Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Gan, Y. Biomass Wastes. Encyclopedia. Available online: https://encyclopedia.pub/entry/9598 (accessed on 04 March 2026).
Gan Y. Biomass Wastes. Encyclopedia. Available at: https://encyclopedia.pub/entry/9598. Accessed March 04, 2026.
Gan, Yong. "Biomass Wastes" Encyclopedia, https://encyclopedia.pub/entry/9598 (accessed March 04, 2026).
Gan, Y. (2021, May 13). Biomass Wastes. In Encyclopedia. https://encyclopedia.pub/entry/9598
Gan, Yong. "Biomass Wastes." Encyclopedia. Web. 13 May, 2021.
Copy Citation
Biomass wastes are abundant around us. They are renewable and inexpensive. Product manufacturing from renewable resources has caught increasing interest recently. Activated carbon preparation from biomass resources, including various trees, leaves, plant roots, fruit peels, and grasses, is a good example. In this paper, an overview of activated carbon production from biomass resources will be given.
Biomass Wastes
Activated Carbon
Porous Materials
Processing
1. Introduction
One of the most important forms of carbon, called activated carbon, has a high surface area and a large volume of micropores. The specific surface area of activated carbon can reach as high as 3000 m2/g, which makes it very effective in the removal of inorganic pollutants such as heavy metals from water [1]. Activated carbon has also been studied for mercury removal from water [2][3]. Activated carbon is sometimes called active carbon because it can participate in chemical reactions or it can be used as the support for catalysis. Recent applications of activated carbons are in the field of energy storage and conversions [4]. What are the differences between porous carbon and activated carbon? Generally speaking, porous carbon is characterized by its physicochemical properties, such as large surface area, large pore size range, relatively low density, etc. Activated carbon refers to carbon materials experienced with the activation of their surfaces or modification on the structures via functionalization, metal or oxide deposition, etc., for well-defined applications. All activated carbons are porous carbons. However, not all porous carbons are activated carbons. The porosity of porous carbons spans a very wide range of pore sizes, while the activated carbons are, in essence, microporous materials. Although this fundamental difference should not be overlooked, sometimes the boundary between the activated carbons and porous carbons may not be so distinct, especially from processing and application perspectives. As will be discussed in next section, the pore generation and activation of carbon materials happen in the same process. Surface functionalization and activation result in pore generation simultaneously.
Traditionally, activated carbon is made from coal or charcoal. However, making activated carbon from renewable resources is more intriguing because it is sustainable. The carbonizing of naturally grown grass and tree leaves has been studied for various potential industrial applications [5]. Date palm-tree branches (DPB) generated from the regular trimming of palm-trees were carbonized to generate an activated carbon product for toluene adsorption [6]. Although some biomass may be directly used for the adsorption of cationic dyes with high concentration at lower cost [7], activated sorbents after carbonization showed higher efficiency in dye removal [8]. Usually, curry tree (Murraya koenigii) stem is considered as an agricultural waste [9]. It is present in various vegetable markets. To convert it to a value-added product, carbonization of curry tree bark was conducted to generate activated carbon. The activated carbon was used to effectively remove the crystal violet dye from wastewater [9].
The abundance and diversity of bioresources are other reasons for the preparation of new activated carbon from woods, tree barks and leaves, grasses, and roots. In the following sections, recent development in various techniques for generating high performance and low cost activated carbon from representative renewable sources will be dealt with. The physical and chemical activation methods will be discussed. In the last part of the paper, typical applications of the activated carbons for gas adsorption, water purification, and energy storage will be presented.
