
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Dana Larocca | + 3276 word(s) | 3276 | 2021-04-27 06:00:16 | | | |
| 2 | Dana Larocca | -19 word(s) | 3257 | 2021-05-02 16:59:06 | | | | |
| 3 | Dana Larocca | + 5 word(s) | 3262 | 2025-06-15 17:09:30 | | |
Video Upload Options
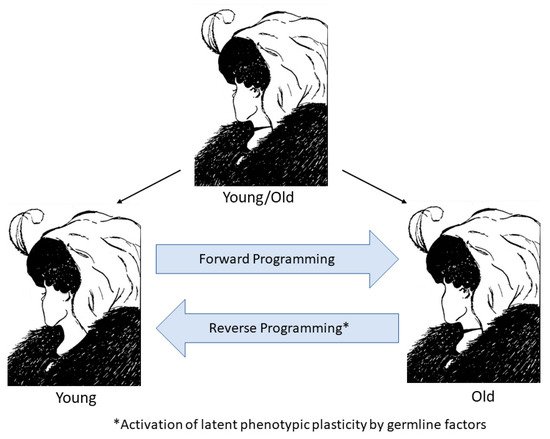
Cellular life evolved from simple unicellular organisms that could replicate indefinitely, being essentially ageless. At this point, life split into two fundamentally different cell types: the immortal germline representing an unbroken lineage of cell division with no intrinsic endpoint and the mortal soma, which ages and dies. We consider aging as a process not fixed to the pace of chronological time but one that can speed up or slow down depending on the rate of intrinsic cellular clocks. Moreover germline factor reprogramming might be used to slow the rate of aging and potentially reverse it by causing the clocks to tick backward. Therefore, reprogramming may eventually lead to therapeutic strategies to treat degenerative diseases by altering aging itself, the one condition common to us all.
1. Introduction
2. Plasticity of Aging and Development
3. Reversal of Aging In Vitro
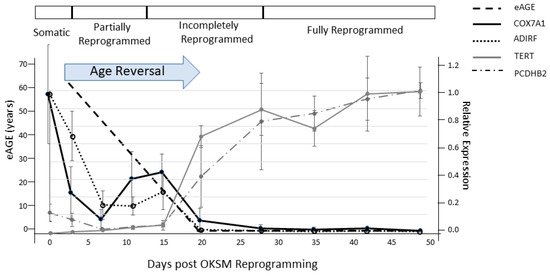
4. Reversal of Aging In Vivo
5. Concluding Remarks
References
- Weiss, M.C.; Preiner, M.; Xavier, J.C.; Zimorski, V.; Martin, W.F.; The last universal common ancestor between ancient Earth chemistry and the onset of genetics.. PLoS Genet. 2018, 14, e1007518.
- Carlos López-Otín; Maria A. Blasco; Linda Partridge; Manuel Serrano; Guido Kroemer; The Hallmarks of Aging. Cell 2013, 153, 1194-1217, 10.1016/j.cell.2013.05.039.
- Strehler, B.L.; Mildvan, A.S.; General theory of mortality and aging. Science 1960, 132, 14-21.
- Calvin B. Harley; A. Bruce Futcher; Carol W. Greider; Telomeres shorten during ageing of human fibroblasts. Nature 1990, 345, 458-460, 10.1038/345458a0.
- Steve Horvath; DNA methylation age of human tissues and cell types. Genome Biology 2012, 14, R115-R115, 10.1186/gb-2013-14-10-r115.
- John M. Sedivy; Jill A. Kreiling; Nicola Neretti; Marco De Cecco; Steven W. Criscione; Jeffrey W. Hofmann; Xiaoai Zhao; Takahiro Ito; Abigail L. Peterson; Death by transposition – the enemy within?. BioEssays 2013, 35, 1035-1043, 10.1002/bies.201300097.
- Dana Larocca; Jieun Lee; Michael West; Ivan Labat; Hal Sternberg; No Time to Age: Uncoupling Aging from Chronological Time. Genes 2021, 12, 611, 10.3390/genes12050611.
- Juulia Jylhävä; Nancy L. Pedersen; Sara Hägg; Biological Age Predictors. EBioMedicine 2017, 21, 29-36, 10.1016/j.ebiom.2017.03.046.
- David H. Meyer; Björn Schumacher; BiT age: A transcriptome‐based aging clock near the theoretical limit of accuracy. Aging Cell 2021, 20, e13320, 10.1111/acel.13320.
- Thomas B. L. Kirkwood; Thomas Cremer; Cytogerontology since 1881: A reappraisal of August Weismann and a review of modern progress. Quality of Life Research 1982, 60, 101-121, 10.1007/bf00569695.
- Finch, C.E.. Longevity, Senescence, and the Genome; University of Chicago Press: London, UK, 1994; pp. 43-247.
