You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Lindsay Dong and Version 1 by Charalampos Proestos.
Cyanidin 3-O-glucoside (C3G) is a secondary metabolite naturally present in plants. It is a water-soluble bioactive compound belonging to the class of monomeric anthocyanin, one of the main classes of the flavonoid family. Recently, tThere has been considerable interest in work on anthocyanin derivatives. There are two main reasons for this increased interest: (i) anthocyanin derivatives are important sources of natural pigments primarily used as food colorants, (ii) and their regular consumption has significant health benefits.
- cyanidin 3-O-glucoside
- extraction
- nanoencapsulation
1. Chemical Structure and Health Properties of Cyanidin 3-O-Glucoside
Cyanidin 3-O-glucoside (C3G) (Figure 1) is an anthocyanin compound derived from the aglycone cyanidin. It is a red pigment of anthocyanin commonly and naturally found in vegetables and fruits. C3G is highly hydrophilic, with a solubility of 0.6 mg/mL, LogP of 0.39, Å2 of 193.4, [M]+ (m/z) of 449, and MS/MS (m/z) of 287 [12][1].
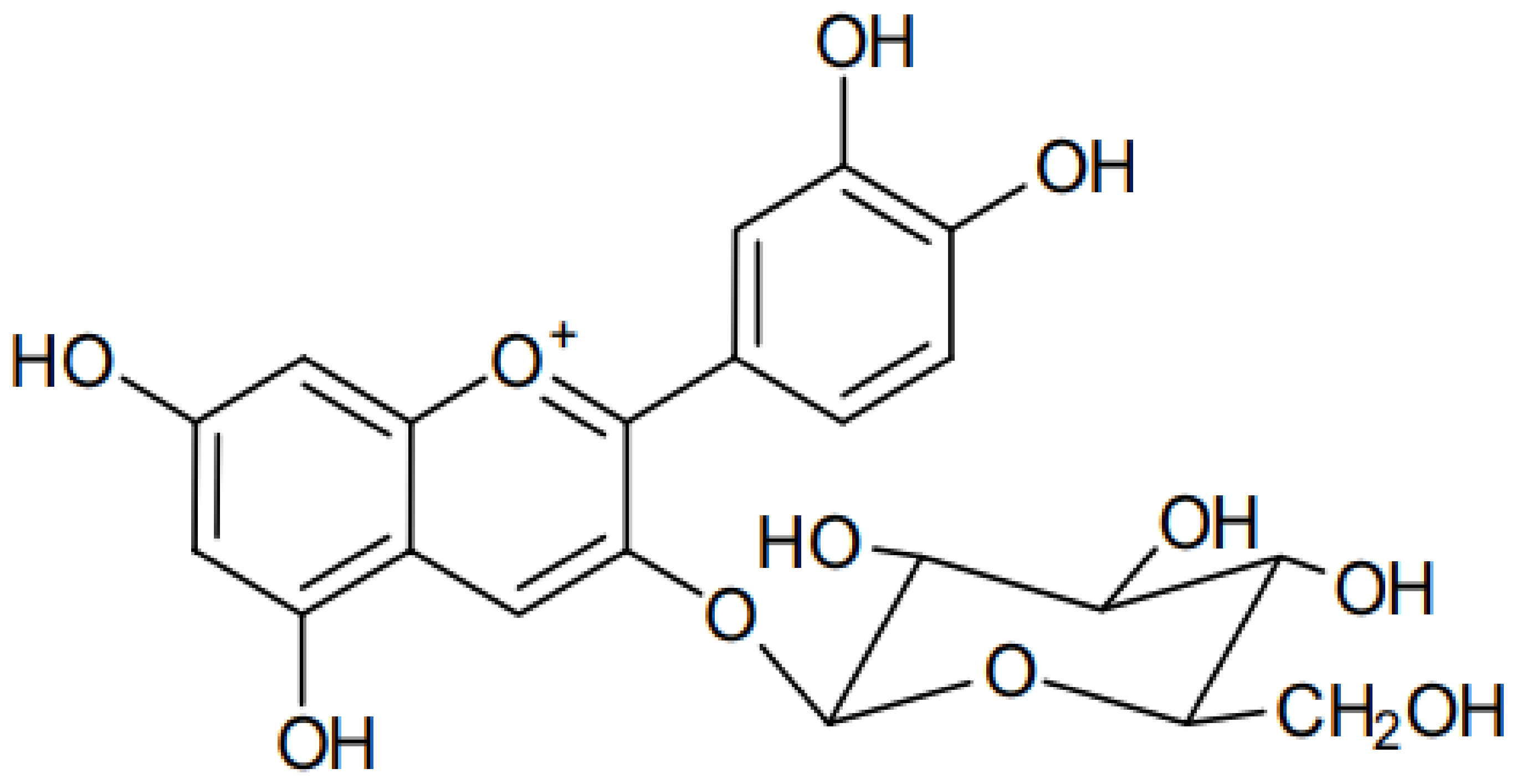
Figure 1.
Chemical structure of cyanidin 3-
O
-glucoside.
The UV-vis spectrum of C3G at different pH (pH 3–8) revealed that C3G is pH-dependent [13][2]. In this case, it was found that the flavylium cation absorbance is dominant at pH 3, whose intensity decreases at pH 4–5, but at pH 5, the quinoidal species starts dominating. At pH 6–8, cyandin-3-glucoside takes the quinoidal and chalcone forms.
C3G is a well-known natural anthocyanin and possesses tremendous health benefits. Wang et al. [14][3] studied the ability of C3G and its phenolic acid metabolites to attenuate the visible light-induced retinal degeneration and found out that the C3G, protocatechuic acid, and ferulic acid caused a higher secretion and expression of heme oxygenase (HO-1) and nuclear factor erythroid-2-related factor 2 (Nrf2), indicating the attenuation of the secretion or expression of inflammation-related genes. Likewise, the treatments attenuated the photo-oxidation-induced apoptosis and angiogenesis in the retina. Du et al. [15][4] reported that the C3G as well as cyanidin chloride hinder the accumulation of cholesterol and inhibit the LXRα pathway-induced inflammatory response. In the study of Chen et al. [16][5], it was found that the C3G and peonidin 3-glucoside exhibited anticancer activity via G2/M arrest with peonidin 3-glucoside treatment, showing downregulation of protein levels of cyclin-dependent kinase (CDK)-1, CDK-2, cyclin B1, and cyclin E, while cyanidin 3-O-glucoside reduced the protein levels of CDK-1, CDK-2, cyclin B1, and cyclin D1. The potency of C3G to improve the hyperglycemia and insulin sensitivity in diabetic mice fed with dietary C3G for 5 weeks was investigated [17][6].