2. Processing Techniques
Activated carbon materials are made through three required processes. The first process is the pretreatment of biomass raw materials. The second process is carbonization. The third process is activation. During the pretreatment process, most of the nutrients and solvable impurities are removed. The carbonization process allows organic lignin and cellulose to be converted into carbonaceous materials. Carbonization also reduces the amount of water, nutrients, oxygen, hydrogen, sulfur, and other elements. Carbon loss may happen in the carbonization process due to heating to temperatures above 400 °C. With the increase of temperature, the grains in raw materials are dehydrated. The oxygen in the raw materials is released in forms of H2O, CO, CO2, etc. Such reaction products facilitate subsequent activation reactions [10]. Typically, the activation process follows carbonization. However, they may be conducted at the same time. The carbonaceous materials (biochars) can be activated by two different approaches: physical activation (PA), and chemical activation (CA). In the physical activation process, a raw material is activated in the temperature range from 750 to 1000 °C in a vacuum or inert gas atmosphere. In the chemical activation process, chemical agents are incorporated into raw materials. They are heated up together in an inert gas, and carbonization and activation occur simultaneously. Recently, chemical activation has been studied for processing high-performance activation carbons. There are some challenges associated with the chemical activation of carbon as well. A washing step is always required to remove byproducts following chemical activation. This washing, followed by drying, is typically time consuming, which is why physical activation is claimed as a more mature process used for producing most commercially available activated carbons. Considering the increased research interest in the chemical activation process, in the following subsections, the activated carbon processing techniques based mainly on the chemical activation approach will be discussed.
2.1. Pretreatment and Carbonization
Activated carbon is made through the general procedures including pre-carbonization, carbonization, and activation. Sometimes a pre-treatment procedure is needed. As shown in [4], tamarisk-tree branches were collected as the starting material, and the pre-treatment was performed by soaking the tamarisk-tree branch in distilled water for 12 h. Then the sample was air dried at 60 °C for a sufficiently long period of time. The whole process for making activated carbon from the tree branch is schematically shown in Figure 1a [4]. The abbreviation of FLC@BC in Figure 1a stands for the “few-layer carbon@bulk carbon”, a unique structure due to the activation treatment of the tamarisk tree sample with the KOH solution. The pre-carbonization was carried out at 320 °C for 5 h. The carbonization and activation of the sample are shown in more detail in [4]. Briefly, a typical pre-carbonized sample with a weight of 1 g was soaked in 20 mL KOH solution for a day. For multiple experiments, the mass ratios of the pre-carbonized product to KOH were kept as 1:1, 2:1, 3:1, 5:1, and 7:1, respectively. The samples were dried and then carbonized at 700 °C for 2 h in N2. Then, the samples were washed by 1 wt% HCl solution and distilled water until the pH value reached 7. For comparative studies, the product carbonized at 700 °C for 2 h without soaking in KOH solution was made. Figure 1b shows optical images of the tamarisk tree and branches. Under scanning electron microscope, the tamarisk tree branch sample demonstrated a porous microstructure, and the ligaments were relatively rough, as shown by the SEM image in Figure 1c [4].
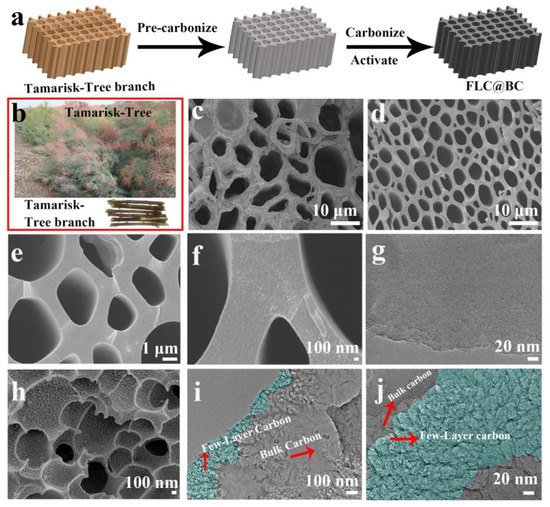
Figure 1. Schematic and images showing (a) the tamarisk tree branches under pre-carbonization, carbonization, and activation treatment procedures; (b) tamarisk tree and branches under visible light; (c) the tamarisk tree branch under scanning electron microscope (SEM); (d–f) carbonized sample without KOH activation under SEM; (g) carbonized sample without KOH activation under transmission electron microscope (TEM); (h) carbonized sample with KOH activation under SEM; (i,j) carbonized sample with KOH activation under TEM. Reproduced with permission from [4], ©2020 Elsevier Ltd.
