
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Athanasios Alexiou | + 696 word(s) | 696 | 2021-04-25 10:16:16 | | | |
| 2 | Catherine Yang | Meta information modification | 696 | 2021-05-17 03:30:04 | | |
Video Upload Options
Many proteins with zinc-binding domains (ZBDs) are involved in epigenetic modifications such as DNA methylation and histone modifications, which regulate transcription in physiological and pathological conditions. Zinc metalloproteins in epigenetics are mainly zinc metalloenzymes and zinc finger proteins (ZFPs), classified into writers, erasers, readers, editors, and feeders. Altogether, these classes of proteins engage in crosstalk that fundamentally maintains the epigenome's modus operandi.
1. Introduction
Zinc is an omnipresent micronutrient essential for healthy prenatal and postnatal developments in humans and the growth and development of plants, animals, and microorganisms [1][2]. In silico data suggest that about 10% of the human proteome potentially binds zinc [3]. Zinc is present in all body tissues and fluids as a component of over 2000 proteins (including epigenetically active enzymes) [4][5][6][7]. A group of writers, erasers, and readers of epigenetic marks are zinc-dependent [8]. Some of these are enzymes such as histone deacetylases (HDACs) having the zinc itself incorporated in their active sites; thus, it directly partakes in the catalytic process [9]. Others are proteins containing zinc within a zinc-binding domain (ZBD). These domains are important in substrate recognition, self-regulation, integrity, crosstalk, and sometimes catalysis [10][11]. With intracellular zinc within the normal range, these proteins work together in a coordinated manner to shape the plastic epigenome. In contrast, fluctuations in zinc levels or its deficiency and loss of function mutations in the ZBDs of these proteins affect their expression and function and may lead to epigenetic perturbations. [12][13]. These aberrant epigenetic changes may increase the risk of non-communicable chronic diseases such as cancer, diabetes, and cardiovascular diseases with possible multigenerational or transgenerational consequences. Intracellular zinc homeostasis is under the tight regulation of two families of zinc transporters and the zinc-binding proteins metallothionines (MTs) [14][15]. In addition to their zinc homeostasis roles, zinc transporters are also emerging as important modulators of the epigenome [4].
