40][20,40]. The pvΜΦ were generated postnatally from sdΜΦ, requiring integrin-signaling and vascular smooth muscle cells (VSMCs) [40]. Regarding the repopulation pattern of BAMs in adulthood, there is a great heterogeneity between BAM clusters; specifically, the sdΜΦ, pvMΦ and cpepiΜΦ exhibit similar longevity with microglial cells as being self-maintained in the CNS independently from blood monocytes’ contribution [8][14][8,14]. The cpepiΜΦ were solely derived from local SALL1+ macrophages [14]. In Ccr2-deficient mice, the number of cpMΦ decreased, revealing their replenishment from Ly6Chi monocytes and shorter turnover [8]. In accordance with these results, Van Hove et al., combining single-cell RNA sequencing with complementary approaches in mice, suggested that dmΜΦ and cpΜΦ were gradually replenished by bone marrow-derived monocytes [14]. As dura mater and choroid plexus stroma are more accessible brain regions than (i) subdural space, (ii) the apical surface of the choroid plexuses, and (iii) brain parenchyma, the tissue permeability may be considered a crucial factor for brain macrophage ontogeny. However, the ablation of BAMs through CSF1R blockade led to the replenishment of cpΜΦ and dmΜΦ via local expansion, indicating their self-renewal capacity, while sdΜΦ presented difficulties in their repopulation [14]. By utilizing the Cx3cr1CreER:R26tdTomato fate mapping system in an experimental autoimmune encephalomyelitis (EAE) mouse model, Jordão et al. proposed that BAMs remained stable and locally self-renewed in addition to the recruitment of bone marrow-derived progenitors [12]. In Cx3cr1gfpCcr2rfp bone marrow chimeric mice, CD169+ BAMs proliferated after ischemia, while a small proportion of BAMs was bone marrow-derived, populating the perivascular and ischemic regions [36]. Both in homeostasis and disease, skull and vertebrae bone marrow constitute a pool of myeloid cells that can invade non-parenchymal and parenchymal CNS regions, transforming into tissue-resident macrophages [41]. A fate-mapping analysis in a mouse model of Alzheimer’s disease (AD) revealed that BAMs are a stable cell population with an unaffected turnover rate and a minimal replenishment from bone marrow-derived cells during this neurodegenerative disease [42]. Summarizing, the origin of BAMs has been extensively studied in the last few years using new genetic tools, e.g., fate mapping analysis. It has been proposed that BAMs originate from early EMPs in the yolk sac during embryogenesis. Although specific BAMs are replenished by peripherally-derived monocytes postnatally, some remain solely derived from the local pool. BAMs have been shown to remain stable and locally self-renewed in both homeostasis and disease. Further investigation is needed to (i) confirm BAM origin, (ii) detect the precise embryonic progenitors of BAMs, especially of the dura mater and choroid plexus macrophages, (iii) determine the timing of each BAM subpopulation’s generation, and (iv) delineate their repopulation pattern.3. Molecular Drives Orchestrating BAM Development
The transcription factor PU.1 (or SFPI) could be essential for the BAM generation during embryonic development since research has showed that in mice with deletion of the Sfpi1 gene, pvMΦ, sdMΦ, and cpMΦ were ablated [8]. Progenitors of BAMs express the runt-related transcription factor 1 (RUNX1), which regulates the expression of PU.1 during embryogenesis [20][43][20,43]. The impairment of PU.1 factor in mice results in a reduced number of A1 (CD45+ c-kitlo CX3CR1− immature cells) and A2 (CD45+ c-kit− CX3CR1+ cells) progenitor cells of the yolk sac, from which both microglial cells and BAMs originate. In contrast, the lack of interferon regulatory factor 8 (IRF8) exclusively decreased the number of A2 cells [44]. Furthermore, the colony-stimulating factor 1 receptor (CSF1R) signaling could be essential for BAM development [5][11][14][5,11,14]. In a zebrafish model carrying the panther mutation, a loss-of-function mutation in the fms gene orthologue which encodes CSF1R, primitive macrophages of the yolk sac could not colonize the embryonic tissues [45]. After progenitors’ migration and invasion in the CNS, BAM generation is initiated (Figure 1). The BAMs may be developed independently of transforming growth factor beta receptor (TGF-βR) signaling. In Tgfbr2-deficient mice, no alteration in cell numbers of BAMs occurred, while transforming growth factor beta (TGF-β) is required for the generation of microglial cells [20][46][20,46]. Three main brain border regions are filled with BAMs, namely, meninges, choroid plexus, and perivascular spaces. The postnatal expansion of sdMΦ was influenced by IRF8 and MAFB [40]. Indeed, in Irf8-deficient mice, a reduction of sdMΦ was observed [8]. The lack of integrin subunit beta 1 (ITGB1) in mice resulted only in a minor change in the numbers of sdΜΦ [40]. Similarly, the absence of insulin-like growth factor 1 (IGF1R) induces transcriptomic changes in BAMs via its implication in RNA processing, growth, migration and intracellular signaling [47]. The MYB, BATF3, and NR4A1 transcription factors were not necessary for BAM development [8].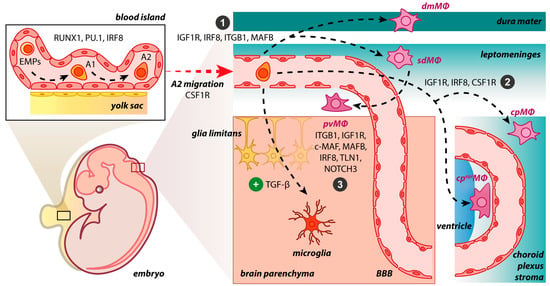