3. Biomass Resources for Activated Carbon
Various raw materials including nutshells, fruit pits, paper mill waste (lignin), wood, charcoal, brown and bituminous coals, lignite, bone, and peat are the some of the starting materials for the production of activated carbon. It is not the intent to list every category of material here. Instead, the discussion will be on certain representative sustainable biomass sources. Nut shells, tree leaves, tree woods, willow catkins, and vegetable wastes will be discussed in the following subsections.
4. Functionalization of Activated Carbon
In this section, the functionalization of activated carbon by adding some elements or compounds will be discussed. These added elements or molecules have special properties to enhance the functions of porous carbons. As an example, incorporating iron into activated carbon was studied to promote the adsorption of As (V) from aqueous solutions [11]. As is well known, arsenic compounds in aqueous environment are highly poisonous and they are harmful to human health. More details on the iron decorated carbon will be discussed below. In addition, titanium oxide and manganese oxide modified activated carbon will also be discussed because they take significant roles in photocatalysis and desalination.
5. Applications
Activated carbons have found wide applications for catalysis [12][13], adsorption [14][15][16][17][18][19][20][21][22][23][24][25][26], templating [27], desalination [28], and electromagnetic interference shielding [29]. In addition, they have been extensively used for making supercapacitors [30][31][32][33][34], battery electrodes [35][36], and solar photothermal energy converters [37]. Activated carbons are accepted adsorbents for the purification of gaseous and aqueous solution systems at a large scale. In addition to purification application, energy conversion and energy storage are some of the most important applications. Some of these applications will be briefly discussed in the following section.
Although in the previous section, the catalysis applications were presented, it is worth mentioning that biochars and activated carbons derived from different woods are efficient catalysts for toluene conversion [12]. In [13], new bio-composite materials consisting of TiO2 (Degussa P25) and activated carbon (AC) of Argania spinosa tree nutshells by calcination and H3PO4 activation were made. The composites were used as photocatalysts for the elimination of pharmaceuticals, including diclofenac (DCF), carbamazepine (CBZ), and sulfamethoxazole (SMX), from aqueous solution. The TiO2 was attached to the AC to form the composite materials by high temperature impregnation. The drug elimination efficiency was evaluated.
The adsorption applications of biomass-derived activated carbons may be divided into several sub-categories. One of the categories is on heavy metal adsorption [14][15][16][17][18][19]. There is also a report on vitamin B adsorption [20]. Another category is about dye adsorption and decomposition [21][22][23][24]. Recently, carbon dioxide adsorption using activated carbon has become an increasingly important branch of research [25][26]. In [25], the porous carbon materials prepared from camellia leaves at the hydrothermal carbonization (HTC) temperature of 240 °C followed by KOH activation showed the microporous structure. From the HTC, the tree leaves were converted to hydrochars or biochars in solid form. The biochars were used as the raw materials for activated carbon preparation. The specific surface area was as high as 1824 m2/g. A maximum CO2 adsorption capacity of 8.30 mmol/g at 25 °C under 0.4 MPa was achieved. Xu et al. [26] prepared nitrogen doped carbons from camphor tree leaves. The tree leaves were carbonized at 500 °C for 2 h to generate chars. The chars were then activated with KOH at 600 °C in nitrogen gas flow. The nitrogen contained in the tree leaves also served as the nitrogen source for doping. The carbon showed a relatively high surface area of 1736 m2/g. A fairly high CO2 uptake of 5.86 mmol/g at 1 bar and 273 K was attained.
