
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | AIKATERINI KOUNTOURI | + 1597 word(s) | 1597 | 2021-05-10 12:10:38 | | | |
| 2 | Vicky Zhou | Meta information modification | 1597 | 2021-05-19 09:54:10 | | |
Video Upload Options
Patients with type 1 diabetes mellitus (T1DM) present elevated levels of cytokines including interleukin-1a (IL), IL-1β, IL-2, IL-6 and tumor necrosis factor alpha (TNF-α), suggesting the pre-existence of chronic inflammation, which, in turn, has been considered the major risk factor of adverse COVID-19 outcomes in many cohorts. Even more importantly, oxidative stress is a key player in COVID-19 pathogenesis and determines disease severity. It is well-known that extreme glucose excursions, the prominent feature of T1DM, are a potent mediator of oxidative stress through several pathways including the activation of protein kinase C (PKC) and the increased production of advanced glycation end products (AGEs). Additionally, chronic endothelial dysfunction and the hypercoagulant state observed in T1DM, in combination with the direct damage of endothelial cells by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), may result in endothelial and microcirculation impairment, which contribute to the pathogenesis of acute respiratory syndrome and multi-organ failure. The binding of SARS-CoV-2 to angiotensin converting enzyme 2 (ACE2) receptors in pancreatic b-cells permits the direct destruction of b-cells, which contributes to the development of new-onset diabetes and the induction of diabetic ketoacidosis (DKA) in patients with T1DM. Large clinical studies are required to clarify the exact pathways through which T1DM results in worse COVID-19 outcomes.
1. Introduction
Diabetes—especially when poorly controlled—has been recognized as a strong and independent predictor of severe outcomes including death during COVID-19 infection [1][2][3][4][5]. In a systematic review of the literature, Apicella et al. [6] summarized the potential risk factors that could lead to worst outcomes in COVID-19-positive subjects with diabetes: hyperglycemia, older age, male sex, non-white ethnic groups, poor socioeconomic status, comorbidities (such as hypertension, cardiovascular/cerebrovascular disease, chronic kidney disease), obesity, inflammation, and coagulation [6]. However, although it can be assumed that most of the patients reported in the literature with severe outcomes from SARS-CoV2 had type 2 diabetes (T2DM), the information concerning type 1 diabetes (T1DM) is still scarce.
The risk for severe COVID-19 disease in patients with T1DM has been investigated in the following studies:
(a) Ebekozien et al. [7] reported clinical outcomes in 33 COVID-19-positive subjects with T1DM—mean age was 24.8 years, median HbA1c was 8.5%, obesity/hypertension/cardiovascular disease were the most prevalent comorbidities and diabetic ketoacidosis the most prevalent adverse outcome (45.5% of the cases).
(b) Two reports by Barron et al. [8] and Holman et al. [9] from NHS UK covering almost all the general practices in England were the first to compare the risk for severe outcomes between T1DM and T2DM during COVID-19 infection addressing all the potential risk factors, including previous glycemic control. The results (adjusted for confounding factors) demonstrated that 1/3 of all in-hospital deaths with COVID-19 occurred in people with diabetes; the overall risk was 3.5 times higher in those with T1DM, while people with T2DM were at about twice the risk, compared to people without a known diagnosis of diabetes. In people with T1DM, preceding hyperglycemia was significantly associated with COVID-19-related mortality at values of HbA1c >10%, whereas in those with T2DM this risk was significant at HbA1c of 7.6% or higher. Obesity (body mass index (BMI) > 30 kg/m2) was almost equally associated with COVID-19-related deaths in both diabetes types. Old age (>70 years), male sex, impaired renal function, previous cardiovascular comorbidities, deprived people in under-privileged communities and people of black ethnicity were also significantly associated with the highest risk of in-hospital death from COVID-19. These results are supported by a previous study in over 100,000 primary care adult patients with diabetes in England: those with T1DM and T2DM had ~2-3-fold higher rates of all infections (including pneumonia, sepsis, and endocarditis) compared to patients without diabetes. A comparison of T1DM and T2DM revealed that the risk of hospitalization and infection-related death in the former versus the latter was 2-fold and 4-fold higher, respectively; older age (≥70 years), morbid obesity (BMI > 40 kg/m2), serious comorbidities (cardiovascular disease, hypertension, chronic kidney failure) and residence in more deprived areas markedly increased the risk for severe infections and adverse outcomes, whereas the duration of diabetes seemed to play a role only in patients with T2DM [10].
