Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Natalia Georgantzoglou | + 4290 word(s) | 4290 | 2021-04-21 10:17:39 | | | |
| 2 | Lily Guo | Meta information modification | 4290 | 2021-04-30 02:46:39 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Georgantzoglou, N. Tumor Microenvironment in Adrenocortical Carcinoma. Encyclopedia. Available online: https://encyclopedia.pub/entry/9200 (accessed on 07 February 2026).
Georgantzoglou N. Tumor Microenvironment in Adrenocortical Carcinoma. Encyclopedia. Available at: https://encyclopedia.pub/entry/9200. Accessed February 07, 2026.
Georgantzoglou, Natalia. "Tumor Microenvironment in Adrenocortical Carcinoma" Encyclopedia, https://encyclopedia.pub/entry/9200 (accessed February 07, 2026).
Georgantzoglou, N. (2021, April 29). Tumor Microenvironment in Adrenocortical Carcinoma. In Encyclopedia. https://encyclopedia.pub/entry/9200
Georgantzoglou, Natalia. "Tumor Microenvironment in Adrenocortical Carcinoma." Encyclopedia. Web. 29 April, 2021.
Copy Citation
Adrenocortical carcinoma is a rare but aggressive malignancy with poor outcomes even for patients with early-stage disease. Several immunotherapy clinical trials, many of which are still in progress, have yielded modest results so far. In-depth understanding of the tumor microenvironment in other cancer types has helped the scientific community to identify novel therapeutic targets and gain a better insight of cancer biology.
adrenocortical carcinoma
1. Introduction
Adrenocortical carcinoma (ACC) is an aggressive malignancy with an annual incidence of 0.5–2 cases per 1 million population. Although it can occur at any age, it has a bimodal distribution, with disease peaks before the age of five and the fifth decade of life, while there is also a predilection for the female gender. Most cases of ACC are considered to be sporadic, however, it can also present as part of hereditary syndromes such as Li–Fraumeni syndrome (LFS), Beckwith–Wiedemann syndrome, Carney complex and Multiple Endocrine Neoplasia(MEN) I [1].
Histologically, malignant cells follow a solid, trabecular, or large nested growth pattern, while tumor necrosis, vascular, and capsular invasion are all commonly present. Weiss score, the most widely used diagnostic system, evaluates the abovementioned features, along with the mitotic rate and nuclear grade, to predict the malignant behavior of adrenocortical neoplasms. Overall, there are three main histologic variants: oncocytic, myxoid, and sarcomatoid, with the oncocytic variant being the most common [1].
Around 50% of the patients present with steroid hormone excess symptoms, and among those, 80% present with Cushing’s syndrome, which is often characterized by remarkably rapid onset. The remaining patients present with tumor growth-related symptoms or are discovered incidentally during imaging studies for unrelated reasons.
Currently, the prognosis for ACC is grim, with the five-year survival rate at 37–47%. For patients with disease at stages I–III, surgical resection is recommended, with adjuvant mitotane (a steroidogenesis inhibitor) therapy in high-risk patients. However, many patients in these stages already have micro-metastases at the time of initial presentation, and therefore surgery is not curative. Approximately one out of two patients treated with surgery will relapse. Adjuvant mitotane treatment has been evaluated in some retrospective studies including heterogeneous populations. It has been demonstrated to improve recurrence-free survival [2], although the results are not consistent across the studies.
For surgically unresectable tumors multi-drug chemotherapy with etoposide, doxorubicin, and cisplatin (EDP), combined with mitotane (M), is currently the mainstay of treatment, while radiofrequency ablation can be employed for local control. EDP-M regimen was evaluated in a prospective phase 2 trial of advanced ACC patients and led to a response rate of 38% and a complete hormonal response in 38% of patients with functional syndrome [3]. The phase 3 First International Randomized Trial in locally advanced and metastatic Adrenocortical Carcinoma Treatment (FIRM-ACT) trial compared first-line treatment with EDP-M versus streptozocin-M. EDP-M was superior in terms of response rate (23.2% versus 9.2%) and median progression-free survival (PFS) (5 versus 2.1 months [4]. Mitotane monotherapy is an option in patients with less aggressive disease. Its use is supported by a German study that reported an objective response rate of 20.5% and a median PFS of 4.1 months [5]). Gemcitabine in combination with capecitabine is usually proposed as a second-line treatment, based on retrospective data lacking a meaningful clinical benefit [6]. Several targeted therapies have also been assessed in small studies with modest results. The poor survival rates, despite the abovementioned therapeutic strategies, highlight the imperative need for novel therapeutic approaches [7].
