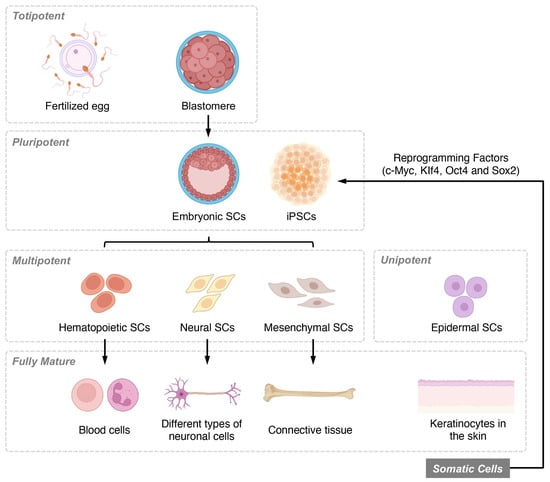
Stem cells can be categorized based on their differentiation potential and developmental stages. Differentiation potential classifications include totipotent, pluripotent, multipotent, and unipotent cells, while developmental stage categories consist of embryonic, fetal, infant or umbilical cord blood, and adult stem cells (Figure 1). Among these, MSCs have garnered considerable interest as a promising candidate for SCI treatment due to their ability to differentiate into various cell types. Recent research has highlighted the multiple molecular mechanisms through which MSCs can promote recovery following SCI.
Figure 1. Overview of stem cell classifications based on differentiation potential and developmental stages. Stem cells are classified based on their differentiation potential, which reflects their ability to form various cell types. Totipotent stem cells, found in early-stage embryos, can differentiate into all three germ layers, as well as extra-embryonic tissues and placental cells. Pluripotent stem cells, present in the blastocyst stage of development, maintain the ability to self-renew and differentiate into the three germ layers and multiple lineages but cannot form extra-embryonic tissues or placental cells. Multipotent stem cells, also known as adult or somatic stem cells, are undifferentiated cells found in postnatal tissues. These specialized cells have limited self-renewal capabilities and are committed to specific lineages. Unipotent stem cells are the most restricted in their differentiation potential, as they can only give rise to a single cell type, although they still retain the capacity for self-renewal.
2.1. Neuroprotection
MSCs secrete a variety of growth factors and cytokines that exhibit neuroprotective effects, playing a pivotal role in supporting the recovery of damaged neurons after SCI
[21]. Among these factors are vascular endothelial growth factor (VEGF), nerve growth factor (NGF), insulin-like growth factor-1 (IGF-1), and brain-derived neurotrophic factor (BDNF), all of which contribute to a supportive microenvironment for neuronal survival and regeneration
[22].
VEGF, for instance, not only promotes angiogenesis but also exerts direct neuroprotective effects by inhibiting apoptosis, reducing oxidative stress, and promoting neurogenesis
[23]. NGF, on the other hand, supports the survival and growth of neurons, particularly those in the peripheral nervous system (PNS), by binding to its receptors, TrkA and p75NTR, and activating intracellular signaling pathways that promote neuronal survival
[24]. IGF-1 contributes to neuroprotection by promoting neuronal survival, synaptic plasticity, and neurogenesis
[25]. It has been shown to reduce inflammation, inhibit neuronal apoptosis, and stimulate the proliferation and differentiation of neural progenitor cells
[26]. BDNF, another critical neurotrophic factor secreted by MSCs, enhances neuronal survival and function by activating the TrkB receptor and downstream signaling pathways, such as the PI3K/Akt and MAPK/ERK pathways
[27].
Furthermore, MSCs can also modulate the expression of pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α) and interleukin-1 beta (IL-1β), in microglia and astrocytes, and promote the release of anti-inflammatory cytokines like interleukin-10 (IL-10) and transforming growth factor-beta (TGF-β) from MSCs themselves as well as from other cell types, such as microglia and macrophages
[28]. This modulation of the inflammatory milieu creates a more favorable environment for neuronal survival and recovery
[29][30][29,30]. Overall, the neuroprotective effects of MSCs are multifaceted and involve a complex interplay of various growth factors and cytokines that work together to support the survival and regeneration of damaged neurons after SCI.