(c) McGurnaghan et al. [11] investigated the risk factors for severe outcomes in COVID-19-infected subjects with T1DM and T2DM in the total population of Scotland. The authors showed that subjects with diabetes had ~40% greater risk of developing fatal or critical care unit-treated COVID-19 disease compared to people without diabetes after adjustment for age and sex; the risk was ~1.75 times higher in T1DM than in T2DM. Male sex, residence in deprived areas, comorbidities (retinopathy, chronic kidney disease), longer diabetes duration, worse glycemic control and previous hospitalization for hypoglycemia or ketoacidosis in the past five years were among the most prevalent risk factors. Adjustment for age, sex and diabetes duration revealed that people with T1DM and T2DM had a similar risk for COVID-19-related severe outcomes [11].
(d) In a prospective cohort study, Gregory et al. [12] identified 40 COVID-19-positive patients with T1DM across a regional healthcare network of 137 service locations using electronic health records at Vanderbilt University Medical Center. The risk for severe illness and hospitalization in T1DM (BMI ~25 kg/m2) was 3- to 4-fold higher than in patients without diabetes and similar to that in T2DM. COVID-19 outcome severity in T1DM was related to poor glycemic control within the past year (HbA1c ~8%), the presence of chronic complications (hypertension, retinopathy, chronic kidney disease, neuropathy), older age, black ethnicity and low socioeconomic status [13].
(e) In a recent study, Ebekozien et al. [14] investigated the prevalence of ketoacidosis among 180 non-Hispanic white, non-Hispanic black and Hispanic subjects with T1DM during COVID-19 infection in 52 clinical sites across the USA. The results showed that non-Hispanic blacks were more prone to develop ketoacidosis, suggesting the role of race and ethnicity. In contrast to non-Hispanic blacks, most of the non-Hispanic white patients were using insulin pumps and continuous glucose monitoring systems (CGMS) in their treatment.
(f) In contrast to these reports, Vangoitsenhoven et al. [14] studied the hospitalization needs of 2336 subjects with T1DM from two diabetes specialist centers in the community during the first 3 months of the pandemic in Belgium. Of this cohort, only 0.21% were admitted to the hospital for treatment, suggesting that their risk for severe outcomes was not increased. BMI and HbA1c in hospitalized vs. non-hospitalized patients were ~24 kg/m2 vs. ~25.5 kg/m2 and ~8% vs. 7.7%, respectively. It should be noted that the vast majority of patients in this study were using CGMS and 25% of them were on insulin pumps. Research data regarding the effect of COVID-19 infection in a pediatric population with T1DM are scarce. At present, evidence suggests that, contrary to adults, children and adolescents with T1DM infected with SARS-CoV-2 have similar clinical outcomes without increased morbidity and mortality compared to peers without diabetes. A pediatric population with T1DM and COVID-19 infection usually did not require hospitalization [15][16]. Two reports by Rabbone et al. [17] and Unsworth et al. [18] demonstrated that children with type 1 diabetes mellitus and PCR-confirmed SARS-CoV-2 infection presented with mild or no symptoms and did not display long-term adverse outcomes. Regarding the association of COVID-19 infection with the risk of new-onset diabetes, research data are inconsistent. Unsworth et al. in a multicenter study in the UK reported an important increase in new-onset T1DM in children [18]. However, Tittel et al. [19] pointed out that the percentage of new-onset TIDM observed across Germany from March to May 2020 did not differ significantly compared to rates based on data collected over the last decade. In general, several potential factors, alone or in combination, may increase the susceptibility of subjects with T1DM to serious illness following COVID-19 infection.