Immunotherapy (IO) constitutes a new systemic therapy in the armamentarium against cancers, and has resulted in remarkable tumor responses and survival prolongation in various cancers, such as melanoma [8], non-small cell lung cancer [9], renal cell carcinoma [10], and squamous cell carcinoma of the head and neck [11]. In contrast to cytotoxic chemotherapy, which directly “kills” tumor cells, IO induces the activation of the patient’s immune system in order to attack cancer cells, mainly through the activation of cytotoxic T-lymphocytes. The most widely used IO drugs belong to immune checkpoint inhibitors (ICI): PD-1 (programmed cell death protein 1) inhibitors and PD-L1 (programmed death-ligand 1) inhibitors. Both drugs are monoclonal antibodies that inhibit the interaction of PD-1 and PD-L1, and therefore prevent T-lymphocytes’ recognition of cancer antigens through their binding to antigen-presenting cells. Although immunotherapy has undoubtedly revolutionized cancer therapy in the preceding years, results from the ongoing immunotherapy trials for ACC have been modest.
Over the years it has been documented that a major determinant of tumor behavior and responsiveness to immunotherapy is the tumor microenvironment (TME), a dynamic and extremely complex milieu of inflammatory cells, cancer cells, cytokines, extracellular matrix, mesenchymal stem cells, and blood vessels. Cancer cells employ various mechanisms, such as downregulation of HLA molecules and co-stimulatory molecules or apoptosis and the dysfunction of T-cells and dendritic cells, in order to evade immune surveillance and, in fact, they are able to subvert the host’s defense mechanisms into an inflammatory milieu that supports the tumor’s growth and progress. Manipulating TME in an attempt to optimize its immunogenicity has captured the scientific interest as a possible way to modulate responsiveness to immunotherapy [12][13].
In the current review, we aim to explore the characteristics of the immune microenvironment (TIME) of ACC in an attempt to elucidate the possible reasons for immunotherapy resistance and provide an overview of potential new prognostic biomarkers and therapeutic targets.
2. Tumor Immune Microenvironment in Adrenocortical Carcinoma
2.1. Immune Cells
Tumor-infiltrating lymphocytes (TILs) constitute a polyclonal lymphocyte population that migrates from the bloodstream to the tumor and target tumor associated antigens, playing, therefore, a key role in the anti-tumor immune response. Numerous studies have demonstrated the association of the density and distribution of TILs with patient response to chemotherapy and their overall prognosis in several solid tumors. Denkert et al. demonstrated that TILs are a favorable prognostic factor in HER2-positive breast cancer and triple negative breast cancer [14], while recently, TILs have emerged as a potential biomarker in highly aggressive tumors, such as melanoma [15].
ACC has been traditionally described as an immune deplete tumor, a fact mainly attributed to adrenal glucocorticoid production. In a pan-cancer analysis utilizing mRNA expression of immune-related genes (including PD-L1) in a TCGA (The Cancer Genome Atlas Program) cohort, Pare et al. showed that immune infiltration in ACC is indeed lower compared to other types of cancer [16]. In agreement with this data, an immune genomic analysis of multiple cancer types revealed that ACC, along with uveal melanoma, displayed the lowest leucocyte fraction (calculated based on data of DNA methylation) [17]. TCGA’s integrative genomic analysis of ACC revealed three distinct molecular subtypes (CoC, or cluster of cluster I, II, and III). The expression of immune-related genes is, in general, low, except for the CoC I subgroup, where immune infiltration is observed [18].
However, expression of immune-related genes, such as ERN1 and CEP55, points out the important role of TME in ACC [19]. An increasing number of studies in recent years highlight the significance of TILs as well as other types of immune cells in ACC patients’ prognosis. A recent study using immunofluorescence to quantify infiltrating cell populations in ACC samples, demonstrated that >80% of the samples were infiltrated by lymphocytes with CD8+ cells being the predominant type [20]. It should be noted, however, that the median number of infiltrating immune cells was rather low, which explains the lower quantitative immune gene-related mRNA expression observed in previous studies [16]. Tian et al. applied gene analysis and CIBERSORT algorithms—a computational method for quantifying cell fractions—to a TGCA cohort and showed that the immune infiltrate mainly consisted of T-cells, natural killer cells, mast cells, and macrophages while infiltration levels by each subtype showed strong correlation with the other subtypes. The number of mast cells correlated with survival, as well as with specific changes in signaling pathways [21]. Both the abovementioned studies proved a significant correlation of TIL levels with overall survival (OS) and recurrence-free survival (RFS). Increased levels of TILs were associated with lower pTNM and AJCC staging [21], and notably, metastatic foci seem to be relatively immune deplete compared to primary tumors. This suggests that immune evasion plays a crucial role in disease progression. The association of TILs, and more specifically CD8+ lymphocytes, with prognosis has been demonstrated in pediatric ACC patients as well [22]. Overall, current data suggest that, although lymphocyte infiltration is present in ACC and correlates with patient prognosis, it is quantitatively smaller comparing to other types of cancers.