2.2. Promoting Neuronal Regeneration
MSCs possess the remarkable ability to differentiate into various neural cell types, including neurons and glial cells, such as astrocytes and oligodendrocytes
[31][32][31,32]. Recent studies have shown that the differentiation of MSCs towards neurons or glial cells is orchestrated by a complex interplay of signaling pathways, transcription factors, and epigenetic modifications. For instance, key signaling pathways implicated in the neural differentiation of MSCs include the Notch, Wnt, and BMP signaling pathways
[33][34][33,34]. Moreover, transcription factors such as Sox2, Pax6, and Neurogenin-2 play essential roles in guiding MSC differentiation toward neuronal and glial lineages
[35][36][37][35,36,37]. The ratio of neurons to glial cells originating from MSCs is determined by the specific combination of signaling molecules and transcription factors present in the local microenvironment, which can be modulated by various extrinsic cues and experimental conditions. For example, the presence of growth factors like epidermal growth factor (EGF) and fibroblast growth factor (FGF) can promote neuronal differentiation
[38][39][38,39], while the addition of ciliary neurotrophic factor (CNTF) can drive glial differentiation. The delicate balance of these factors ultimately influences the cell fate of MSCs and their potential to contribute to neural regeneration following CNS injury.
The pro-inflammatory niche within the SCI lesion can indeed affect the differentiation of MSCs. For instance, inflammatory cytokines, such as TNF-α and IL-1β, have been reported to influence MSC differentiation, potentially biasing MSCs towards a particular cell phenotype, such as astrocytes. High levels of these pro-inflammatory cytokines have been shown to inhibit neuronal differentiation while promoting the differentiation of MSCs into astrocytes
[40][41][40,41]. On the other hand, anti-inflammatory cytokines, such as IL-4 and IL-10, have been reported to promote neuronal differentiation and inhibit glial differentiation of MSCs
[42]. Importantly, MSCs also possess immunomodulatory properties, which may help mitigate inflammation and create a more favorable environment for tissue repair and regeneration
[43][44][43,44]. MSCs can secrete various anti-inflammatory factors, such as TGF-β and IL-10, as well as modulate the function of immune cells, such as T cells and macrophages, to reduce inflammation and create a more permissive environment for tissue repair
[45][46][45,46]. These MSC-mediated immunomodulatory effects can potentially counteract the negative influence of the pro-inflammatory niche on MSC differentiation, thus supporting their therapeutic potential in SCI.
Most studies have demonstrated that the preferential differentiation of MSCs towards a particular cell phenotype in the context of the pro-inflammatory niche within the SCI lesion could be a potential hurdle in using MSCs for post-SCI tissue regeneration. For example, excessive differentiation of MSCs into astrocytes, potentially exacerbated by the overexpression of transcription factors like STAT3, may contribute to glial scar formation, impeding axonal regeneration and functional recovery
[47][48][47,48]. On the other hand, insufficient differentiation into oligodendrocytes, possibly hindered by the presence of inhibitory factors such as chondroitin sulfate proteoglycans (CSPGs), might limit remyelination and restoration of neuronal connectivity
[49][50][49,50]. To address these challenges, it is crucial to gain a better understanding of the factors that influence MSC differentiation in the SCI environment and develop strategies, such as genetic modifications or controlled release of growth factors, to direct MSC differentiation towards the desired cell types. For instance, overexpressing transcription factors like Olig2 in MSCs can enhance their differentiation into oligodendrocytes and promote remyelination
[51][52][51,52], while engineering MSCs to secrete specific growth factors, such as brain-derived neurotrophic factor (BDNF) or glial cell-derived neurotrophic factor (GDNF), can support neuronal survival and regeneration
[53][54][53,54]. These examples highlight the importance of understanding and controlling MSC differentiation to maximize their therapeutic potential in post-SCI tissue regeneration.