2. The Association of T1DM with SARS-CoV2 Infection: Pathophysiological Mechanisms
The dysregulated metabolic milieu in T1DM exceeds far beyond hyperglycemia; in fact, T1DM is a combination of chronic inflammation and immune dysfunction, which exerts deleterious effects on the vasculature and the coagulation cascade, among others. On the other hand, COVID-19 is a hyperinflammatory condition that ends up as a multi-organ disease. Below, the most prominent connections between the pathophysiology of T1DM and adverse COVID-19 clinical outcomes are presented (Figure 1).
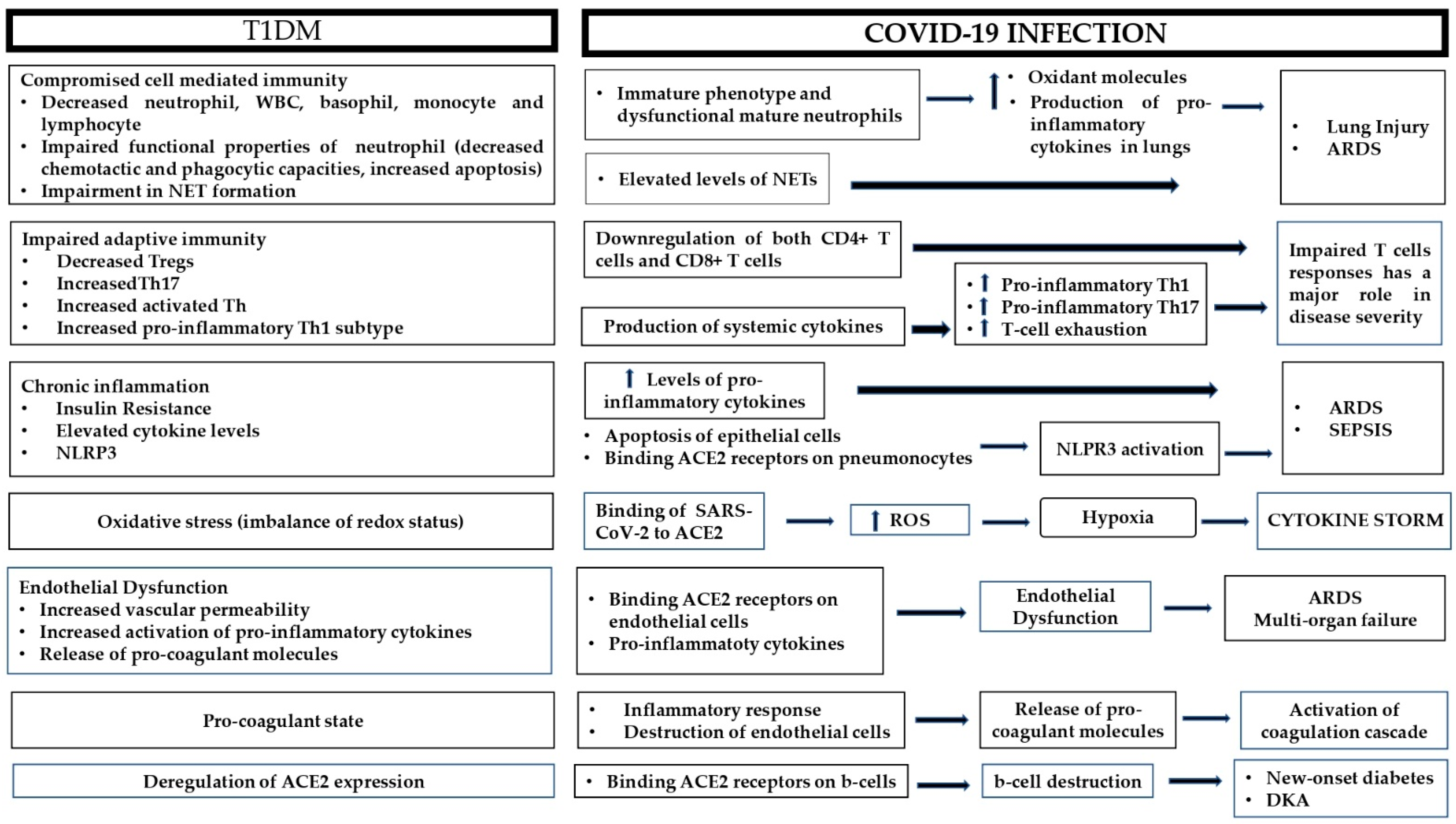
Figure 1. Possible pathophysiological mechanisms responsible for adverse outcomes during COVID-19 infection in patients with type 1 diabetes. ARDS: acute respiratory distress syndrome; ACE2: angiotensin converting enzyme 2; DKA: diabetic ketoacidosis; NET: neutrophil extracellular trap; NLRP3: NOD-like receptor family pyrin domain-containing 3; ROS: reactive oxygen species; Th: T-helper; Tregs: regulatory T-cells; WBC: white blood cells.