2. Extraction of Cyanidin 3-O-Glucoside
The recovery of anthocyanins is affected by the extraction techniques and extraction conditions, such as temperature, type of solvents, solvent ratio, and extraction time [24,25][7][8]. The influence of extraction techniques, type of solvents. Conventional techniques such as Soxhlet extraction and maceration are widely used for extraction of phenolic compounds such as C3G. For example, Santos et al. [26][9] successfully applied the Soxhlet extraction to recover C3G from Jabuticaba (Myrciaria cauliflora) skins. Likewise, Ćujić et al. [27][10] and Meregalli et al. [28][11] determined that the maceration extraction can be used to extract the C3G from various plant materials. Nonetheless, considering these studies on the extraction of C3G by conventional techniques, it can be evidenced that they are time-consuming and mainly depend on the type of solvent. The emerging extraction technologies proved to be more efficient regarding the extraction yield of C3G. A comparative study implying ultrasound-assisted extraction, agitated bed extraction, combined ultrasound-assisted extraction + agitated bed extraction, and Soxhlet extraction has been carried out for the extraction of C3G, and the results showed that the use of shorter ultrasonic irradiation is viable and increased the extraction performance [26][9]. Although the use of ultrasound-assisted extraction can imply high energy consumption, it is more efficient, rapid, and selective regarding the extraction of anthocyanin than the conventional extraction techniques such as agitated bed extraction, maceration, and Soxhlet extraction [29,30][12][13]. Microwave-assisted extraction, which uses the energy of microwaves to induce the molecular movement and rotation of liquids with a permanent dipole, is also an emerging extraction technology for the recovery of C3G [31][14]. The implementation of microwave-assisted extraction causes, through the energy of microwaves, a rapid heating of the solvent and the sample, leading to the release of bioactive compounds. Microwave-assisted extraction has been found highly efficient for the extraction of anthocyanin derivatives such as C3G, pelargonidin 3-glucoside, and peonidin 3-glucoside from purple corn when compared to the conventional extraction procedure [32][15]. Ohmic heating technology is also based on the application of electric fields, and use of its thermal nature allows for a controllable heating rate, unrestricted treatment time scale, unrestricted type of waveform and frequency, and the presence of alternating moderate to low electric fields [34][16]. It is generally used as a pre-treatment prior to the extraction to increase the efficiency of the extraction technique. The grape skins have been pre-treated by ohmic heating, followed by aqueous extraction under a gentle orbital shaking system [34][16]. It resulted that ohmic heating allowed to boost extraction levels of total phenolic compounds, delphinidin 3-O-glucoside, C3G, petunidin 3-glucoside, peonidin 3-O-glucoside-3-glucoside, and malvidin 3-O-glucoside, as well as the conductivity, soluble solids, and red color intensity of the obtained extracts. The application of ohmic heating allows not only the increase of extraction yield but also reduces the extraction time [35][17]. High hydrostatic pressure is a cold pasteurization method used as a good alternative to heat-based treatments, consisting of subjecting samples to pressures up to 1000 MPa inside a vessel filled mainly with water, acting as a pressure-transmitting medium. During the high hydrostatic pressure processing, the pressure is transferred into the sample in an isostatic and quasi-instantaneous manner [36][18]. Recently, a comparative study of ultrasound-assisted, microwave-assisted, high hydrostatic pressure, and conventional extraction methods has been conducted for the extraction of C3G, and the results showed that the high hydrostatic pressure extraction technique can be used for the recovery of C3G, although ultrasound- and microwave-assisted extractions are more efficient [36][18]. Supercritical CO2 extraction uses carbon dioxide above its critical point and exhibits liquid- and gas-like proprieties. Supercritical and subcritical CO2 extractions have been commonly used to improve the extraction and selectivity of plant bioactive compounds. Babova et al. [38][19] applied the supercritical and subcritical CO2 extractions to maximize the recovery of anthocyanin from bilberry. These techniques have selectively and highly extracted C3G, as well as cyanidin 3-O-arabinoside, delphinidin 3-O-glucoside, ellagic acid pentoside, feruloyl hexoside, and quercetin glycosides. Moreover, the use of co-solvents such as water and ethanol in the supercritical CO2 extraction increases the recovery C3G [39][20]. Pressurized fluid extraction is a nonthermal technique that exposes the fluid to pressures above 6 bar and temperatures above 100 °C in the case of water [40][21].3. Different Nanocarriers of Cyanidin 3-O-Glucoside
The stability, controlled release, intestinal absorption, and bioavailability of nutraceuticals can be improved by a key strategy depending on the creation of nanocarriers. As preferable carrying materials, proteins, polysaccharides, and phospholipids obtained from food are biocompatible, biodegradable, and “generally recognized as safe” (Figure 2) [6][22].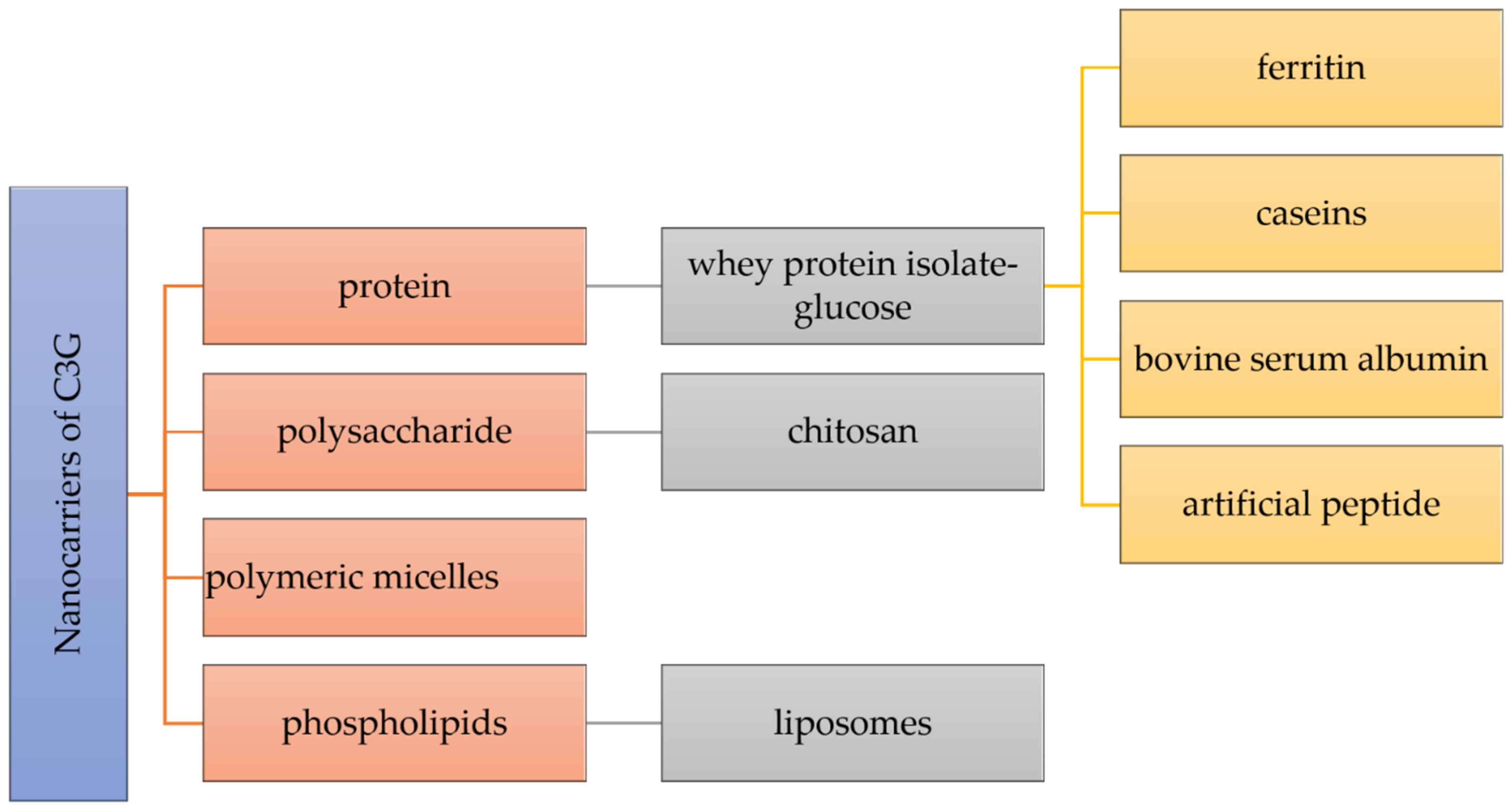
Figure 2.