- J B Gurdon; The developmental capacity of nuclei taken from intestinal epithelium cells of feeding tadpoles.. Journal of embryology and experimental morphology 1962, 10, 622-640.
- K. H. S. Campbell; J. McWhir; W. A. Ritchie; I. Wilmut; Sheep cloned by nuclear transfer from a cultured cell line. Nature 1996, 380, 64-66, 10.1038/380064a0.
- Masahito Tachibana; Paula Amato; Michelle Sparman; Nuria Marti Gutierrez; Rebecca Tippner-Hedges; Hong Ma; Eunju Kang; Alimujiang Fulati; Hyo-Sang Lee; Hathaitip Sritanaudomchai; et al.Keith MastersonJanine LarsonDeborah EatonKaren Sadler-FreddDavid BattagliaDavid LeeDiana WuJeffrey JensenPhillip PattonSumita GokhaleRichard L. StoufferN WolfShoukhrat Mitalipov Human Embryonic Stem Cells Derived by Somatic Cell Nuclear Transfer. Cell 2013, 154, 465-466, 10.1016/j.cell.2013.06.042.
- Dean H. Betts; Vilceu Bordignon; Jonathan R. Hill; Quinton Winger; Mark E. Westhusin; Lawrence C. Smith; W. Allan King; Reprogramming of telomerase activity and rebuilding of telomere length in cloned cattle. Proceedings of the National Academy of Sciences 2001, 98, 1077-1082, 10.1073/pnas.031559298.
- Robert P. Lanza; Jose B. Cibelli; Catherine Blackwell; Vincent J. Cristofalo; Mary Kay Francis; Gabriela M. Baerlocher; Jennifer Mak; Michael Schertzer; Elizabeth A. Chavez; Nancy Sawyer; et al.Peter M. LansdorpMichael D. West Extension of Cell Life-Span and Telomere Length in Animals Cloned from Senescent Somatic Cells. Science 2000, 288, 665-669, 10.1126/science.288.5466.665.
- R. P. Lanza; Cloned Cattle Can Be Healthy and Normal. Science 2001, 294, 1893-1894, 10.1126/science.1063440.
- Kazutoshi Takahashi; Koji Tanabe; Mari Ohnuki; Megumi Narita; Tomoko Ichisaka; Kiichiro Tomoda; Shinya Yamanaka; Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861-872, 10.1016/j.cell.2007.11.019.
- Bao-Yang Hu; Jason P. Weick; Junying Yu; Li-Xiang Ma; Xiao-Qing Zhang; James A. Thomson; Su-Chun Zhang; Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proceedings of the National Academy of Sciences 2010, 107, 4335-4340, 10.1073/pnas.0910012107.
- Qiang Feng; Irina Klimanskaya; Ignatius Gomes; Hoon Kim; Young Chung; George R. Honig; Kwang-Soo Kim; Robert Lanza; Shi-Jiang Lu; Hemangioblastic Derivatives from Human Induced Pluripotent Stem Cells Exhibit Limited Expansion and Early Senescence. STEM CELLS 2010, 28, 704-712, 10.1002/stem.321.
- H Vaziri; Kb Chapman; A Guigova; J Teichroeb; M D Lacher; H Sternberg; I Singec; L Briggs; J Wheeler; J Sampathkumar; et al.R GonzalezD LaroccaJ MuraiE SnyderWh AndrewsWd FunkM D West Spontaneous reversal of the developmental aging of normal human cells following transcriptional reprogramming. Regenerative Medicine 2010, 5, 345-363, 10.2217/rme.10.21.
- Laure Lapasset; Ollivier Milhavet; Alexandre Prieur; Emilie Besnard; Amelie Babled; Nafissa Aït-Hamou; Julia Leschik; Franck Pellestor; Jean-Marie Ramirez; John De Vos; et al.Sylvain LehmannJean-Marc Lemaitre Rejuvenating senescent and centenarian human cells by reprogramming through the pluripotent state. Genes & Development 2011, 25, 2248-2253, 10.1101/gad.173922.111.
- Takuya Yagi; Arifumi Kosakai; Daisuke Ito; Yohei Okada; Wado Akamatsu; Yoshihiro Nihei; Akira Nabetani; Fuyuki Ishikawa; Yasumichi Arai; Nobuyoshi Hirose; et al.Hideyuki OkanoNorihiro Suzuki Establishment of Induced Pluripotent Stem Cells from Centenarians for Neurodegenerative Disease Research. PLOS ONE 2012, 7, e41572, 10.1371/journal.pone.0041572.
- Jieun Lee; Paola A. Bignone; L.S. Coles; Yang Liu; Evan Snyder; Dana Larocca; Induced pluripotency and spontaneous reversal of cellular aging in supercentenarian donor cells. Biochemical and Biophysical Research Communications 2020, 525, 563-569, 10.1016/j.bbrc.2020.02.092.