The mechanical properties of extracellular matrix (ECM), such as stiffness, topography, and 3D architecture, can significantly affect MSC differentiation
[55]. MSCs sense and respond to their mechanical environment through mechanotransduction, which involves converting mechanical signals into biochemical and cellular responses
[56]. For instance, studies have shown that MSCs cultured on softer substrates with a stiffness similar to that of the brain tend to differentiate into neural lineages, whereas those cultured on stiffer substrates resembling bone tissue preferentially differentiate into osteogenic lineages
[57]. Additionally, the topography and 3D architecture of the ECM can guide MSC alignment, migration, and differentiation by providing physical cues that influence cell shape and cytoskeletal organization
[58][59][58,59]. In the context of SCI, optimizing the mechanical properties of the ECM could potentially enhance the therapeutic efficacy of MSCs by promoting their differentiation into the desired neural cell types and improving their integration with the host tissue.
This unique characteristic enables MSCs to replace damaged neural tissue, promote the regeneration of neuronal circuits, and ultimately contribute to functional recovery after SCI
[27]. The process of neuronal regeneration is facilitated by the secretion of several trophic factors, such as BDNF, GDNF, NGF, and CNTF, which stimulate the growth, differentiation, and survival of neural cells
[60]. Additionally, MSCs can promote the activation and proliferation of endogenous neural stem cells (NSCs) and progenitor cells within the injured spinal cord, further enhancing the regenerative process
[61]. While it is clear that MSCs can differentiate into the component parts of neural circuits, the evidence for their ability to reestablish the correct neural circuits that existed prior to injury is still emerging. A few studies have reported that MSCs, either directly or indirectly, can contribute to the formation of functional neural circuits after SCI. For instance, Zeng et al. (2015) demonstrated that MSCs transplanted into the injured spinal cord were able to differentiate into neurons and form synapses with host neurons, contributing to the restoration of motor function
[62]. Another study by Nakajima et al. (2012) showed that MSC transplantation promoted the growth of host corticospinal tract axons and the formation of new synapses
[63].
Another crucial aspect of MSC-mediated neuronal regeneration involves the modulation of ECM components, such as CSPGs and matrix metalloproteinases (MMPs)
[64][65][64,65]. By regulating the balance between ECM deposition and degradation, MSCs can create a more permissive environment for axonal growth and neural regeneration
[66]. MSCs can also exert paracrine effects, which involve the release of extracellular vesicles (EVs) containing various bioactive molecules, such as proteins, lipids, and nucleic acids
[67]. These EVs can transfer their cargo to recipient cells in the injured spinal cord, influencing their gene expression, proliferation, and differentiation, ultimately contributing to neuronal regeneration
[68].
In summary, MSCs promote neuronal regeneration through various mechanisms, including their capacity to differentiate into neural cell types, the secretion of trophic factors, stimulation of endogenous NSCs, modulation of ECM components, and paracrine effects. These concerted actions of MSCs contribute to the restoration of neuronal circuits and functional recovery following SCI.
2.3. Angiogenesis
MSCs play a critical role in promoting the formation of new blood vessels by secreting pro-angiogenic factors, such as VEGF, angiopoietin-1, and basic fibroblast growth factor (bFGF)
[69][70][69,70]. The process of angiogenesis is crucial for the recovery of injured spinal cord tissue, as it improves blood supply, accelerates tissue repair, and supports the survival of neural cells
[71][72][71,72]. In addition to the secretion of pro-angiogenic factors, MSCs can also modulate the expression of various cell adhesion molecules and integrins, such as intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1), which facilitate the recruitment and migration of endothelial cells to the injury site
[73][74][73,74]. This process contributes to the formation of new blood vessels and enhances the overall regenerative capacity of the injured spinal cord
[75].
MSC-derived EVs also contribute to the angiogenic process by transferring bioactive molecules, such as microRNAs (miRNAs) and proteins, to the recipient endothelial cells
[76]. These transferred molecules can regulate gene expression, promote endothelial cell proliferation, migration, and tube formation, ultimately stimulating angiogenesis in the injured spinal cord
[77]. Additionally, MSCs can establish communication with other cell types, such as pericytes and astrocytes, which play essential roles in the stabilization and maturation of newly formed blood vessels
[78]. By engaging in crosstalk with these cells, MSCs can ensure the proper development and functionality of the newly generated vascular network within the injured spinal cord
[79].
In summary, MSCs contribute to angiogenesis through various mechanisms, including the secretion of pro-angiogenic factors, modulation of cell adhesion molecules, release of EVs, and interaction with other cell types involved in vascular development. These collective actions of MSCs help improve blood supply to the injured spinal cord, facilitate tissue repair, and support neural cell survival, ultimately contributing to functional recovery after SCI.