Different nanocarriers used for the encapsulation of cyanidin 3-
O
Many types of nanocarriers have been utilized for the delivery of C3G, including whey protein isolate-glucose (WPI-Glu) nanoparticles, which demonstrated a unique protein delivery method that provides high stability of C3G in acidic and high-temperature conditions [43][23]. C3G molecules were encapsulated within a ferritin nanocage in order to increase their stability [44][24]. By encapsulating nanotechnology, protein cage designs such as ferritins provide a strong potential to increase the stability of C3G. The first protein with a shell-like structure employed for the creation of nanomaterials is ferritin, which is a particular kind of widely distributed iron-storage protein [44][24]. The molecule ferritin, which has a molecular mass of about 500 kDa and external and internal diameters of 12 and 8 nm, is made up of 24 subunits that self-assemble into a shell-like structure with 432 symmetries. Ferritin is used to oxidize and store iron as microcrystalline, hydrated ferric oxide particles. The protein shell of ferritin has a remarkable resistance to chemical and physical denaturants, and the reassembly technique might be utilized to encapsulate such unstable small species, such as C3G, that could not enter the protein cavity through the subunit junction pores [44][24]. Therefore, C3G was encapsulated in a ferritin nanocage using the reassembling ability of ferritin. It was discovered that such encapsulation significantly improved anthocyanin stability while facilitating C3G transport through Caco-2 cell monolayers [44][24].
Chitosan has attracted a lot of attention for the encapsulation and delivery of bioactive substances because of its exceptional functionality. However, in the latest mentioned study, in order to increase the stability of C3G, chitosan, chitosan oligosaccharides, and carboxymethyl chitosan were combined with ionic crosslinking agents γ-polyglutamic acid nanoparticles [49][25]. Similarly, Ge et al. generated new nanocomplexes in their work to stabilize C3G utilizing the chitosan derivatives chitosan hydrochloride (CHC) and carboxymethyl chitosan (CMC). These two distinct water-soluble chitosan derivatives, which have significant surface positive and negative charges, respectively, are able to increase the stability of C3G via intermolecular electrostatic interactions [52,53][26][27]. Chitosan is a linear cationic polysaccharide composed of β-(1–4)-linked D-glucosamine and N-acetyl-D-glucose-amine, obtained by the deacetylation of crustacean chitin. Chitosan is a non-toxic, biodegradable, biocompatible, film-forming, antioxidant, and antibacterial natural biopolymer that is second in abundance after cellulose in terms of natural biopolymers [11,52][26][28]. Moreover, the effects of C3G alone and encapsulated in chitosan nanoparticles (Nano-C3G) in a UVB-induced acute 4 photodamage animal model were assessed in another study for the encapsulation of C3G within chitosan [54][29].
-glucoside.
4. Nanoencapsulation Techniques of Cyanidin 3-O-Glucoside
4.1. Spray-Drying Encapsulation of Cyanidin 3-O-Glucoside
Spray-drying is considered one of the oldest and most popular processes used for the nano/encapsulation of anthocyanin’s components, including C3G, found in fruits and vegetables [70][30]. The data of encapsulation methods collected by some researchers showed that 31.37% of investigations were based on the spray-drying technique [69][31]. When compared to other encapsulation processes, spray-drying is known as an effective low-cost method, capable to obtain superior-quality dry particles using largely accessible equipment [67][32]. This process can be used to generate anthocyanin powder loaded with C3G with increased storage stability and favors a faster production and better control over the particle size dispersion [71][33]. The choice of barrier materials is fundamentally the most important step in this process, where some factors should be considered for strong protection of the core material based on its physicochemical properties [67][32]. For an effective bioactive compound protection, the spray-drying process is generally combined with biopolymer agents such as maltodextrin, cyclodextrin, gums, modified starch, lipids, and protein nanoparticles. Inulin and sodium alginate combined with spray-drying in the encapsulation of maqui juice showed encapsulation performances of 68.6 and 47.3%, respectively.4.2. Freeze-Drying Encapsulation of Cyanidin 3-O-Glucoside
Anthocyanin derivatives comprising C3G are very sensitive to high-temperature and heat treatments, and thus spray-drying application is sometime detrimental to anthocyanin components. Freeze-drying is regarded as one of the best drying candidates for anthocyanin, and especially for C3G encapsulation. This drying process offers the dehydration of frozen sample mixtures of core and nano/micro-carrier agents via sublimation under vacuum and reduced temperature, resulting in great chemical structure preservation and a well-reduced risk of unwanted changes in dried samples [75][34]. Thanks to the low operating temperature, it is a suitable method for anthocyanin and related phenolic components’ encapsulation as they are thermosensitive materials. This process yields high-quality products with acceptable sensory properties, preserves the bio-accessibility and functionality, extends the shelf-life for bioactive compounds, and improves the thermal and color stability of anthocyanin components through diverse carrier agents [76,77][35][36].4.3. Lipid-Based Encapsulation of Cyanidin 3-O-Glucoside