In addition to the levels of infiltration, the pattern of distribution of infiltrating lymphocytes within the tumor could potentially play a significant role in the dynamics of the TME. The diffuse pattern of CD8+ lymphocytes seems to predominate in ACC compared to the multifocal pattern, which is dominant in benign adenomas [22].
Further investigation regarding the role of immune cells, other than the lymphocytes, in the microenvironment of ACC is warranted. Mast cells constitute a potential target population as they have been positively correlated with prognosis and their presence might exert a regulatory effect on lymph cell populations. Furthermore, emerging data highlight the significance of neutrophils in the microenvironment of many solid tumors, rendering them a potential target in immunotherapy. In a multicenter study, Bagante et al. reported that increased preoperative neutrophil/lymphocyte ratio (NLR) and platelet/lymphocyte ratio (PLR) are associated with decreased post-operative RFS in patients with ACC [23]. Neutrophils are responsible for releasing several chemokines such as TNF-a and VEGF, which are implicated in angiogenesis, steroidogenesis, tumor growth, and metastasis, while, at the same time, interfering with normal T-cell response. It should be noted that the elevated NLR was significantly associated with tumor secretion of glucocorticoids, suggesting that corticosteroid-induced lymphocyte depletion could also be contributing to the observed increased ratio.
2.2. Cancer Cells
2.2.1. Altered MHC II Expression
MHC II expression by cortical cells of the innermost zones of the adrenal gland has been well established by previous studies. It is possible that MHC II molecules on cortical cells of zona reticularis play a role in cell-to-cell interactions with androgen producing cells, orchestrating the crosstalk between steroid hormones and infiltrating immune cells. DHEA and DHEA-S exhibit anabolic effects and potentially augment immune response, mitigating, therefore, the effects of locally produced corticosteroids—their exact role, however, remains to be further elucidated, as explained later in this review.
The role of MHC II cortical cell expression in human disease has been well documented. As early as 1988, Jackson et al. described increased expression of MHC II in cortical cells of patients with Addison’s disease [24]. In contrary to autoimmune disease, cancer has been associated with a markedly decreased expression of MHC II in the adrenal gland. Wolkersdorfer et al. reported the absence of MHC II expression on ACC cells, which could result in attenuated interaction of cancer cells with the TCR receptor of T-cells, while at the same time they investigated the interaction of MHC II with Fas/Fas-L in ACC tissue. According to this study, ACC cells exhibit increased expression of Fas-L and decreased expression of Fas receptor [25]. The significance of increased Fas-L expression in immune evasion has been suggested in many different types of cancers, such as melanoma and myeloma, in which Fas-L seems to mediate infiltrating T-cell apoptosis [26]. On the other hand, downregulation of the Fas receptor renders tumor cells resistant to apoptosis, and, according to recent studies, is associated with reduced survival and decreased response to immunotherapy. Xiao et al. reported decreased OS and response to immunotherapy in colon cancer patients with significantly decreased Fas expression [27], while Shibakita et al. demonstrated that Fas expression was an independent negative prognostic factor for RFS in patients with esophageal cancer [28].
In addition, the prognostic role of MHC II has been documented in pediatric ACC as well. Pinto et al. showed that increased MHC II expression correlates with improved prognosis in pediatric patients [29].
2.2.2. TLR4 and CD14 Expression
TLR4 is a trans-membrane protein that belongs to the Toll-like-receptor (TLR) family. TLRs recognize pathogen-associated molecular patterns, with Gram negative derived lipopolysaccharide (LPS) being the most significant among those. Apart from their well-documented role in innate immunity against microbial pathogens, accumulating data highlights the role of TLRs in cancer, which is mostly mediated through receptor stimulation by damage-associated molecular patterns (DAMPs) [30][31]. In order for signal transduction to occur, TLR4 cooperates with co-receptor CD14 and myeloid differentiation factor 2 (MD2), activating ultimately, among others, the NF-κβ pathway.
Depending on the cell type on which TLR4 is expressed, it can either enhance immune response or promote tumorigenesis. More specifically, it has been shown that TLR4 plays a role in promoting the maturation of dendritic cells, thus boosting anti-tumor immunity, while, on the other hand, it stimulates angiogenesis and induces the accumulation of tumor associated macrophages and mesenchymal stem cells, expediting this manner of tumor growth and invasion [32].