4. BAMs vs. Microglia
Although microglia and BAMs are immune-competent cells of the CNS with common progenitors, their different localization may contribute to variations in their biological roles. The microglial populations’ functions have been reviewed in detail [57][58][59][65,66,67]. Concisely, microglial cells are involved in developmental processes, including cell positioning, survival, myelinogenesis, synaptic patterning, and axonal dynamics [60][68]. In adult CNS, microglia, as the regulators of acute and chronic immune responses, are implicated in removing pathogens and noxious particles, scavenging cellular debris and synapses, protecting neural tissue, and mediating neurogenesis in CNS injury [24][58][61][24,66,69]. The unique localization of BAMs between brain parenchyma and peripheral tissues pinpoint their pivotal role in the immune surveillance of pathological antigens [16]. Their antigen-presenting capacity is attributed to MHC II molecules on some BAM surfaces [12][32][62][63][12,32,70,71]. Furthermore, the pvΜΦ and dmΜΦ mainly phagocytose intruding pathogens and any foreign molecule or substance that can be detected in the bloodstream and cerebrospinal fluid [64][64][72,72]. The pvΜΦ also appear to regulate the accessibility of brain parenchyma to circulating cells and molecules by increasing the contractility of regional vessels and capillaries or diminishing the BBB permeability [65][66][67][68][73,74,75,76]. Interestingly, the latest approaches demonstrate the involvement of BAMs in ensuring a well-balanced metabolic environment for neurons, especially in the course of systemic perturbations [69][70][77,78]. Data regarding morphology, motility, and molecular identity of microglia and BAMs are summarized in Table 1.|
Cell Type | Cell Type |
Morphology | Morphology |
Motility | Motility |
Cell-Specific Markers | Cell-Specific Markers |
|---|---|---|---|---|---|---|---|
|
Microglia | Microglia |
Ramified in homeostasis; Amoeboid in inflammation | Ramified in homeostasis; Amoeboid in inflammation |
Cell bodies with limited-motility but highly dynamic processes in homeostasis; Highly phagocytic in inflammation | Cell bodies with limited-motility but highly dynamic processes in homeostasis; Highly phagocytic in inflammation |
SIGLEC-H+, P2RY12+, HEXB+, TMEM119+, ANXA3+, SALL1+ | SIGLEC-H+, P2RY12+, HEXB+, TMEM119+, ANXA3+, SALL1+ |
|
pvΜΦ | pvΜΦ |
Slightly elongated cell bodies | Slightly elongated cell bodies |
Non-motile cell bodies with extending and retracting projections through the blood vessel wall in homeostasis; Dendritic-like processes in inflammation | Non-motile cell bodies with extending and retracting projections through the blood vessel wall in homeostasis; Dendritic-like processes in inflammation |
CD206+, CD38+, LYVE1+, CD36+, CD163+, CD169+ | CD206+, CD38+, LYVE1+, CD36+, CD163+, CD169+ |
|
dmΜΦ | dmΜΦ |
Elongated; Spindle-shaped cells; Few thick membrane projections; Dendriform | Elongated; Spindle-shaped cells; Few thick membrane projections; Dendriform |
Limited motility and highly dynamic protrusions in homeostasis; Extending projections in inflammation | Limited motility and highly dynamic protrusions in homeostasis; Extending projections in inflammation |
||
|
sdΜΦ | sdΜΦ |
Elongated; Amoeboid; Spindle-shaped cells; Few thick membrane projections | Elongated; Amoeboid; Spindle-shaped cells; Few thick membrane projections |
Limited motility and highly dynamic protrusions in homeostasis; Extending projections in inflammation | Limited motility and highly dynamic protrusions in homeostasis; Extending projections in inflammation |
||
|
cpΜΦ | cpΜΦ |
Star-like shape | Star-like shape |
Unknown | Unknown | ||
|
cpepiΜΦ | cpepiΜΦ |
Round; Bipolar; Stellate | Round; Bipolar; Stellate |
Unknown | Unknown |
5. BAMs in Neurological Diseases and Promising Therapies
The implication of BAMs in the pathogenesis of CNS diseases, especially in neurodegeneration and neuroinflammation, is a rapidly emerging field of research. Although the precise role of BAMs in diseases is not yet elucidated, recent studies have addressed their potential involvement in several pathological conditions such as Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, and stroke. Further experimental studies are needed to delineate the exact pathophysiological mechanism through which methodical manipulation of BAMs can halt or even reverse the progression of the aforementioned debilitating CNS diseases.