- Guang-Hui Liu; Basam Z. Barkho; Sergio Zamudio Ruiz; Dinh Diep; Jing Qu; Sheng-Lian Yang; Athanasia D. Panopoulos; Keiichiro Suzuki; Leo Kurian; Christopher T Walsh; et al.James ThompsonStephanie BoueHo Lim FungIgnacio Sancho-MartinezKun ZhangJohn Yates IiiJuan Carlos Izpisua Belmonte Recapitulation of premature ageing with iPSCs from Hutchinson–Gilford progeria syndrome. Nature 2011, 472, 221-225, 10.1038/nature09879.
- Hoi-Hung Cheung; Xiaozhuo Liu; Lucile Canterel-Thouennon; Lu Li; Catherine Edmonson; Owen M. Rennert; Telomerase Protects Werner Syndrome Lineage-Specific Stem Cells from Premature Aging. Stem Cell Reports 2014, 2, 534-546, 10.1016/j.stemcr.2014.02.006.
- Guang-Hui Liu; Basam Z. Barkho; Sergio Zamudio Ruiz; Dinh Diep; Jing Qu; Sheng-Lian Yang; Athanasia D. Panopoulos; Keiichiro Suzuki; Leo Kurian; Christopher T Walsh; et al.James ThompsonStephanie BoueHo Lim FungIgnacio Sancho-MartinezKun ZhangJohn Yates IiiJuan Carlos Izpisua Belmonte Recapitulation of premature ageing with iPSCs from Hutchinson–Gilford progeria syndrome. Nature 2011, 472, 221-225, 10.1038/nature09879.
- Steven T. Suhr; Eun Ah Chang; Jonathan Tjong; Nathan Alcasid; Guy A. Perkins; Marcelo D. Goissis; Mark H. Ellisman; Gloria I. Perez; Jose B. Cibelli; Mitochondrial Rejuvenation After Induced Pluripotency. PLOS ONE 2010, 5, e14095, 10.1371/journal.pone.0014095.
- Joana Frobel; Hatim Hemeda; Michael Lenz; Giulio Abagnale; Sylvia Joussen; Bernd Denecke; Tomo Šarić; Martin Zenke; Wolfgang Wagner; Epigenetic Rejuvenation of Mesenchymal Stromal Cells Derived from Induced Pluripotent Stem Cells. Stem Cell Reports 2014, 3, 414-422, 10.1016/j.stemcr.2014.07.003.
- Martin Wahlestedt; Eva Erlandsson; Trine Kristiansen; Rong Lu; Cord Brakebusch; Irving L. Weissman; Joan Yuan; Javier Martin-Gonzalez; David Bryder; Clonal reversal of ageing-associated stem cell lineage bias via a pluripotent intermediate. Nature Communications 2017, 8, 14533, 10.1038/ncomms14533.
- Zhao Cheng; Hong-Ling Peng; Rong Zhang; Xian-Ming Fu; Guang-Sen Zhang; Rejuvenation of Cardiac Tissue Developed from Reprogrammed Aged Somatic Cells. Rejuvenation Research 2017, 20, 389-400, 10.1089/rej.2017.1930.
- Weissman, A. Die Entstehung der Sexualzellen Bei Den Hydromedusen: Zugleich Ein Betrag zur Kenntniss des Baues und der Lebenserscheinungen Dieser Gruppe; Gustav Fischer Verlag: Jena, Germany, 1883.
- Matsumoto, Y.; Piraino, S.; Miglietta, M.P. Transcriptome Characterization of Reverse Development in Turritopsis dohrnii (Hydrozoa, Cnidaria). G3 Genes Genomes Genet. 2019, 9, 4127–4138.
- Bryan B. Teefy; Stefan Siebert; Jack F. Cazet; Haifan Lin; Celina E. Juliano; PIWI–piRNA pathway-mediated transposable element repression in Hydra somatic stem cells. RNA 2020, 26, 550-563, 10.1261/rna.072835.119.
- Daniel Elsner; Karen Meusemann; Judith Korb; Longevity and transposon defense, the case of termite reproductives. Proceedings of the National Academy of Sciences 2018, 115, 5504-5509, 10.1073/pnas.1804046115.
- Todd, E.V.; Ortega-Recalde, O.; Liu, H.; Lamm, M.S.; Rutherford, K.M.; Cross, H.; Black, M.A.; Kardailsky, O.; Graves, J.A.M.; Hore, T.A.; et al. Stress, novel sex genes, and epigenetic reprogramming orchestrate socially controlled sex change. Sci. Adv. 2019, 5, eaaw7006.
- Uhlenhaut, N.H.; Jakob, S.; Anlag, K.; Eisenberger, T.; Sekido, R.; Kress, J.; Treier, A.C.; Klugmann, C.; Klasen, C.; Holter, N.I.; et al. Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation. Cell 2009, 139, 1130–1142.