Several studies have demonstrated the expression of TLR4 in the normal adrenal cortex and elucidated its role in the adrenal response to sepsis. The significance of TLR4 in adrenal TME, however, had not been widely studied until recently. In a study using immunohistochemical analysis of adrenocortical tumors and real-time PCR in ACC cell lines, Kanczkowski et al. reported a 115-fold decrease in the expression of TLR4 and a 38-fold decrease in the expression of CD14 in ACC tissue when comparing to normal adrenal tissue. The expression of MD2 was reduced as well. In contrast to ACC tissue, adrenocortical adenoma samples seemed to retain, at least partially, TLR4 and CD14 expression [33].
2.2.3. CD276 (B7-H3) Expression
CD276 is a member of the B7 family, with structural similarities to PD-L1 that harbors a dual immunoregulatory role: either co-stimulating APC-induced T-cell activation, or acting as a co-inhibitor of T-cells, contributing therefore to tumor immune evasion. Indeed, accumulating evidence suggests that overexpression of CD276 is implicated in the pathogenesis of many different solid tumors such as lung, prostate, and breast cancer, and has been emerging as a potential immunotherapy target. Apart from suppressing T-cell proliferation and activation, experiments have shown that CD276 downregulates several cytokines, such as IFN-γ, TNF-a, and IL-2, while in vitro studies suggest its potential role in tumor invasion and metastasis as well [34][35].
In a recent study, Liang et al. used immunohistochemistry in 48 ACC samples to assess the overall expression of CD276 in ACC, as well as explore a possible correlation between CD276 expression and patient prognosis. The results of this study showed that >90% of the samples expressed CD276, and more than half of the samples showed moderate to strong expression. Furthermore, higher intensity of CD276 in tumor cells correlated with increased recurrence risk and overall worse prognosis, while at the same time, increased expression of CD276 in tumor vasculature was associated with tumor invasion of adjacent structures and more advanced disease stage [36].
2.3. Adipose Stem Cells
Over the previous years, increasing importance has been attributed to the role of adipose stem cells in the TME and their association with increased tumor growth and invasiveness. The correlation of certain malignancies with obesity is well established. The crosstalk between adipose stem cells (ASC) and cancer cells seems to be mediated through the release of several growth factors (VEGF, PDGF, TGF-β), cytokines (IL-6, Il-8, IFN-γ, TNF-a, CXCL2, CXC12), and leptin by the former. Furthermore, several in vitro studies have highlighted the increased expression of matrix metalloproteinases in ASC and cancer cell cocultures, which could be pivotal in a tumor’s potential for migration and invasion [37][38].
Recently, Armignacco et al. performed similar co-culture experiments using human ASC cells and ACC cells (H295R cell line). According to this study, cancer cells showed increased proliferation and invasiveness when cocultured with ASCs in comparison to cancer cell cultures alone. On the other hand, ASCs showed decreased maturation and lipid content in the presence of H295R cells. The chemokine and molecular profile of the cocultures was investigated further, and it was demonstrated that the cocultures showed increased levels of CXC12 and CXC7-chemokines consistently associated with increased tumor migration. In addition, increased levels of leptin and IL-8 seemed to be implicated in the more invasive phenotype of the cancer cells in the coculture, compared to the control [39]. Indeed, several studies in the past have associated leptin with tumor aggressiveness in other solid tumors. Strong et al. reported increased proliferation and metastatic dynamics in ER-positive breast cancer in response to leptin produced from ASCs isolated from obese women [40], while Chen et al. reached similar conclusions regarding proliferation in ovarian cancer cell lines [41].
2.4. Immunosuppressive Role of Glucocorticoids on TME
Approximately 60% of ACC patients present with hormonal excess syndromes. Among those, the majority present with either Cushing’s syndrome alone, or a mixed virilization-Cushing’s syndrome, inducing endogenous hypercortisolism. Furthermore, exogenous glucocorticoids are administered to many patients following adrenalectomy or during mitotane treatment. Even in the absence of high serum concentration, intratumoral glucocorticoid concentrations may be high due to activation of steroid synthesis pathway in some tumors [42]. Indeed, CoC II and III molecular subtypes have a steroid-high phenotype and render a worse prognosis, whereas CoC I is characterized by a significant up-regulation of genes in immune-mediated pathways [43].