Among the nano/encapsulation methods, lipid-based micro/nanocarrier techniques are also one of the most applied processes for anthocyanin derivatives’ encapsulation. Two classes of lipid-based nanoencapsulation techniques can be distinguished. Emulsions, fabricated with oil, water, and surfactants in various forms. These emulsions can be formulated using a single oil in water (O/W), or vice versa (W/O), double-oil in water (O/W/O), or water in oil in water (W/O/W) emulsions [63][37]. These emulsions, including Pickering emulsions, are promising lipid-based encapsulation processes for food and related industries as anthocyanin-encompassing C3G distribution systems. The emulsion droplets used to trap these bioactive compounds may be obtained in a large variety of food-grade constituents, such as polysaccharides and proteins. Recent studies on emulsions’ encapsulation indicated that 5 out of 51 cases, covering 10%, focused on establishing water in oil in water (W/O/W) emulsions for encapsulating anthocyanin derivatives. Several agents, such as guar gum and whey protein, have been reported in producing W/O/W emulsions for encapsulating anthocyanin, including C3G, in plant-based food [69][31]. The results of Bamba et al. [82][38] revealed that increasing the time of water/oil emulsion from 5 to 10 min at 10,000 rpm ensured a higher anthocyanin encapsulation performance by decreasing the droplets’ dimeter.4.4. Biopolymer-Based Encapsulation of Cyanidin 3-O-Glucoside
Another widely use encapsulation technique is the ionic gelation process, which is a part of a biopolymer-based encapsulation method to fabricate micro/nanocarriers to encapsulate C3G and associated anthocyanin derivatives. Ionic nano/microgels could be developed via a combination of two distinct surface-charged biopolymers [87,88][39][40]. Biopolymer nano/microcarriers have been produced by a specific biopolymer, such as carbohydrates (chitosan, maltodextrin, β-cyclodextrin, alginates, and starch, etc.), proteins (whey and soy proteins, etc.), and plant-based hydrophilic gums [89,90][41][42]. Mar et al. [91][43] reported that among the nano/microgels used to encapsulate anthocyanin constituents, including C3G, alginate hydrogel beads and samples coated with chitosan were more effective in encapsulating and preserving the anthocyanin. They observed higher antioxidant potential with alginate hydrogel compared to samples with whey protein concentrate and gelatine.4.5. Electrohydrodynamic Encapsulation of Cyanidin 3-O-Glucoside
This encapsulation process composed of electrospinning and electro-spraying is regarded as an alternative technique to conventional ones. For instance, the electrohydrodynamic process has been newly proposed as a beneficial, cost-effective, simple, and flexible technique for fabricating encapsulation materials for different bioactive constituents such as anthocyanin, mainly C3G [66,94,95][44][45][46]. This process is especially advantageous for the encapsulation and preservation of thermo/photosensitive molecules such as cyanidin 3-O-glucoside and other anthocyanin components. Different electrohydrodynamic production processes which are able to generate encapsulation barriers in one step, with low temperature, nontoxic reagents, and lower interaction with bioactive components, yielding high loading efficiencies, have been reported in previous studies [96][47]. The electrospinning and electro-spraying techniques are almost similar in functionality and apparatus design, and the main differences between these two processes are the final products generated, namely nanodroplets for the electro-spraying process and nanofibers for the electrospinning method. The distinction of the nanofibers and nanodroplets obtained is evaluated by the intrinsic viscosity of the solution and biomaterials’ concentration [66][44].5. Bioavailability and Bio-Accessibility of Cyanidin 3-O-Glucoside
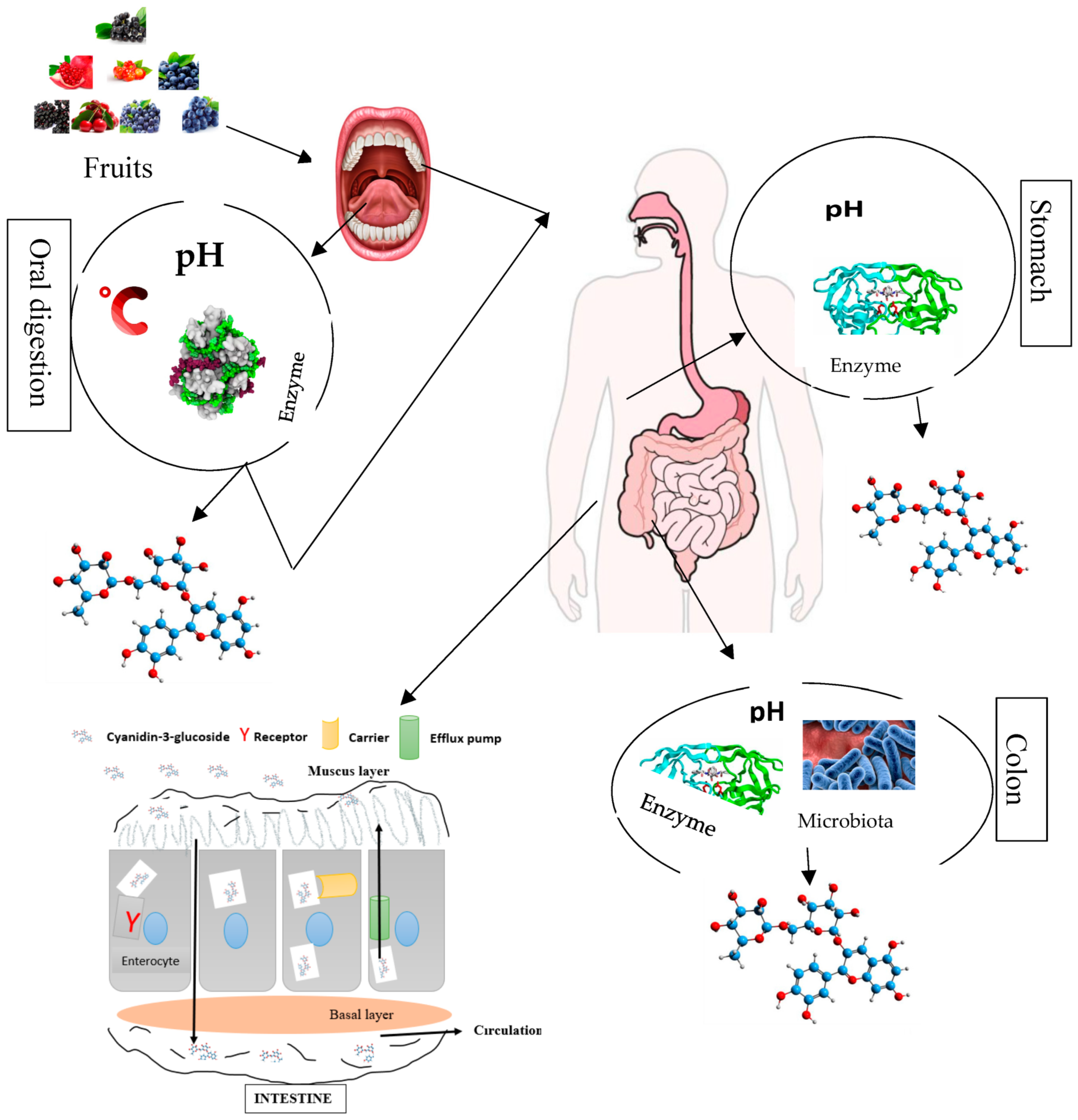
The bio-accessibility of anthocyanin components comprising C3G through the different compartments of the digestive tract firstly start from their release from the food matrix. C3G and other anthocyanin components after liberation enter in contact with some micro-molecules, enzymes, and other phenolic compounds, which might affect their bio-accessibility [99,100,101,102,103][48][49][50][51][52]. Anthocyanin components including C3G have frequently been reported to poorly assimilate in the different compartments of the digestive tract or gastrointestinal track, and they have a low diffusion rate, which limits their application in functional food (foods with physiological benefits). The effect of anthocyanin constituents’, especially cyanidin 3-O-glucoside, health properties might largely depend on their bioavailability, encompassing bio-accessibility, absorption, diffusion, metabolism, distribution, and excretion (Figure 3). The thermo/photosensitivity, instability, the neutral or basic pH sensitivity, oxygen, enzymes, metal ions, and organic acids cause C3G to rapidly degrade [104][53].