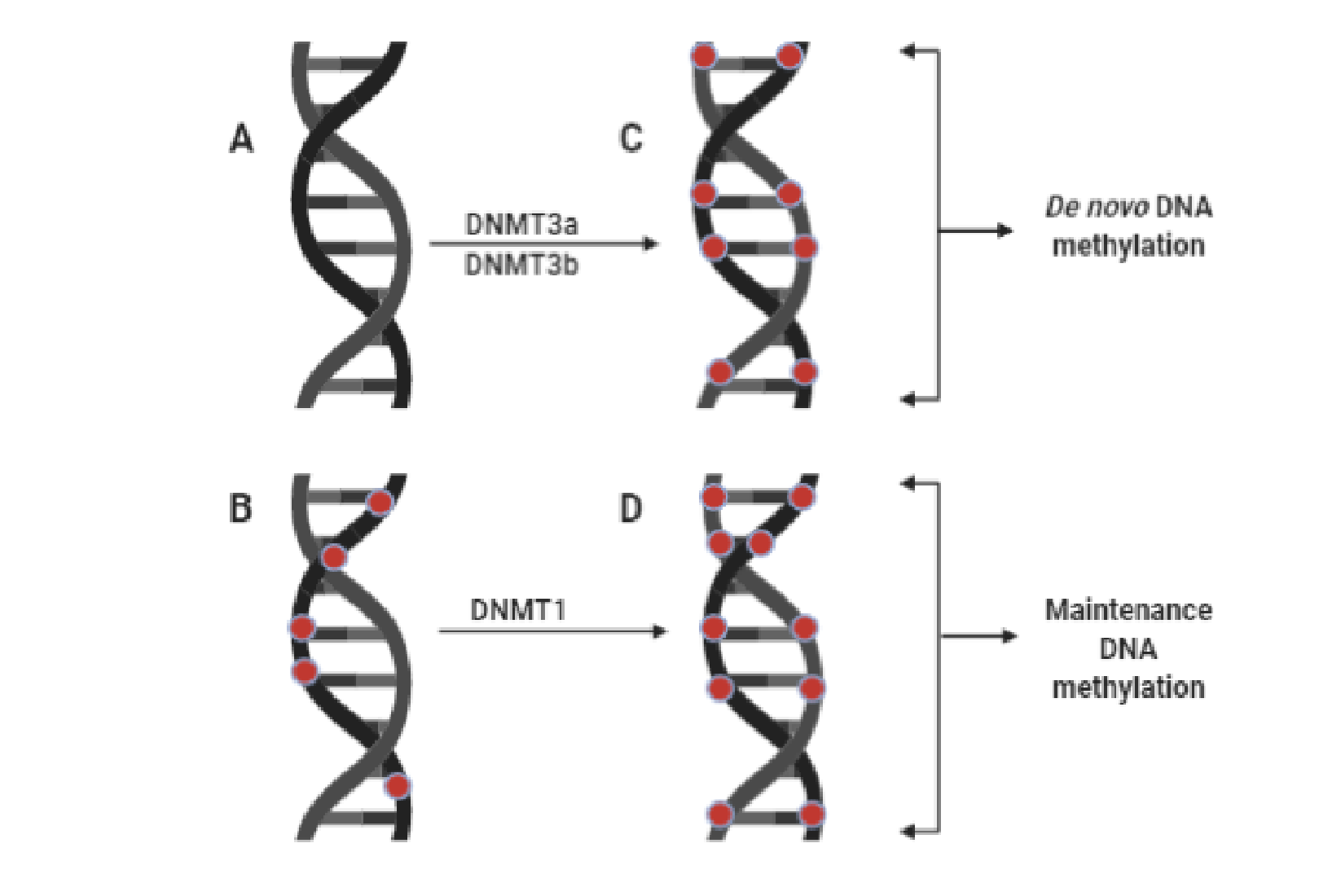 Figure 1. Comparison between de novo DNA methylation and maintenance DNA methylation. The figure compares de novo DNA methylation and maintenance DNA methylation. The two forms of DNA methylation catalyzed by DNMTs. (A) = unmethylated DNA; (B) = hemimethylated DNA; (C) and (D) = fully methylated DNA; DNMT3a/DNMT3b = DNA methyltransferases 3a/3b, both catalyze de novo DNA methylation (methylation of a newly formed double-stranded DNA during gametogenesis and embryogenesis). DNMT1 = DNA methyltransferase 1: It catalyzes replication-dependent maintenance of DNA methylation existing on hemimethylated DNA. Red dots stand for methyl groups.
Figure 1. Comparison between de novo DNA methylation and maintenance DNA methylation. The figure compares de novo DNA methylation and maintenance DNA methylation. The two forms of DNA methylation catalyzed by DNMTs. (A) = unmethylated DNA; (B) = hemimethylated DNA; (C) and (D) = fully methylated DNA; DNMT3a/DNMT3b = DNA methyltransferases 3a/3b, both catalyze de novo DNA methylation (methylation of a newly formed double-stranded DNA during gametogenesis and embryogenesis). DNMT1 = DNA methyltransferase 1: It catalyzes replication-dependent maintenance of DNA methylation existing on hemimethylated DNA. Red dots stand for methyl groups.

Figure 2. Role of zinc in DNA and histone methylation through the folate-mediated one-carbon metabolism. This figure summarizes the pathways linking one-carbon metabolism with DNA and histone methylation. Zinc-dependent enzymes and key intermediates are highlighted in different colors. BHMT (orange) = Betaine homocysteine methyltransferase: A zinc-dependent enzyme, which catalyzes the alternate reaction of methionine synthesis; DMG = Dimethylglycine; DNMTS (dark green) = DNA methyltransferases: Catalyze DNA methylation and have zinc-binding domains that regulate their activities; HMTs (dark green) = histone methyltransferases: Catalyze histone, lysine, and arginine methylation; MTR (pink) = Methionine synthase: It is zinc-dependent and catalyzes the direct synthesis of methionine (the direct precursor of SAM) from homocysteine and N5-methyl THF; SAH = S-Adenosylhomocysteine; SAM (light green) = S-Adenosylmethionine: The activated methyl donor for methylation reactions; THF = Tetrahydrofolate: The active form of folate that supplies methyl groups to homocysteine to form methionine. Zn2+ (red) = Zinc ion.