2.4. Immunomodulation
MSCs possess remarkable immunomodulatory properties that contribute to their therapeutic potential in SCI treatment
[80]. Their ability to modulate the activity of various immune cells, such as macrophages, T-cells, B-cells, and natural killer (NK) cells, helps control inflammation, prevent autoimmune responses, and create a more favorable environment for tissue repair
[81][82][81,82]. MSCs can regulate the polarization of macrophages, promoting a switch from the pro-inflammatory M1 phenotype to the anti-inflammatory M2 phenotype
[83]. This shift in macrophage polarization is essential for controlling inflammation and fostering an environment that supports tissue repair and regeneration
[84]. Additionally, MSCs can suppress the activation and proliferation of T-cells, modulate their cytokine secretion profile, and induce the generation of regulatory T-cells (Tregs), which play a crucial role in maintaining immune tolerance and preventing autoimmune responses
[85][86][85,86]. MSCs can also inhibit B-cell activation, proliferation, and antibody production, further dampening the potential for harmful immune reactions
[87].
MSCs can directly interact with NK cells, downregulating their cytotoxic activity and pro-inflammatory cytokine production
[44][88][44,88]. Moreover, MSCs can secrete various soluble factors, such as TGF-β, prostaglandin E2 (PGE2), and indoleamine 2,3-dioxygenase (IDO), which contribute to their immunomodulatory effects
[89]. Another important aspect of MSC-mediated immunomodulation is the release of EVs, which contain bioactive molecules, such as proteins, lipids, and nucleic acids
[90][91][90,91]. These EVs can mediate intercellular communication and modulate the function of recipient immune cells, thus contributing to the overall immunomodulatory effects of MSCs
[92].
In summary, MSCs exert their immunomodulatory effects through various mechanisms, including the modulation of immune cell polarization, secretion of soluble factors, and release of EVs. These actions help control inflammation, prevent autoimmune responses, and create a more favorable environment for tissue repair and regeneration following SCI.
2.5. Axonal Regeneration
MSCs play a crucial role in promoting axonal regeneration after SCI through various mechanisms, including the secretion of diverse neurotrophic factors, such as NGF, CNTF, and FGF
[93][94][95][93,94,95]. These factors not only stimulate the growth of new axons but also support the survival and differentiation of neurons, ultimately leading to improved connectivity and functionality in the injured spinal cord
[96]. In addition to secreting neurotrophic factors, MSCs can influence the local cellular environment by releasing cytokines and chemokines that recruit endogenous stem cells to the site of injury
[97][98][97,98]. This recruitment of stem cells can further support axonal regeneration and promote tissue repair by providing additional cellular resources for the formation of new neuronal connections
[99].
MSCs can also promote axonal regeneration by directly interacting with neurons and fostering the extension of growth cones
[100]. This interaction can be mediated by various cell adhesion molecules and extracellular signaling molecules, such as N-cadherin and ephrin family members, which help guide axonal growth and encourage the formation of new synaptic connections
[101]. Moreover, MSCs can enhance the intrinsic growth capacity of injured neurons, including both central nervous system (CNS) and PNS neurons, such as dorsal root ganglion (DRG) neurons, by upregulating the expression of regeneration-associated genes (RAGs), such as growth-associated protein-43 (GAP-43), arginase-1 (Arg-1), and activating transcription factor-3 (ATF3)
[102][103][104][105][102,103,104,105]. These RAGs play a critical role in the regenerative process by supporting the growth and guidance of axons and promoting synaptic plasticity
[106]. Furthermore, MSCs can form cellular bridges at the injury site, which help guide regenerating axons across the lesion and re-establish connections with target neurons
[107]. This scaffold-like structure created by MSCs can enhance the overall regenerative capacity of the injured spinal cord, leading to improved functional recovery.
In summary, MSCs promote axonal regeneration through multiple mechanisms, including the secretion of neurotrophic factors, recruitment of endogenous stem cells, direct interaction with neurons, upregulation of regeneration-associated genes, and formation of cellular bridges. These combined actions contribute to enhanced axonal growth and improved functional recovery after SCI.