Porous carbon can be used as the template for fabricating nanostructures with different compositions. Activated carbon from biomass through physical activation in an inert atmosphere was chemically treated using tetraethyl orthosilicate (TEOS) [27]. Porous carbons were obtained from carbonization of the Platanus orientalis L. plane tree fruit (PTF) precursor and activated at 850 °C. The activated carbon as a template allowed the creation of highly porous and spatially ordered bio-SiC ceramics. The SiC nanostructures were generated at several processing temperatures. The carbothermal reduction occurred at 1400 °C. The increase in the temperature and the duration of processing promoted the generation of the SiC particles inside the porous structure. β-SiC with the cubic structure was the major portion, and the remainder was α-SiC with a hexagonal structure [27].
Activated carbon plays an important role in capacitive deionization and helps in the making of biologically-inspired desalination systems. As described in [28], the growth of mangrove trees in brackish swamps represents an amazing biologic adaptation to saltwater. Through water desalination, the mangrove maintains a near freshwater flow from roots to leaves to maintain growth. One-step carbonization of a plant with developed aerenchyma tissue to enable highly-permeable, freestanding flow-through capacitive deionization electrodes was performed [28]. The resistance to water flow through the electrode made by carbonized aerenchyma from red mangrove roots was more than 60 times lower than that through the electrode from carbonized common woody biomass. The practical use of the intact carbonized red mangrove roots as electrodes in a flow-through capacitive deionization system was illustrated [28].
Farhan, Wang, and Li [29] made a green carbon foam from the fibrous fruits of Platanus orientalis L. (plane) along with the tar oil as binder via the powder molding route. The porous carbon derived from biomaterials showed a considerably high strength. Various physical, thermal, and electromagnetic shielding properties were investigated. The application for electromagnetic interference shielding was proposed because the carbon foam exhibited shielding effectiveness of more than 20 dB over the X-band frequency. A fast carbonization approach was performed at 1000 °C under the cover of the pyrolyzed tree seeds without using extra protective gas. In some samples, 5 wt.% iron chloride was added during the molding process. Iron chloride is a graphitization catalyst and activating agent, which helped increasing the specific surface area from 88 to 294 m2/g, but the flexural strength of the carbon foam was decreased by 25%. Thermal stability was improved due to the incorporation of more graphitic phases in the sample. The thermal conductivity was increased slightly from 0.22 to 0.67 W/(m·K) due to the graphitization catalyzed by the iron chloride. In an electromagnetic (EM) field, the EM wave absorption by the carbon foam was dominant with only 8–10% reflection. This indicates that the EM wave absorption is the dominant shielding mechanism. The new carbon foam material preserved the light weight and was highly porous with interconnected pore morphology from the original biomaterial. It is suggested for high temperature thermal insulation as well [29].
Activated carbons have long been studied for energy storage and conversions [30][31][32][33][34][35][36][37]. A lot of researchers investigated the supercapacitors made from activated carbons [30][31][32][33][34]. In [30], a symmetric ionic liquid-based supercapacitor was fabricated with porous carbon derived from capsicum (bell pepper) seeds. The porous carbon with the nickname of “peppered”-activated carbon (ppAC) was obtained through the carbonization at 850 °C using KHCO3 as the activating agent. The ppAC-based supercapacitor operated at a maximum cell voltage of 3.20 V and was filled with an ionic liquid electrolyte, 1-ethyl-3-methylimidazolium bistrifluorosulfonylimide (EMIM-TFSI). The highest specific energy was 37 Wh/kg with a power density of 0.6 kW/kg at 0.5 A/g. A specific energy of 26 Wh/kg was obtained when the applied current was increased to 1.0 A/g. After being tested for 25,000 cycles, the capacitor was proven to have a high cyclic stability. The coulombic efficiency was kept at 99% after the cycling. He, Huang, and Wang [31] introduced porous nitrogen and oxygen co-doped carbon microtubes (PCMTs) generated from the carbonization and activation of plane tree fruit fluffs (PTFFs). The PCMTs were proposed as high-performance supercapacitor electrode materials. The pore structures, surface chemistry, and degree of graphitization of the porous carbon tubes can be tailored by varying the activation temperature in a range from 650 to 900 °C. The PCMT obtained from the 850 °C activation, named as PCMT-850, showed a specific surface area of 1533 m2/g), with the highest mesopore ratio of 9.13%. It contains 2.2 at% nitrogen, which is the highest N content achieved among all the PCMTs. It also has the highest degree of graphitization, leading to excellent electrical conductivity. In 6 M KOH, the PCMT-850 electrode attained the lowest internal resistance and highest charge storage capacity. The specific capacitance was 257.6 F/g at a current of 1A/g.