A growing number of studies have demonstrated that T1DM is an important risk factor affecting the clinical severity of COVID-19 disease. Immune and inflammatory dysregulation in conjunction with increased oxidative stress render patients with T1DM susceptible to severe COVID-19 infection. Additionally, the chronic endothelial dysfunction and the hypercoagulant state observed in T1DM account for the generalized endothelitis and thrombotic events that culminate in multi-organ failure and death. The deregulation of ACE2 expression in T1DM results in both increased risk of COVID-19 infection and in adverse outcomes of the disease. Apart from its role in viral transmission, the ACE2 receptor could also contribute to the development of new-onset diabetes and the induction of DKA in patients with T1DM. Large clinical studies are required to identify the clinical and biochemical parameters that could help identify patients at greater risk so that, along with a better understanding of the underlying pathophysiological mechanisms, more precise, timely and individualized therapeutic decisions can be made.
References
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020, 584, 430–436.
- Bode, B.; Garrett, V.; Messler, J.; McFarland, R.; Crowe, J.; Booth, R.; Klonoff, D.C. Glycemic Characteristics and Clinical Outcomes of COVID-19 Patients Hospitalized in the United States. J. Diabetes Sci. Technol. 2020, 14, 813–821.
- Huang, I.; Lim, M.A.; Pranata, R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia-A systematic review, meta-analysis, and meta-regression. Diabetes Metab. Syndr. 2020, 14, 395–403.
- Seiglie, J.; Platt, J.; Cromer, S.J.; Bunda, B.; Foulkes, A.S.; Bassett, I.V.; Hsu, J.; Meigs, J.B.; Leong, A.; Putman, M.S.; et al. Diabetes as a Risk Factor for Poor Early Outcomes in Patients Hospitalized With COVID-19. Diabetes Care 2020, 43, 2938–2944.
- Scheen, A.J.; Marre, M.; Thivolet, C. Prognostic factors in patients with diabetes hospitalized for COVID-19: Findings from the CORONADO study and other recent reports. Diabetes Metab. 2020, 46, 265–271.
- Apicella, M.; Campopiano, M.C.; Mantuano, M.; Mazoni, L.; Coppelli, A.; Del Prato, S. COVID-19 in people with diabetes: Understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 2020, 8, 782–792.
- Ebekozien, O.A.; Noor, N.; Gallagher, M.P.; Alonso, G.T. Type 1 Diabetes and COVID-19: Preliminary Findings from a Multicenter Surveillance Study in the U.S. Diabetes Care 2020, 43, e83–e85.
- Barron, E.; Bakhai, C.; Kar, P.; Weaver, A.; Bradley, D.; Ismail, H.; Knighton, P.; Holman, N.; Khunti, K.; Sattar, N.; et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: A whole-population study. Lancet Diabetes Endocrinol. 2020, 8, 813–822.
- Holman, N.; Knighton, P.; Kar, P.; O’Keefe, J.; Curley, M.; Weaver, A.; Barron, E.; Bakhai, C.; Khunti, K.; Wareham, N.J.; et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: A population-based cohort study. Lancet Diabetes Endocrinol. 2020, 8, 823–833.