Figure 3. Overview of anthocyanin (cyanidin 3-O-glucoside) bio-transformations (degradation and absorption) in different compartments of the human gastrointestinal–digestive system.
7. Storage, Thermal, pH, and Light Stabilities of the Encapsulated Cyanidin 3-O-Glucoside
By monitoring the changes in color and the anthocyanin concentration during the accelerated tests, the stability of natural anthocyanin pigments may be assessed [45][54]. However, C3G is chemically unstable. The phenolic hydroxyl groups in the structure of C3G prevent it from steadily existing for a very long time [109][55]. C3G degradation was likely caused by the hydrolysis of the carbon atom at position C2, which caused the pyrylium ring of anthocyanin to open and generate a chalcone structure [45][54]. The stability of C3G is influenced by a number of variables. It degrades rapidly during food processing when exposed to neutral or basic pH values, elevated temperatures, oxygen, enzymes, light, pressure, and other reactive substances, such as ascorbic acid and metallic ions [6,49,110][22][25][56]. However, C3G and other compounds that are susceptible to heat and light are stabilized and given a longer shelf-life through microencapsulation [101][50].7.1. Storage Stability
As discussed earlier, C3G is easily impacted by a variety of reactions happening in food products. The biggest concern with the preservation of C3G is their instability caused by temperature, oxygen, light, and certain enzymes during the storage of the product. According to the evidence, C3G liposomes produced using the pH gradient loading approach have much better stability (85.4%), antioxidant activity, and skin permeability and may remain stable for 14 days in vitro under physiological circumstances [112][57].7.2. Thermal Stability
It is well-known that C3G is a notoriously unstable plant pigment that may deteriorate very quickly when exposed to heat. According to some theories, the rapid decomposition of anthocyanin at higher temperatures may be caused by the hydrolysis of the 3-glycoside structure, which acts as a barrier for unstable anthocyanin. The other hypothesis is that the hydrolysis of the pyrilium ring produced chalcone, which is what gives foods containing anthocyanin their brown color [113][58]. However, the encapsulation of C3G within the ferritin cage demonstrated great thermal stability upon examination by UV-vis spectrophotometry in combination with HPLC, using free anthocyanin as a reference. When compared to free C3G, the thermal and photo-stabilities of the protein nanocage-enclosed C3G were significantly increased. This results from the contact between C3G molecules and amino acid residues on the inner surface of apoferritin, which protects the molecules of C3G within it [44][24].7.3. Light Stability
Light is another factor that affects the color stability of C3G. The effect of light on encapsulated and non-encapsulated C3G within maltodextrin and chitosan was investigated. The encapsulated powders were stored in dark and light conditions for 15 days, and then their light stability was assessed using UV and IR spectroscopic methods. Data showed that maltodextrin and chitosan can increase the light stability of C3G because the encapsulated polymeric matrices protect the C3G by inhibiting oxidation, which may explain why non-encapsulated C3G degraded more quickly than encapsulated C3G [114][59]. In comparison to non-encapsulated C3G samples, the half-life of all encapsulated C3G samples increased during storage [117][60].7.4. pH Sensitivity
The pH is a significant additional factor that affects the stability of C3G. Anthocyanin nanoparticles are pH-sensitive in vitro, as demonstrated by Sun et al.’s research, which showed that, in comparison to pH 6.8 and pH 7.4, C3G encapsulated with chitosan nanoparticles at pH 5.3 demonstrated the greatest release ratio [49][25]. According to the observation of a few in vitro studies, at a pH of around 3 or lower, the anthocyanin is orange or red and exists as a flavylium cation [113][58]. The hydration process of the flavylium cation and the proton transfer reactions linked to the acidic hydroxyl groups of the aglycone compete kinetically and thermodynamically when the pH is raised. While the initial reaction produces an inert carbinol pseudo-base that can undergo ring opening to become a chalcone pseudo-base, the second reaction can produce quinonoidal bases at pH levels between 6 and 7 with the generation of purple, resonance-stabilized quinonoid anions [113][58]. As a result of pH-dependent equilibrium forms, they may exhibit different colors in aqueous solutions: at very acidic pH, anthocyanin are primarily in their red cationic form (AH+), but as pH increases, the flavylium cation immediately undergoes deprotonation to give rise to quinoidal bases (A), and at the same time, but more slowly, it may hydrate to form colorless hemiketal species (B2), which then quickly tautomerize to yield cis-chalcone. The species in question might then isomerize into trans-chalcone [9][61].8. Uses of the Encapsulated Cyanidin 3-O-Glucoside
Numerous studies have demonstrated that C3G-encapsulated nanoparticles have strong encapsulation efficiency as well as the potential to further enhance and provide a framework for the investigation of C3G nanoparticle applications in functional foods [109,119,120][55][62][63]. Converging research has recently shown that the natural pigment C3G has an impact on several physiological processes, including inflammation, cardiovascular disease, cancer, antioxidant activity, and antidiabetic effects [121][64]. C3G is widely used as a food additive because of its appealing hue and advantageous bioactivity, which enhances both food color and health functions [53][27]. C3G could possess the ability to scavenge free radicals, which might stop the oxidation of low-density lipoproteins and have a good impact on obesity, inflammation, and chronic gut inflammatory disorders [59][65]. According to a number of studies, C3G prevents the negative effects of UV-B radiation, regulates important components of carcinogenesis, stops cancer cells from proliferating, and triggers the death of cancer cells, decreases oxidative stress, prevents oxidative stress brought on by H2O2 in human embryonic kidney (HEK 293) cells, triggers cell death, and prevents cell migration in TNF-α-challenged RASMCs in vitro [59][65]. C3G was shown to inhibit UVB-induced apoptosis in human HaCaT keratinocytes [54][29]. In vivo, C3G can guard against UVB-induced epidermal deterioration. However, the therapeutic use of C3G and its industrial applications as functional food components has been constrained by how quickly it degrades. Nowadays, polymeric nanoparticles are currently playing a crucial role in the advancement of therapeutic and calleidic systems in the new wave of the development of cosmetic or pharmaceutical dosage forms due to their capacity for regulating drug release and enhancing the stability of pharmaceuticals [54][29]. The nano-C3G encapsulation within chitosan according to the animal experiment showed that nano-C3G could efficiently lower the levels of lipid peroxidation, malondialdehyde, and 8-hydroxy-2′-deoxyguanosine caused by UVB exposure, as well as downregulate the expression of p53, Bcl-2-associated X (Bax), caspase-3 and -9, and balance the B-cell lymphoma-2/leukemia-2 ratio [54][29]. Liang et al. investigated the effect of C3G encapsulated with liposomes on normal GES-1 cells by evaluating cell viability and mitochondrial structure. The primary cell line in the human stomach is the human gastric epithelial cell line (GES-1 cells), and the health of the stomach is crucial for body health since it is the primary organ through which many biochemical interactions occur [111][66].9. Conclusions
Anthocyanin C3G is a secondary metabolite naturally present in herbal origin sources. It exerts tremendous health benefits, including regulation of cholesterol, antioxidant, anti-inflammatory, hepatoprotective, anticancer, anti-obesity, and antidiabetic effects. The recovery of C3G is influenced by many factors, such as extraction techniques, extraction conditions, and raw materials. Likewise, C3G is sensitive to many factors and its stability is affected by pH, light, temperature, and storage time. The nanoencapsulation improves the stability and bioavailability of C3G. Most nanocarriers used for its nanoencapsulation are proteins, polysaccharides, polymeric micelles, and phospholipid-based materials. Furthermore, the encapsulation techniques are also determinant factors that influence the production yield and availability of C3G. C3G is used as a food additive and colorant, as well as for therapeutic purposes.References
- Olivas-Aguirre, F.J.; Rodrigo-García, J.; Martínez-Ruiz, N.D.R.; Cárdenas-Robles, A.I.; Mendoza-Díaz, S.O.; Álvarez-Parrilla, E.; González-Aguilar, G.A.; De la Rosa, L.A.; Ramos-Jiménez, A.; Wall-Medrano, A. Cyanidin-3-O-glucoside: Physical-Chemistry, Foodomics and Health Effects. Molecules 2016, 21, 1264.