Kumar et al. [32] used a new activating agent (NaCl: KCl = 1: 1) for making a nanoporous carbon from Java Kapok tree shell. The nanoporous carbon showed a specific surface area of 1260 m2/g, pore volume of 0.439 cm3/g, pore size of 1.241 nm, and microspore volume of 0.314 cm3/g. The capacitor electrode using the nanoporous carbon demonstrated a specific capacitance of 169 F/g with 97% capacity retention after 10,000 cycles at 1 A/g. Barzegar et al. [33] prepared low-cost carbons from expanded graphite (EG) and pinecone (PC) biomass using KOH as the activation agent. The final carbonization was carried out in argon and hydrogen atmosphere. A specific surface area of 808 and 457 m2/g were obtained for the activated pinecone carbon (APC) and the activated expanded graphite (AEG), respectively. The activated carbon was used to make the electrode for asymmetric supercapacitors. A specific capacitance of 69 F/g was reported.
Nitrogen-doped porous carbon nanosheets prepared from eucalyptus tree leaves by simply mixing the leaf powders with KHCO3 and subsequent carbonization were used for electrodes in supercapacitors and lithium batteries [34]. The specific surface area of the porous carbon nanosheets was as high as 2133 m2/g. For supercapacitor application, the porous carbon nanosheet electrode exhibited a supercapacitance of 372 F/g at a current density of 500 mA/g in 1 M H2SO4 aqueous electrolyte and excellent cycling stability over 15,000 cycles. In an organic electrolyte, the nanosheet electrode demonstrated stable cycling behavior with a specific capacitance of 71 F/g at a current density of 2A/g. When applied as the anode material for lithium ion batteries, the carbon nanosheets showed good rate capability and stable cycling performance with a high specific capacity of 819 mAh/g at a current density of 100 mA/g [34].
Another area of energy storage research is in utilizing activated carbon for battery electrodes, because the biomass-derived carbon electrodes have low cost [34][35][36]. There are various carbon-based electrodes for lithium–sulfur batteries [35][36]. Zhang et al. [35] carbonized and activated palm tree fibers with KOH to obtain novel highly ordered carbon tube (OCT) arrays. The OCT was taken as the host in lithium–sulfur batteries. The electrode made from OCT was found effective on sulfur storage. The large specific area and pore volume were also found. The S@OCT composite with 65% (w/w) sulfur exhibited satisfactory electrochemical performance. It delivered an initial discharge capacity of 1255.2 mAh/g or 1.8 mAh/cm2 and retained 756.9 mAh/g after 100 cycles with a high coulomb efficiency [35].
Selva et al. [36] also showed that biomass-derived porous carbon could be a promising sulfur host material for lithium sulfur batteries because it is highly conductive and has large porosity. Two different carbons were prepared from oak tree fruit shells by carbonization with and without KOH activation. It was found that the KOH activated carbon (AC) revealed a much higher surface area of 796 m2/g than the pyrolyzed carbon (PC) (334 m2/g) without KOH activation. The activated-carbon contains more single-layer sheets with a lower degree of graphitization. The biomass-derived porous carbon was coated onto a separator, which led to an improved electrochemical performance in Li–S cells. The Li–S cell assembled with porous carbon modified separator demonstrated an initial capacity of 1324 mAh/g. This value for the cell with the uncoated separator was 875 mAh/g. The charge transfer resistance measurement confirmed the high ionic conductivity nature of porous carbon modified separator. The biomass-derived activated carbon can be considered as an alternative material for the polysulfide inhibition in Li–S batteries [36].