The various mechanisms by which glucocorticoids impair immunity have been described in detail in numerous studies over the years. In regard to the T-cell response, which has a cardinal role in orchestrating anti-tumor immunity, glucocorticoids deplete T-cells by directly inducing apoptosis, downregulating IL-2 production, and inhibiting their release from lymphoid organs [44]. They also hinder T-cell antitumor function. It is obvious that successful ICI therapy requires intact cytotoxic T-lymphocytes. In addition, high glucocorticoids levels promote tumor cells’ survival and proliferation in vivo [45].
It is of no surprise, therefore, that adrenocortical tumors with glucocorticoid hypersecretion display diminished numbers of TILs, with CD3+ CD4+ being the predominantly affected subset. Landwehr et al. analyzed 146 ACCs and demonstrated a negative correlation between hypercortisolism and overall survival, with immune depleted cortisol-secreting tumors displaying the lowest survival rates (compared to non-immune depleted cortisol-secreting, non-immune depleted non-secreting, and immune depleted non-secreting tumors) [20]. Other studies have demonstrated that glucocorticoids interfere with anti-tumor immune response by downregulating MHCII and TLR-4 [46][47].
In support of the unfavorable glucocorticoid milieu, pembrolizumab administration to a patient with metastatic ACC, Lynch syndrome, and tumor-associated Cushing’s syndrome led rapidly to disease progression (PD) [48]. On the contrary, an impressive response to the drug was reported in another patient with normal cortisol levels, due to concomitant mitotane treatment [49]. Therefore, suppression of glucocorticoids’ negative effect on the immune system seems important to produce meaningful clinical benefit of IO in ACC patients.
Mitotane is effective in controlling glucocorticoid excess by inhibiting glucocorticoid biosynthesis and inducing increased steroid clearance and cortisol-binding globulin, but it takes several weeks [7]. In the case of severe Cushing’s syndrome requiring a rapid control, other agents can be used that inhibit adrenal steroidogenesis. Metyrapone is a steroidogenesis enzyme blocker that can be used concomitantly to chemotherapy and mitotane during the first weeks of treatment until mitotane therapeutic levels are obtained, leading to the rapid resolution of symptoms [50]. Another steroidogenesis inhibitor, which is also administered in patients with Cushing’s syndrome, is ketoconazole. It is less effective than metyrapone though, and it cannot be used in combination with mitotane due to hepatotoxicity. It is also effective in androgen excess. Finally, mifepristone is a synthetic steroid with progesterone receptor antagonist activity and glucocorticoid receptor antagonist activity at higher doses [51] that could also be used. Improvement in 66% of patients during the first month was observed in an international retrospective study [52]. However, there are concerns about mineralocorticoid adverse effects requiring monitoring. Apart from mitotane, all these drugs could be used in combination with IO to overcome hypercortisolism in ACC.
2.5. Locally Produced Androgens
The immune modulatory role of androgens is extremely complicated and remains to be fully elucidated. There are reports in the literature that DHEA-S counterbalances the effects of glucocorticoids by interfering with the function of 11β-HSD1, which is responsible for cortisone to cortisol conversion [53]. It has also been suggested that it can potentially augment T-cell response by upregulating Il-2 production, and therefore boost T-cell proliferation, as well as potentiate T-cell mediated cytotoxicity [54]. Canning et al. have also documented a possible positive net effect of DHEA-S on the maturation of dendritic cells [55]. On the other hand, DHEA-S has been reported to downregulate the production of several inflammatory cytokines, such as TNF-a and IL-6 [56].
Further research, therefore, is warranted to clarify the role of DHEA-S in the glucocorticoid-rich microenvironment of adrenal cancer. It should also be noted that pure androgen hypersecreting ACC is extremely rare, as, in most cases, androgen hypersecretion is accompanied by Cushing’s syndrome. Identifying, therefore, the role of androgen hypersecretion on survival could be confounded by the increased cortisol concentrations in the same patients.
2.6. Alteration of Oncogenic Pathways
The landscape of genomic alterations of ACC is complex. Activation of WNT/β-catenin signaling has been recognized as an oncogenic driver in a large subset of ACC patients, mainly in CoC II and CoC III tumors [16]. β-catenin regulates development and homeostasis in different tissues. Preclinical data in melanoma shed light on the effects of WNT/β-catenin pathway activation on tumor immune microenvironment (TIME), characterized by a reduced production of some chemokines, such as CCL4 and subsequent reduced BATF3 dendritic cells. As a result, decreased T-cells recruitment and a defective effector T-cell trafficking into the TME were observed, preventing anti-tumor immunity [57]. Similarly, limited clinical data in human ovarian carcinoma and adenoid cystic carcinomas revealed an association of WNT signaling activation and lack of T-cell infiltration [58]. According to a pan-cancer integrative genomic analysis using the TCGA, activation of WNT/β-catenin signaling was enriched in non-T-cell-inflamed tumors, whereas ACC demonstrated the strongest correlation [59]. In silico analysis of the TCGA ACC tumors revealed that high Catenin β 1B (CTNNB1) gene expression correlated with cortisol excess, worsened survival, and decreased immunity, as manifested by fewer TILs [60]. The different elements of the TIME are depicted in Figure 1.