- Tirupula, K.C.; Balem, F.; Yanamala, N.; Klein-Seetharaman, J. pH-dependent Interaction of Rhodopsin with Cyanidin-3-glucoside. 2. Functional Aspects. Photochem. Photobiol. 2009, 85, 463–470.
- Wang, Y.; Huo, Y.; Zhao, L.; Lu, F.; Wang, O.; Yang, X.; Ji, B.; Zhou, F. Cyanidin-3-glucoside and its phenolic acid metabolites attenuate visible light-induced retinal degeneration in vivo via activation of Nrf2/HO-1 pathway and NF-κB suppression. Mol. Nutr. Food Res. 2016, 60, 1564–1577.
- Du, C.; Shi, Y.; Ren, Y.; Wu, H.; Yao, F.; Wei, J.; Wu, M.; Hou, Y.; Duan, H. Anthocyanins inhibit high-glucose-induced cholesterol accumulation and inflammation by activating LXRα pathway in HK-2 cells. Drug Des. Dev. Ther. 2015, 9, 5099–5113.
- Chen, P.-N.; Chu, S.-C.; Chiou, H.-L.; Chiang, C.-L.; Yang, S.-F.; Hsieh, Y.-S. Cyanidin 3-Glucoside and Peonidin 3-Glucoside Inhibit Tumor Cell Growth and Induce Apoptosis In Vitro and Suppress Tumor Growth In Vivo. Nutr. Cancer 2005, 53, 232–243.
- Sasaki, R.; Nishimura, N.; Hoshino, H.; Isa, Y.; Kadowaki, M.; Ichi, T.; Tanaka, A.; Nishiumi, S.; Fukuda, I.; Ashida, H.; et al. Cyanidin 3-glucoside ameliorates hyperglycemia and insulin sensitivity due to downregulation of retinol binding protein 4 expression in diabetic mice. Biochem. Pharmacol. 2007, 74, 1619–1627.
- Cristianini, M.; Sánchez, J.S.G. Extraction of bioactive compounds from purple corn using emerging technologies: A review. J. Food Sci. 2020, 85, 862–869.
- Zannou, O.; Pashazadeh, H.; Galanakis, C.M.; Alamri, A.S.; Koca, I. Carboxylic acid-based deep eutectic solvents combined with innovative extraction techniques for greener extraction of phenolic compounds from sumac (Rhus coriaria L.). J. Appl. Res. Med. Aromat. Plants 2022, 30, 100380.
- Santos, D.T.; Veggi, P.C.; Meireles, M.A.A. Extraction of antioxidant compounds from Jabuticaba (Myrciaria cauliflora) skins: Yield, composition and economical evaluation. J. Food Eng. 2010, 101, 23–31.
- Ćujić, N.; Šavikin, K.; Janković, T.; Pljevljakušić, D.; Zdunić, G.; Ibrić, S. Optimization of polyphenols extraction from dried chokeberry using maceration as traditional technique. Food Chem. 2016, 194, 135–142.
- Meregalli, M.M.; Puton, B.M.S.; Camera, F.D.; Amaral, A.U.; Zeni, J.; Cansian, R.L.; Mignoni, M.L.; Backes, G.T. Conventional and ultrasound-assisted methods for extraction of bioactive compounds from red araçá peel (Psidium cattleianum Sabine). Arab. J. Chem. 2020, 13, 5800–5809.
- Zannou, O.; Koca, I. Greener extraction of anthocyanins and antioxidant activity from blackberry (Rubus spp) using natural deep eutectic solvents. LWT 2022, 158, 113184.
- Santos, D.T.; Cavalcanti, R.N.; Rostagno, M.A.; Queiroga, C.L.; Eberlin, M.N.; Meireles, M.A.A. Extraction of Polyphenols and Anthocyanins from the Jambul (Syzygium cumini) Fruit Peels. Food Public Health 2013, 3, 12–20.
- Jafari, S.M.; Khazaei, K.M.; Assadpour, E. Production of a natural color through microwave-assisted extraction of saffron tepal’s anthocyanins. Food Sci. Nutr. 2019, 7, 1438–1445.
- Yang, Z.; Zhai, W. Optimization of microwave-assisted extraction of anthocyanins from purple corn (Zea mays L.) cob and identification with HPLC–MS. Innov. Food Sci. Emerg. Technol. 2010, 11, 470–476.
- Pereira, R.N.; Coelho, M.I.; Genisheva, Z.; Fernandes, J.M.; Vicente, A.A.; Pintado, M.E.; Teixeira, E.J.A. Using Ohmic Heating effect on grape skins as a pretreatment for anthocyanins extraction. Food Bioprod. Process. 2020, 124, 320–328.
- Kutlu, N.; Isci, A.; Sakiyan, O.; Yilmaz, A.E. Effect of ohmic heating on ultrasound extraction of phenolic compounds from cornelian cherry (Cornus mas). J. Food Process. Preserv. 2021, 45, e15818.
- Okur, I.; Baltacıoğlu, C.; Ağçam, E.; Baltacıoğlu, H.; Alpas, H. Evaluation of the Effect of Different Extraction Techniques on Sour Cherry Pomace Phenolic Content and Antioxidant Activity and Determination of Phenolic Compounds by FTIR and HPLC. Waste Biomass Valorization 2019, 10, 3545–3555.