- Matson, C.K.; Murphy, M.W.; Sarver, A.L.; Griswold, M.D.; Bardwell, V.J.; Zarkower, D. DMRT1 prevents female reprogramming in the postnatal mammalian testis. Nature 2011, 476, 101–104.
- Olova, N.; Simpson, D.J.; Marioni, R.E.; Chandra, T. Partial reprogramming induces a steady decline in epigenetic age before loss of somatic identity. Aging Cell 2019, 18, e12877.
- West, M.D.; Labat, I.; Sternberg, H.; Larocca, D.; Nasonkin, I.; Chapman, K.B.; Singh, R.; Makarev, E.; Aliper, A.; Kazennov, A.; et al. Use of deep neural network ensembles to identify embryonic-fetal transition markers: Repression of COX7A1 in embryonic and cancer cells. Oncotarget 2018, 9, 7796–7811.
- Gill, D.; Parry, A.; Santos, F.; Hernando-Herraez, I.; Stubbs, T.M.; Milagre, I.; Reik, W. Multi-omic rejuvenation of human cells by maturation phase transient reprogramming. bioRxiv 2021.
- Ohnishi, K.; Semi, K.; Yamamoto, T.; Shimizu, M.; Tanaka, A.; Mitsunaga, K.; Okita, K.; Osafune, K.; Arioka, Y.; Maeda, T.; et al. Premature termination of reprogramming in vivo leads to cancer development through altered epigenetic regulation. Cell 2014, 156, 663–677.
- Abad, M.; Mosteiro, L.; Pantoja, C.; Canamero, M.; Rayon, T.; Ors, I.; Grana, O.; Megias, D.; Dominguez, O.; Martinez, D.; et al. Reprogramming in vivo produces teratomas and iPS cells with totipotency features. Nature 2013, 502, 340–345.
- Ocampo, A.; Reddy, P.; Martinez-Redondo, P.; Platero-Luengo, A.; Hatanaka, F.; Hishida, T.; Li, M.; Lam, D.; Kurita, M.; Beyret, E.; et al. In Vivo Amelioration of Age-Associated Hallmarks by Partial Reprogramming. Cell 2016, 167, 1719–1733.e1712.
- Doeser, M.C.; Scholer, H.R.; Wu, G. Reduction of Fibrosis and Scar Formation by Partial Reprogramming In Vivo. Stem Cells 2018, 36, 1216–1225.
- Tang, Y.; Cheng, L. Cocktail of chemical compounds robustly promoting cell reprogramming protects liver against acute injury. Protein Cell 2017, 8, 273–283.
- Lu, Y.; Brommer, B.; Tian, X.; Krishnan, A.; Meer, M.; Wang, C.; Vera, D.L.; Zeng, Q.; Yu, D.; Bonkowski, M.S.; et al. Reprogramming to recover youthful epigenetic information and restore vision. Nature 2020, 588, 124–129.
- Sarkar, T.J.; Quarta, M.; Mukherjee, S.; Colville, A.; Paine, P.; Doan, L.; Tran, C.M.; Chu, C.R.; Horvath, S.; Qi, L.S.; et al. Transient non-integrative expression of nuclear reprogramming factors promotes multifaceted amelioration of aging in human cells. Nat. Commun. 2020, 11, 1545.
- Agarwal, S.; Agarwal, V.; Agarwal, M.; Singh, M. Exosomes: Structure, Biogenesis, Types and Application in Diagnosis and Gene and Drug Delivery. Curr. Gene Ther. 2020, 20, 195–206.
- Mendt, M.; Kamerkar, S.; Sugimoto, H.; McAndrews, K.M.; Wu, C.C.; Gagea, M.; Yang, S.; Blanko EV, R.; Peng, Q.; Ma, X.; et al. Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI Insight 2018, 3, e99263.
- Dorronsoro, A.; Santiago, F.E.; Grassi, D.; Zhang, T.; Lai, R.C.; McGowan, S.J.; Angelini, L.; Lavasani, M.; Corbo, L.; Lu, A.; et al. Mesenchymal stem cell-derived extracellular vesicles reduce senescence and extend health span in mouse models of aging. Aging Cell 2021, 20, e13337.
- Walsh, E.E.; Frenck, R.W., Jr.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N. Engl. J. Med. 2020, 383, 2439–2450.
- Justice, J.N.; Niedernhofer, L.; Robbins, P.D.; Aroda, V.R.; Espeland, M.A.; Kritchevsky, S.B.; Kuchel, G.A.; Barzilai, N. Development of Clinical Trials to Extend Healthy Lifespan. Cardiovasc. Endocrinol. Metab. 2018, 7, 80–83.