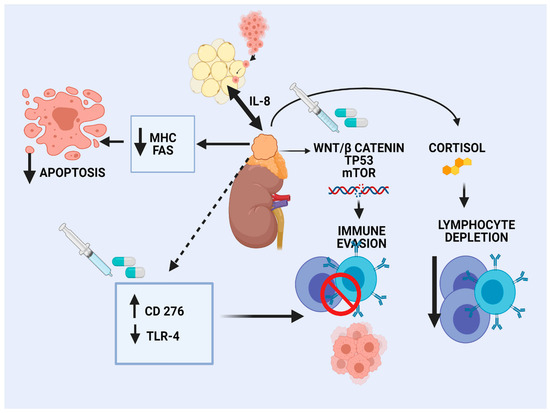
Figure 1. Immune microenvironment in adrenocortical carcinoma.
Given the immune exclusion caused by WNT/β-catenin activation, drugs inhibiting the pathway seem rational combination partners for future immune checkpoint inhibitor (ICI) trials. Targeting WNT/ β-catenin signaling is challenging, since it has a critical role in normal tissues homeostasis like bones and various physiological processes. There are no approved drugs in oncology. However, several effectors and inhibitors of WNT/β-catenin pathway have been tested at the preclinical level, as well as in early phase clinical trials of solid tumors and hematologic malignancies [61]. As an example, Porcupine inhibitors act by blocking the secretion of WNT ligands. The frequent deletions or loss of function mutations in the ZNRF3 gene, a gene which fosters turnover of cell surface Frizzled receptors of WNT, may render ACC harboring these alterations sensitive to these drugs. OMP18RS (vandictumab) is a monoclonal antibody against the Frizzled receptor that has been tested in a phase 1 study of breast pancreatic cancer with promising activity [62], whereas a combination phase 1 study with chemotherapy in pancreatic cancer was terminated due to bone-related adverse events [63]. PRI-724 is a small molecule inhibitor of WNT/β-catenin/CBP (a transcriptional coactivator) signaling, and was evaluated in early phase trials of pancreatic cancer [64].
There are no clinical data on these agents in ACC patients. In vitro studies in ACC models demonstrated that β-catenin targeting inhibited cell proliferation [65][66]. It should be noted that the WNT pathway cross talks with the Notch and Sonic Hedgehog pathways, and thus it is possible that multiple targeting is necessary.
Another important proportion of ACC displays a spectrum of mutations in the p53/Rb pathway, which have been correlated to aggressive histotype and bad prognosis [67][68]. These mutations have also been described as pathogenic in other cancers and represent the most relevant prognostic biomarker in ACC. TP53 is a tumor suppressor gene encoding for p53 protein, an important regulator of cellular stress response. The majority of TP53 mutations lead to stabilization and accumulation of the mutant protein, which is protected from ubiquitin-mediated degradation [69]. TP53 mutations that were identified in ACC result in either a negative function or absence of protein expression. Germline TP53 mutations are associated with pediatric ACC, as a component of LFS, whereas somatic mutations are observed mainly in adult cases [70].
TP53 inactivating mutations have been shown to confer an immunosuppressive phenotype, and, due to decreased MHC-I presentation, profound changes in chemokine/cytokine secretion increasing the recruitment and activity of myeloid and Treg cells [71]. Therefore, effector T-cells recruitment in the tumor is suppressed, promoting immune evasion. In the same direction, TP53 mutations were recently found to downregulate immune-related genes in hepatocellular carcinoma [72]. TP53 mutations and inactivation have also been reported in cancer-associated fibroblasts (CAF), affecting immune cell composition and function in TIME and leading to pro-inflammatory molecule production [73].
Strategies aiming at restoring p53 function in tumors and CAF could inverse immunosuppressive TME, which is observed in TP53-mutated tumors. Pharmacological p53 activation, viral vector-mediated p53 reintroduction, and restoration gene therapy have not provided satisfactory results so far [73]. Given the broad spectrum of TP53 mutations, mutant-specific reactivating drugs are probably required. Another approach to target p53 is the disruption of the chaperone machinery involved in its impaired degradation.