Activated carbons have been studied for energy converters, for example, solar thermal convertors or solar steam generators [38][37]. In [37], a photothermal generator inspired from banyan tree using the synthetic material, polyester, was prepared. However, sustainable resources, for example, willow catkin-derived porous carbon membrane demonstrated the potential for efficient solar steam generation [38]. Activated carbon possesses hydrophilic properties, allowing solar energy to be converted into thermal energy to heat the surrounding water flowing in a porous water channel under capillary action.
References
- Bolisetty, S.; Peydayesh, M.; Mezzenga, R. Sustainable technologies for water purification from heavy metals: Review and analysis. Chem. Soc. Rev. 2019, 48, 463–487.
- Liakos, E.V.; Rekos, K.; Giannakoudakis, D.A.; Mitropoulos, A.C.; Fu, J.; Kyzas, G.Z. Activated porous carbon derived from tea and plane tree leaves biomass for the removal of pharmaceutical compounds from wastewaters. Antibiotics 2021, 10, 65.
- Egirani, D.; Latif, M.T.; Wessey, N.; Poyi, N.R.; Shehata, N. Preparation and characterization of powdered and granular activated carbon from Palmae biomass for mercury removal. Appl. Water Sci. 2021, 11.
- Tan, Y.T.; Li, Y.; Wang, W.C.; Ran, F. High performance electrode of synthesized via controlling diffusion depth from liquid phase to solid phase for supercapacitors. J. Energy Storage 2020, 32, 101672.
- Khorasgani, N.B.; Sengul, A.B.; Asmatulu, E. Briquetting grass and tree leaf biomass for sustainable production of future fuels. Biomass Conv. Bioref. 2020, 10, 915–924.
- Vohra, M.; Al-Suwaiyan, M.; Hussaini, M. Gas phase toluene adsorption using date palm-tree branches based activated carbon. Int. J. Env. Res. Public Health 2020, 17, 9287.
- Peng, L.C.; Gao, J.; Yao, S.; Lan, X.Q.; Li, H.P.; Song, H. Modified ginkgo leaves for adsorption of methyl violet and malachite green dyes in their aqueous system. Desal. Water Treat. 2020, 206, 358–370.
- Yargic, A.S. Evaluation of poplar tree-based sorbents in dye uptake via 2(5) full factorial experimental design and statistical analysis of %removal efficiency. J. Polytech. Politek. Derg. 2020, 23, 941–954.
- Saniya, A.; Sathya, K.; Nagarajan, K.; Yogesh, M.; Jayalakshmi, H.; Praveena, P.; Bharathi, S. Modelling of the removal of crystal violet dye from textile effluent using Murraya koenigii stem biochar. Desal. Water Treat. 2020, 203, 356–365.
- Yang, H.M.; Zhang, D.H.; Chen, Y.; Ran, M.J.; Gu, J.C. Study on the application of KOH to produce activated carbon to realize the utilization of distiller’s grains. In Proceedings of the 3rd International Conference on Advances in Energy, Environment and Chemical Engineering, Chengdu, China, 26–28 May 2017; p. 012051.
- Sawood, G.M.; Gupta, S.K. Kinetic equilibrium and thermodynamic analyses of As (V) removal from aqueous solution using iron-impregnated Azadirachta indica carbon. Appl Water Sci. 2020, 10, 131.
- Korus, A.; Samson, A.; Szlek, A. Catalytic conversion of toluene over a biochar bed under an inert atmosphere—The comparison of chars from different types of wood and the role of selected metals. Fuel 2020, 279, 118468.
- El Mouchtari, E.; Daou, C.; Rafqah, S.; Najjar, F.; Anane, H.; Piram, A.; Hamade, A.; Briche, S.; Wong-Wah-Chung, P. TiO2 and activated carbon of Argania Spinosa tree nutshells composites for the adsorption photocatalysis removal of pharmaceuticals from aqueous solution. J. Photochem. Photobiol. A Chem. 2020, 388, 112183.