2. Results
Zinc metalloproteins contain ZBDs critical for substrate recognition, self-regulation, and catalysis. Most importantly, there is an interplay involving the ZBDs of these proteins that maintains the highly plastic epigenome in a dynamic state. Therefore, zinc is an essential trace metal in epigenetics. However, despite the plethora of ZBD-containing proteins identified and still being discovered, only a few of them have been employed to manipulate the epigenome. Non-communicable chronic diseases such as cancers, diabetes mellitus, and cardiovascular diseases have been associated with aberrant epigenetic changes in the genes related to these diseases; targeting these genes with customized ZBDs could make a remarkable difference in minimizing their burden. For instance, the hypermethylation of oncogenes or demethylation of tumor suppressor genes by epigenetic editors could help control various cancers. Furthermore, studies on transgenerational epigenetic effects at different doses of parental zinc exposure on offspring could unveil how zinc deficiency affects future generations’ health. Thus, the epigenetic burden of diseases could be minimized.
References
- Chasapis, C.T.; Loutsidou, A.C.; Spiliopoulou, C.A.; Stefanidou, M.E. Zinc and human health: An update. Arch. Toxicol. 2012, 86, 521–534.
- Hambidge, K.M.; Krebs, N.F. Zinc Deficiency: A Special Challenge. J. Nutr. 2007, 137, 1101–1105.
- Andreini, C.; Banci, L.; Bertini, I.; Rosato, A. Counting the Zinc-Proteins Encoded in the Human Genome. J. Proteome Res. 2006, 5, 196–201.
- Brito, S.; Lee, M.-G.; Bin, B.-H.; Lee, J.-S. Zinc and Its Transporters in Epigenetics. Mol. Cells 2020, 43, 323–330.
- Chasapis, C.T.; Ntoupa, P.-S.A.; Spiliopoulou, C.A.; Stefanidou, M.E. Recent aspects of the effects of zinc on human health. Arch. Toxicol. 2020, 94, 1443–1460.
- Roohani, N.; Hurrell, R.; Kelishadi, R.; Schulin, R. Zinc and its importance for human health: An integrative review. J. Res. Med. Sci. 2013, 18, 144–157.
- Foster, M.; Samman, S. Zinc and Regulation of Inflammatory Cytokines: Implications for Cardiometabolic Disease. Nutrients 2012, 4, 676–694.
- Blanquart, C.; Linot, C.; Cartron, P.-F.; Tomaselli, D.; Mai, A.; Bertrand, P. Epigenetic Metalloenzymes. Curr. Med. Chem. 2019, 26, 2748–2785.
- Porter, N.J.; Christianson, D.W. Structure, mechanism, and inhibition of the zinc-dependent histone deacetylases. Curr. Opin. Struct. Biol. 2019, 59, 9–18.
- Hodges, A.J.; Hudson, N.O.; Buck-Koehntop, B.A. Cys2His2 Zinc Finger Methyl-CpG Binding Proteins: Getting a Handle on Methylated DNA. J. Mol. Biol. 2020, 432, 1640–1660.
- Matthews, J.M.; Bhati, M.; Lehtomaki, E.; Mansfield, R.E.; Cubeddu, L.; Mackay, J.P. It Takes Two to Tango: The Structure and Function of LIM, RING, PHD and MYND Domains. Curr. Pharm. Des. 2009, 15, 3681–3696.
- Wessels, I.; Maywald, M.; Rink, L. Zinc as a Gatekeeper of Immune Function. Nutrients 2017, 9, 1286.
- Coneyworth, L.J.; Jackson, K.A.; Tyson, J.; Bosomworth, H.J.; Van Der Hagen, E.; Hann, G.M.; Ogo, O.A.; Swann, D.C.; Mathers, J.C.; Valentine, R.A.; et al. Identification of the human zinc transcriptional regulatory element (ZTRE): A palindromic protein-binding DNA se-quence responsible for zinc-induced transcriptional repression. J. Biol. Chem. 2012, 287, 36567–36581.
- Bin, B.-H.; Seo, J.; Kim, S.T. Function, Structure, and Transport Aspects of ZIP and ZnT Zinc Transporters in Immune Cells. J. Immunol. Res. 2018, 2018, 9365747.
- Myers, S.A.; Nield, A.; Myers, M. Zinc Transporters, Mechanisms of Action and Therapeutic Utility: Implications for Type 2 Diabetes Mellitus. J. Nutr. Metab. 2012, 2012, 1–13.