- Babova, O.; Occhipinti, A.; Capuzzo, A.; Maffei, M.E. Extraction of bilberry (Vaccinium myrtillus) antioxidants using supercritical/subcritical CO2 and ethanol as co-solvent. J. Supercrit. Fluids 2016, 107, 358–363.
- Monroy, Y.M.; Rodrigues, R.A.; Sartoratto, A.; Cabral, F.A. Influence of ethanol, water, and their mixtures as co-solvents of the supercritical carbon dioxide in the extraction of phenolics from purple corn cob (Zea mays L.). J. Supercrit. Fluids 2016, 118, 11–18.
- Saldaña, M.D.; Martinez, E.R.; Sekhon, J.K.; Vo, H. The effect of different pressurized fluids on the extraction of anthocyanins and total phenolics from cranberry pomace. J. Supercrit. Fluids 2021, 175, 105279.
- Feng, J.; Wu, Y.; Zhang, L.; Li, Y.; Liu, S.; Wang, H.; Li, C. Enhanced Chemical Stability, Intestinal Absorption, and Intracellular Antioxidant Activity of Cyanidin-3-O-glucoside by Composite Nanogel Encapsulation. J. Agric. Food Chem. 2019, 67, 10432–10447.
- Qin, X.; Yuan, D.; Wang, Q.; Hu, Z.; Wu, Y.; Cai, J.; Huang, Q.; Li, S.; Liu, G. Maillard-Reacted Whey Protein Isolates Enhance Thermal Stability of Anthocyanins over a Wide pH Range. J. Agric. Food Chem. 2018, 66, 9556–9564.
- Zhang, T.; Lv, C.; Chen, L.; Bai, G.; Zhao, G.; Xu, C. Encapsulation of anthocyanin molecules within a ferritin nanocage increases their stability and cell uptake efficiency. Food Res. Int. 2014, 62, 183–192.
- Sun, J.; Chen, J.; Mei, Z.; Luo, Z.; Ding, L.; Jiang, X.; Bai, W. Synthesis, structural characterization, and evaluation of cyanidin-3-O-glucoside-loaded chitosan nanoparticles. Food Chem. 2020, 330, 127239.
- Oz, F.; Zaman, A.; Kaya, M. Effect of Chitosan on the Formation of Heterocyclic Aromatic Amines and Some Quality Properties of Meatball. J. Food Process. Preserv. 2016, 41, e13065.
- Ge, J.; Yue, P.; Chi, J.; Liang, J.; Gao, X. Formation and stability of anthocyanins-loaded nanocomplexes prepared with chitosan hydrochloride and carboxymethyl chitosan. Food Hydrocoll. 2018, 74, 23–31.
- Shishir, M.R.I.; Xie, L.; Sun, C.; Zheng, X.; Chen, W. Advances in micro and nano-encapsulation of bioactive compounds using biopolymer and lipid-based transporters. Trends Food Sci. Technol. 2018, 78, 34–60.
- Liu, Z.; Hu, Y.; Li, X.; Mei, Z.; Wu, S.; He, Y.; Jiang, X.; Sun, J.; Xiao, J.; Deng, L.; et al. Nanoencapsulation of Cyanidin-3-O-glucoside Enhances Protection Against UVB-Induced Epidermal Damage through Regulation of p53-Mediated Apoptosis in Mice. J. Agric. Food Chem. 2018, 66, 5359–5367.
- Mahdavi, S.A.; Jafari, S.M.; Ghorbani, M.; Assadpoor, E. Spray-Drying Microencapsulation of Anthocyanins by Natural Biopolymers: A Review. Dry. Technol. 2014, 32, 509–518.
- Sharif, N.; Khoshnoudi-Nia, S.; Jafari, S.M. Nano/microencapsulation of anthocyanins; A systematic review and meta-analysis. Food Res. Int. 2020, 132, 109077.
- Mahdavi, S.A.; Jafari, S.M.; Assadpoor, E.; Dehnad, D. Microencapsulation optimization of natural anthocyanins with maltodextrin, gum Arabic and gelatin. Int. J. Biol. Macromol. 2016, 85, 379–385.
- Moalemiyan, M.; Ramaswamy, H.S.; Maftoonazad, N. Pectin-Based Edible Coating for Shelf-Life Extension of Ataulfo Mango. J. Food Process. Eng. 2011, 35, 572–600.
- Ezhilarasi, P.N.; Karthik, P.; Chhanwal, N.; Anandharamakrishnan, C. Nanoencapsulation Techniques for Food Bioactive Components: A Review. Food Bioprocess Technol. 2013, 6, 628–647.
- Garavand, F.; Rahaee, S.; Vahedikia, N.; Jafari, S.M. Different techniques for extraction and micro/nanoencapsulation of saffron bioactive ingredients. Trends Food Sci. Technol. 2019, 89, 26–44.
- Souza, A.C.P.; Gurak, P.D.; Marczak, L.D.F. Maltodextrin, pectin and soy protein isolate as carrier agents in the encapsulation of anthocyanins-rich extract from jaboticaba pomace. Food Bioprod. Process. 2017, 102, 186–194.
- Assadpour, E.; Mahdi Jafari, S. A systematic review on nanoencapsulation of food bioactive ingredients and nutraceuticals by various nanocarriers. Crit. Rev. Food Sci. Nutr. 2019, 59, 3129–3151.
- Bamba, B.; Shi, J.; Tranchant, C.; Xue, S.; Forney, C.; Lim, L.-T.; Xu, W.; Xu, G. Coencapsulation of Polyphenols and Anthocyanins from Blueberry Pomace by Double Emulsion Stabilized by Whey Proteins: Effect of Homogenization Parameters. Molecules 2018, 23, 2525.
- Rajabi, H.; Jafari, S.M.; Rajabzadeh, G.; Sarfarazi, M.; Sedaghati, S. Chitosan-gum Arabic complex nanocarriers for encapsulation of saffron bioactive components. Colloids Surf. A 2019, 578, 123644.
- Abaee, A.; Mohammadian, M.; Jafari, S.M. Whey and soy protein-based hydrogels and nano-hydrogels as bioactive delivery systems. Trends Food Sci. Technol. 2017, 70, 69–81.
- Qidwai, A.; Khan, S.; Shadab, M.; Fazil, M.; Baboota, S.; Narang, J.K.; Ali, J. Nanostructured lipid carrier in photodynamic therapy for the treatment of basal-cell carcinoma. Drug Deliv. 2016, 23, 1476–1485.
- Da Rosa, J.R.; Nunes, G.L.; Motta, M.H.; Fortes, J.P.; Weis, G.C.C.; Hecktheuer, L.H.R.; Muller, E.I.; de Menezes, C.R.; da Rosa, C.S. Microencapsulation of anthocyanin compounds extracted from blueberry (Vaccinium spp.) by spray drying: Characterization, stability and simulated gastrointestinal conditions. Food Hydrocoll. 2019, 89, 742–748.