- Hasanpour, M.; Hatami, M. Application of three-dimensional porous aerogels as adsorbent for removal of heavy metal ions from water/wastewater: A review study. Adv. Colloid Interf. Sci. 2020, 284, 102247.
- Ullah, M.; Nazir, R.; Khan, M.; Khan, W.; Shah, M.; Afridi, S.G.; Zada, A. The effective removal of heavy metals from water by activated carbon adsorbents of Albizia lebbeck and Melia azedarach seed shells. Soil Water Res. 2020, 15, 30–37.
- Liang, S.; Shi, S.Q.; Zhang, H.H.; Qiu, J.J.; Yu, W.H.; Li, M.Y.; Gan, Q.; Yu, W.B.; Xiao, K.K.; Liu, B.C.; et al. One-pot solvothermal synthesis of magnetic biochar from waste biomass: Formation mechanism and efficient adsorption of Cr(VI) in an aqueous solution. Sci. Total Environ. 2019, 695, 133886.
- Vo, A.T.; Nguyen, V.P.; Ouakouak, A.; Nieva, A.; Doma, B.T.; Tran, H.N.; Chao, H.P. Efficient Removal of Cr(VI) from water by biochar and activated carbon prepared through hydrothermal carbonization and pyrolysis: Adsorption-coupled reduction mechanism. Water 2019, 11, 1164.
- Shi, S.Q.; Yang, J.K.; Liang, S.; Li, M.Y.; Gan, Q.; Xiao, K.K.; Hu, J.P. Enhanced Cr(VI) removal from acidic solutions using biochar modified by Fe3O4@SiO2-NH2 particles. Sci. Total Environ. 2018, 628–629, 499–508.
- Egirani, D.E.; Poyi, N.R.; Shehata, N. Preparation and characterization of powdered and granular activated carbon from Palmae biomass for cadmium removal. Int. J. Environ. Sci. Technol. 2020, 17, 2443–2454.
- Lupascu, T.; Petuhov, O.; Timbaliuc, N.; Cibotaru, S.; Rotaru, A. Adsorption capacity of vitamin B(12) and creatinine on highly-mesoporous activated carbons obtained from lignocellulosic raw materials. Molecules 2020, 25, 3095.
- Chong, M.Y.; Tam, Y.J. Bioremediation of dyes using coconut parts via adsorption: A review. SN Appl. Sci. 2020, 2, 187.
- Tolosa, N.C.; Mendoza, K.D.; Dumayas, D.L.P.; De Silva, J.M.D.F. Preparation and characterization of activated carbon derived from antidesma bunius L. in methylene blue removal from wastewater. J. Environ. Sci. Manag. 2020, 2020, 18–28.
- Ghaedi, A.M.; Baneshi, M.M.; Vafaei, A.; Nejad, A.R.S.; Tyagi, I.; Kumar, N.; Galunin, E.; Tkachev, A.G.; Agarwal, S.; Gupta, V.K. Comparison of multiple linear regression and group method of data handling models for predicting sunset yellow dye removal onto activated carbon from oak tree wood. Environ. Technol. Innov. 2018, 11, 262–275.
- Khafri, H.Z.; Ghaedi, M.; Asfaram, A.; Safarpoor, M. Synthesis and characterization of ZnS:Ni-NPs loaded on AC derived from apple tree wood and their applicability for the ultrasound assisted comparative adsorption of cationic dyes based on the experimental design. Ultrason. Sonochem. 2017, 38, 371–380.
- Yang, G.Z.; Song, S.; Li, J.; Tang, Z.H.; Ye, J.Y.; Yang, J.H. Preparation and CO2 adsorption properties of porous carbon by hydrothermal carbonization of tree leaves. J. Mater. Sci. Technol. 2019, 35, 875–884.