Additional genetic alterations have been described in different subsets of ACC patients, such as the dysregulation of the mTOR pathway [74]. Substantial evidence supports that the PI3K/AΚΤ/mTOR cascade plays a central role in immune cells’ homeostasis and activation. It can regulate chemokine-mediated immune cells, with diverse roles from promoting T-cell accumulation to immune evasion [75]. The PI3K/AΚΤ/mTOR pathway has been shown to regulate PD-L1 in different cancers, Treg cells, and myeloid-derived suppressor cells infiltration [76]. Therefore, further investigation is required to determine the role of mTOR inhibitors like everolimus and temsirolimus in the immune modulation of selected ACC subgroups. The mTOR pathway has already been suggested as a potential therapeutic target for ACC [77]. Although in vitro testing of these drugs led to the inhibition of ACC cells’ proliferation [78], preliminary clinical data failed to demonstrate efficacy as monotherapy [79].
Combination of IO with drugs targeting the above pathways is a promising strategy to overcome immune exclusion in ACC and warrants extensive research. Furthermore, injection of mature dendritic cells into tumors with β-catenin alterations could also be explored.
References
- WHO. Classification of Tumors of Endocrine Organs WHO/IARC Classification of Tumors, 4th ed.; Lloyd, R.V., Osamura, R.Y., Kloppel, G., Rosai, J., Eds.; WHO: Geneva, Switzerland, 2017; Volume 10.
- Berruti, A.; Grisanti, S.; Pulzer, A.; Claps, M.; Daffara, F.; Loli, P.; Mannelli, M.; Boscaro, M.; Arvat, E.; Tiberio, G.; et al. Long-Term Outcomes of Adjuvant Mitotane Therapy in Patients with Radically Resected Adrenocortical Carcinoma. J. Clin. Endocrinol. Metab. 2017, 102, 1358–1365.
- Berruti, A.; Terzolo, M.; Sperone, P.; Pia, A.; Della Casa, S.; Gross, D.J.; Carnaghi, C.; Casali, P.; Porpiglia, F.; Mantero, F.; et al. Etoposide, doxorubicin and cisplatin plus mitotane in the treatment of advanced adrenocortical carcinoma: A large prospective phase II trial. Endocr. Relat. Cancer 2005, 12, 657–666.
- Fassnacht, M.; Terzolo, M.; Allolio, B.; Baudin, E.; Haak, H.; Berruti, A.; Welin, S.; Schade-Brittinger, C.; Lacroix, A.; Jarzab, B.; et al. Combination Chemotherapy in Advanced Adrenocortical Carcinoma. N. Engl. J. Med. 2012, 366, 2189–2197.
- Megerle, F.; Herrmann, W.; Schloetelburg, W.; Ronchi, C.L.; Pulzer, A.; Quinkler, M.; Beuschlein, F.; Hahner, S.; Kroiss, M.; Fassnacht, M.; et al. Mitotane Monotherapy in Patients With Advanced Adrenocortical Carcinoma. J. Clin. Endocrinol. Metab. 2018, 103, 1686–1695.
- Henning, J.E.K.; Deutschbein, T.; Altieri, B.; Steinhauer, S.; Kircher, S.; Sbiera, S.; Wild, V.; Schlötelburg, W.; Kroiss, M.; Perotti, P.; et al. Gemcitabine-Based Chemotherapy in Adrenocortical Carcinoma: A Multicenter Study of Efficacy and Predictive Factors. J. Clin. Endocrinol. Metab. 2017, 102, 4323–4332.
- Fassnacht, M. European Journal of Endocrinology European Society of Endocrinology Clinical Practice Guidelines on the management of adrenocortical carcinoma in adults, in collaboration with the European Net-work for the Study of Adrenal Tumors. Eur. J. Endocrinol. 2018, 179, G1–G46.
- Ribas, A.; Hamid, O.; Daud, A.; Hodi, F.S.; Wolchok, J.D.; Kefford, R.; Joshua, A.M.; Patnaik, A.; Hwu, W.-J.; Weber, J.S.; et al. Association of Pembrolizumab With Tumor Response and Survival Among Patients with Advanced Melanoma. JAMA 2016, 315, 1600–1609.
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639.
- Motzer, R.J.; Escudier, B.; McDermott, D.F.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Procopio, G.; Plimack, E.R.; et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2015, 373, 1803–1813.
- Cohen, E.E.W.; Bell, R.B.; Bifulco, C.B.; Burtness, B.; Gillison, M.L.; Harrington, K.J.; Le, Q.-T.; Lee, N.Y.; Leidner, R.; Lewis, R.L.; et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of squamous cell carcinoma of the head and neck (HNSCC). J. Immunother. Cancer 2019, 7, 184.