- Mar, J.M.; da Silva, L.S.; Rabello, M.D.S.; Biondo, M.M.; Kinupp, V.F.; Campelo, P.H.; Bruginski, E.; Campos, F.R.; Bezerra, J.D.A.; Sanches, E.A. Development of alginate/inulin carrier systems containing non-conventional Amazonian berry extracts. Food Res. Int. 2021, 139, 109838.
- Jia, Z.; Dumont, M.-J.; Orsat, V. Encapsulation of phenolic compounds present in plants using protein matrices. Food Biosci. 2016, 15, 87–104.
- Atay, E.; Fabra, M.J.; Martínez-Sanz, M.; Gomez-Mascaraque, L.G.; Altan, A.; Lopez-Rubio, A. Development and characterization of chitosan/gelatin electrosprayed microparticles as food grade delivery vehicles for anthocyanin extracts. Food Hydrocoll. 2018, 77, 699–710.
- Pérez-Masiá, R.; Lagaron, J.M.; López-Rubio, A. Development and Optimization of Novel Encapsulation Structures of Interest in Functional Foods Through Electrospraying. Food Bioprocess Technol. 2014, 7, 3236–3245.
- Pisoschi, A.M.; Pop, A.; Cimpeanu, C.; Turcuş, V.; Predoi, G.; Iordache, F. Nanoencapsulation techniques for compounds and products with antioxidant and antimicrobial activity–A critical view. Eur. J. Med. Chem. 2018, 157, 1326–1345.
- Cassidy, A.; Rogers, G.; Peterson, J.J.; Dwyer, J.T.; Lin, H.; Jacques, P.F. Higher dietary anthocyanin and flavonol intakes are associated with anti-inflammatory effects in a population of US adults. Am. J. Clin. Nutr. 2015, 102, 172–181.
- Li, D.; Wang, P.; Luo, Y.; Zhao, M.; Chen, F. Health benefits of anthocyanins and molecular mechanisms: Update from recent decade. Crit. Rev. Food Sci. Nutr. 2017, 57, 1729–1741.
- Oidtmann, J.; Schantz, M.; Mäder, K.; Baum, M.; Berg, S.; Betz, M.; Kulozik, U.; Leick, S.; Rehage, H.; Schwarz, K.; et al. Preparation and Comparative Release Characteristics of Three Anthocyanin Encapsulation Systems. J. Agric. Food Chem. 2012, 60, 844–851.
- Fernandes, A.; Rocha, M.A.; Santos, L.; Brás, J.; Oliveira, J.; Mateus, N.; Freitas, V. Blackberry anthocyanins: β-Cyclodextrin fortification for thermal and gastrointestinal stabilization. Food Chem. 2018, 245, 426–431.
- Rupasinghe, H.V.; Arumuggam, N.; Amararathna, M.; De Silva, A. The potential health benefits of haskap (Lonicera caerulea L.): Role of cyanidin-3-O-glucoside. J. Funct. Foods 2018, 44, 24–39.
- Sun, J.; Luo, H.; Li, X.; Li, X.; Lu, Y.; Bai, W. Effects of low power ultrasonic treatment on the transformation of cyanidin-3-O-glucoside to methylpyranocyanidin-3-O-glucoside and its stability evaluation. Food Chem. 2019, 276, 240–246.
- Ouyang, Y.; Chen, L.; Qian, L.; Lin, X.; Fan, X.; Teng, H.; Cao, H. Fabrication of caseins nanoparticles to improve the stability of cyanidin 3-O-glucoside. Food Chem. 2020, 317, 126418.
- Hao, X.; Guan, R.; Huang, H.; Yang, K.; Wang, L.; Wu, Y. Anti-inflammatory activity of cyanidin-3-O-glucoside and cyanidin-3-O-glucoside liposomes in THP-1 macrophages. Food Sci. Nutr. 2021, 9, 6480–6491.
- Tian, L.; Tan, Y.; Chen, G.; Wang, G.; Sun, J.; Ou, S.; Chen, W.; Bai, W. Metabolism of anthocyanins and consequent effects on the gut microbiota. Crit. Rev. Food Sci. Nutr. 2019, 59, 982–991.
- Lee, C.; Na, K. Anthocyanin-Loaded Liposomes Prepared by the pH-Gradient Loading Method to Enhance the Anthocyanin Stability, Antioxidation Effect and Skin Permeability. Macromol. Res. 2019, 28, 289–297.
- Jenshi Roobha, J.; Saravanakumar, M.; Aravindhan, K.M.; Suganya Devi, P. The effect of light, temperature, pH on stability of anthocyanin pigments in Musa acuminata bract. Res. Plant Biol. 2011, 1, 5–12.
- Vichit, W.; Saewan, N.; Vichit, W.; Thitipromote, N. Stability of freeze dried encapsulated anthocyanins from black glutinous rice extract. In Proceedings of the Pure and Applied Chemistry International Conference 2012, Chiang Mai, Thailand, 11–13 January 2012; p. 5.
- Mahdavi, S.A.; Jafari, S.M.; Assadpour, E.; Ghorbani, M. Storage stability of encapsulated barberry’s anthocyanin and its application in jelly formulation. J. Food Eng. 2016, 181, 59–66.
- Cruz, L.; Basílio, N.; de Freitas, V. Color stabilization of cyanidin-3-glucoside-based dyes by encapsulation with biocompatible PEGylated phospholipid micelles. Dyes Pigments 2020, 181, 108592.
- Sivasinprasasn, S.; Pantan, R.; Thummayot, S.; Tocharus, J.; Suksamrarn, A.; Tocharus, C. Cyanidin-3-glucoside attenuates angiotensin II-induced oxidative stress and inflammation in vascular endothelial cells. Chem. Biol. Interact. 2016, 260, 67–74.
- De Figueiredo Paes Barretto, F.J.; Clemente, H.A.; Santana, A.L.B.D.; da Silva Vasconcelo, M.A. Stability of encapsulated and non-encapsulated anthocyanin in yogurt produced with natural dye obtained from Solanum melongena L. Bark. Rev. Bras. Frutic. 2020, 42, 1–13.
- Li, W.; Chen, S.; Zhou, G.; Li, H.; Zhong, L.; Liu, S. Potential role of cyanidin 3-glucoside (C3G) in diabetic cardiomyopathy in diabetic rats: An in vivo approach. Saudi J. Biol. Sci. 2016, 25, 500–506.
- Liang, T.; Guan, R.; Wang, Z.; Shen, H.; Xia, Q.; Liu, M. Comparison of anticancer activity and antioxidant activity between cyanidin-3-O-glucoside liposomes and cyanidin-3-O-glucoside in Caco-2 cells in vitro. RSC Adv. 2017, 7, 37359–37368.
- Liang, T.; Guan, R.; Quan, Z.; Tao, Q.; Liu, Z.; Hu, Q. Cyanidin-3-O-glucoside liposome: Preparation via a green method and antioxidant activity in GES-1 cells. Food Res. Int. 2019, 125, 108648.
More