- Carey, I.M.; Critchley, J.A.; DeWilde, S.; Harris, T.; Hosking, F.J.; Cook, D.G. Risk of Infection in Type 1 and Type 2 Diabetes Compared With the General Population: A Matched Cohort Study. Diabetes Care 2018, 41, 513–521.
- McGurnaghan, S.J.; Weir, A.; Bishop, J.; Kennedy, S.; Blackbourn, L.A.K.; McAllister, D.A.; Hutchinson, S.; Caparrotta, T.M.; Mellor, J.; Jeyam, A.; et al. Public Health Scotland COVID-19 Health Protection Study Group; Scottish Diabetes Research Network Epidemiology Group. Risks of and risk factors for COVID-19 disease in people with diabetes: A cohort study of the total population of Scotland. Lancet Diabetes Endocrinol. 2021, 9, 82–93.
- Gregory, J.M.; Slaughter, J.C.; Duffus, S.H.; Smith, T.J.; LeStourgeon, L.M.; Jaser, S.S.; McCoy, A.B.; Luther, J.M.; Giovannetti, E.R.; Boeder, S.; et al. COVID-19 Severity Is Tripled in the Diabetes Community: A Prospective Analysis of the Pandemic’s Impact in Type 1 and Type 2 Diabetes. Diabetes Care 2021, 44, 526–532.
- Vangoitsenhoven, R.; Martens, P.J.; van Nes, F.; Moyson, C.; Nobels, F.; Van Crombrugge, P.; Wierckx, K.; van Pottelbergh, I.; Van Huffel, L.; Gillard, P.; et al. No Evidence of Increased Hospitalization Rate for COVID-19 in Community-Dwelling Patients with Type 1 Diabetes. Diabetes Care 2020, 43, e118–e119.
- Ebekozien, O.; Agarwal, S.; Noor, N.; Albanese-O’Neill, A.; Wong, J.C.; Seeherunvong, T.; Sanchez, J.; DeSalvo, D.; Lyons, S.K.; Majidi, S.; et al. Inequities in Diabetic Ketoacidosis Among Patients With Type 1 Diabetes and COVID-19: Data From 52 US Clinical Centers. J. Clin. Endocrinol. Metab. 2021, 106, e1755–e1762.
- DiMeglio, L.A.; Albanese-O’Neill, A.; Muñoz, C.E.; Maahs, D.M. COVID-19 and Children With Diabetes-Updates, Unknowns, and Next Steps: First, Do No Extrapolation. Diabetes Care 2020, 43, 2631–2634.
- D’Annunzio, G.; Maffeis, C.; Cherubini, V.; Rabbone, I.; Scaramuzza, A.; Schiaffini, R.; Minuto, N.; Piccolo, G.; Maghnie, M. Caring for children and adolescents with type 1 diabetes mellitus: Italian Society for Pediatric Endocrinology and Diabetology (ISPED) statements during COVID-19 pandemia. Diabetes Res. Clin. Pract. 2020, 168, 108372.
- Rabbone, I.; Schiaffini, R.; Cherubini, V.; Maffeis, C.; Scaramuzza, A. Diabetes Study Group of the Italian Society for Pediatric Endocrinology and Diabetes. Has COVID-19 Delayed the Diagnosis and Worsened the Presentation of Type 1 Diabetes in Children? Diabetes Care 2020, 43, 2870–2872.
- Unsworth, R.; Wallace, S.; Oliver, N.S.; Yeung, S.; Kshirsagar, A.; Naidu, H.; Kwong, R.M.W.; Kumar, P.; Logan, K.M. New-Onset Type 1 Diabetes in Children During COVID-19: Multicenter Regional Findings in the U.K. Diabetes Care 2020, 43, e170–e171.
- Tittel, S.R.; Rosenbauer, J.; Kamrath, C.; Ziegler, J.; Reschke, F.; Hammersen, J.; Mönkemöller, K.; Pappa, A.; Kapellen, T.; Holl, R.W. DPV Initiative. Did the COVID-19 lockdown affect the incidence of pediatric type 1 diabetes in Germany? Diabetes Care 2020, 43, e172–e173.