- Xu, J.G.; Shi, J.S.; Cui, H.M.; Yan, N.F.; Liu, Y.W. Preparation of nitrogen doped carbon from tree leaves as efficient CO2 adsorbent. Chem. Phys. Lett. 2018, 711, 107–112.
- Dodevski, V.; Pagnacco, M.C.; Radovic, I.; Rosic, M.; Jankovic, B.; Stojmenovic, M.; Mitic, V.V. Characterization of silicon carbide ceramics obtained from porous carbon structure achieved by plant carbonization. Mater. Chem. Phys. 2020, 245, 122768.
- Wood, A.R.; Garg, R.; Justus, K.; Cohen-Karni, T.; LeDuc, P.; Russell, A.J. Intact mangrove root electrodes for desalination. RSC Adv. 2019, 9, 4735–4743.
- Farhan, S.; Wang, R.M.; Li, K.Z. Physical and electromagnetic shielding properties of green carbon foam prepared from biomaterials. Trans. Nonferr. Met. Soc. China 2018, 28, 103–113.
- Momodu, D.; Sylla, N.F.; Mutuma, B.; Bello, A.; Masikhwa, T.; Lindberg, S.; Matic, A.; Manyala, N. Stable ionic-liquid-based symmetric supercapacitors from Capsicum seed-porous carbons. J. Electroanalyt. Chem. 2019, 838, 119–128.
- He, D.; Huang, Z.H.; Wang, M.X. Porous nitrogen and oxygen co-doped carbon microtubes derived from plane tree fruit fluff for high-performance supercapacitors. J. Mater. Sci. Mater. Electron. 2019, 30, 1468–1479.
- Kumar, K.T.; Sundari, G.S.; Kumar, E.S.; Ashwini, A.; Ramya, M.; Varsha, P.; Kalaivani, R.; Andikkadu, M.S.; Kumaran, V.; Gnanamuthu, R.; et al. Synthesis of nanoporous carbon with new activating agent for high-performance supercapacitor. Mater. Lett. 2018, 218, 181–184.
- Barzegar, F.; Bello, A.; Dangbegnon, J.K.; Manyala, N.; Xia, X.H. Asymmetric supercapacitor based on activated expanded graphite and pinecone tree activated carbon with excellent stability. Appl. Energy 2017, 207, 417–426.
- Mondal, A.K.; Kretschmer, K.; Zhao, Y.F.; Liu, H.; Wang, C.Y.; Sun, B.; Wang, G.X. Nitrogen-doped porous carbon nanosheets from eco-friendly eucalyptus leaves as high performance electrode materials for supercapacitors and lithium ion batteries. Chem. A Euro. J. 2017, 23, 3683–3690.
- Zhang, M.Y.; You, X.L.; Liu, L.J.; Walle, M.D.; Li, Y.J.; Liu, Y.N. Biomass derived highly-ordered carbon tube as athode material for high performance lithium-sulfur batteries. Chin. J. Inorg. Chem. 2019, 35, 1493–1499.
- Selva, R.K.; Zhu, P.; Yan, C.I.; Zhu, J.D.; Dirican, M.; Shanmugavani, A.; Lee, Y.S.; Zhang, X.W. Biomass-derived porous carbon modified glass fiber separator as polysulfide reservoir for Li-S batteries. J. Colloid Interf. Sci. 2018, 513, 231–239.
- Zhang, Q.; Hu, R.; Chen, Y.L.; Xiao, X.F.; Zhao, G.M.; Yang, H.J.; Li, J.H.; Xu, W.L.; Wang, X.B. Banyan-inspired hierarchical evaporators for efficient solar photothermal conversion. Appl. Energy 2020, 276, 115545.
- Zhang, S.C.; Zang, L.L.; Dou, T.W.; Zou, J.L.; Zhang, Y.H.; Sun, L.G. Willow catkins-derived porous carbon membrane with hydrophilic property for efficient solar steam generation. ACS Omega 2020, 5, 2878–2885.
More
Information
Subjects:
Others
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
13 May 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