- Murciano-Goroff, Y.R.; Warner, A.B.; Wolchok, J.D. The future of cancer immunotherapy: Microenvironment-targeting combinations. Cell Res. 2020, 30, 507–519.
- Whiteside, T.L. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008, 27, 5904–5912.
- Denkert, C.; Von Minckwitz, G.; Darb-Esfahani, S.; Lederer, B.; Heppner, B.I.; Weber, K.E.; Budczies, J.; Huober, J.; Klauschen, F.; Furlanetto, J.; et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: A pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018, 19, 40–50.
- Fu, Q.; Chen, N.; Ge, C.; Li, R.; Li, Z.; Zeng, B.; Li, C.; Wang, Y.; Xue, Y.; Song, X.; et al. Prognostic value of tumor-infiltrating lymphocytes in melanoma: A systematic review and meta-analysis. OncoImmunology 2019, 8, e1593806.
- Paré, L.; Pascual, T.; Seguí, E.; Teixidó, C.; Gonzalez-Cao, M.; Galván, P.; Rodríguez, A.; González, B.; Cuatrecasas, M.; Pineda, E.; et al. Association between PD1 mRNA and response to anti-PD1 monotherapy across multiple cancer types. Ann. Oncol. 2018, 29, 2121–2128.
- Thorsson, V.; Gibbs, D.L.; Brown, S.; Wolf, D.; Bortone, D.S.; Ouyang, T.-H.; Porta-Pardo, E.; Gao, G.F.; Plaisier, C.L.; Eddy, J.A.; et al. The Immune Landscape of Cancer. Immunity 2018, 48, 812–830.e14.
- Mohan, D.R.; Lerario, A.M.; Hammer, G.D. Therapeutic Targets for Adrenocortical Carcinoma in the Genomics Era. J. Endocr. Soc. 2018, 2, 1259–1274.
- Peng, Y.; Song, Y.; Ding, J.; Li, N.; Zhang, Z.; Wang, H. Identification of immune-related biomarkers in adrenocortical carcinoma. Int. Immunopharmacol. 2020, 88, 106930.
- Landwehr, L.-S.; Altieri, B.; Schreiner, J.; Sbiera, I.; Weigand, I.; Kroiss, M.; Fassnacht, M.; Sbiera, S. Interplay between glucocorticoids and tumor-infiltrating lymphocytes on the prognosis of adrenocortical carcinoma. J. Immunother. Cancer 2019, 8, e000469.
- Tian, X.; Xu, W.; Wang, Y.; Anwaier, A.; Wang, H.; Wan, F.; Zhu, Y.; Cao, D.; Shi, G.; Zhu, Y.; et al. Identification of tumor-infiltrating immune cells and prognostic validation of tumor-infiltrating mast cells in adrenocortical carcinoma: Results from bioinformatics and real-world data. OncoImmunology 2020, 9, 1784529.
- Parise, I.Z.S.; Parise, G.A.; Noronha, L.; Surakhy, M.; Woiski, T.D.; Silva, D.B.; Costa, T.E.-J.B.; Del-Valle, M.H.C.P.; Komechen, H.; Rosati, R.; et al. The Prognostic Role of CD8+ T Lymphocytes in Childhood Adrenocortical Carcinomas Compared to Ki-67, PD-1, PD-L1, and the Weiss Score. Cancers 2019, 11, 1730.
- Bagante, F.; Tran, T.B.; Postlewait, L.M.; Maithel, S.K.; Wang, T.S.; Evans, D.B.; Hatzaras, I.; Shenoy, R.; Phay, J.E.; Keplinger, K.; et al. Neutrophil-lymphocyte and platelet-lymphocyte ratio as predictors of disease specific survival after resection of adrenocortical carcinoma. J. Surg. Oncol. 2015, 112, 164–172.
- Jackson, R.; McNicol, A.M.; Farquharson, M.; Foulis, A.K. Class II MHC expression in normal adrenal cortex and cortical cells in autoimmune Addison’s disease. J. Pathol. 1988, 155, 113–120.
- Wolkersdörfer, G.W.; Marx, C.; Brown, J.; Schröder, S.; Füssel, M.; Rieber, E.P.; Kuhlisch, E.; Ehninger, G.; Bornstein, S.R. Prevalence of HLA-DRB1 Genotype and Altered Fas/Fas Ligand Expression in Adrenocortical Carcinoma. J. Clin. Endocrinol. Metab. 2005, 90, 1768–1774.
- Hahne, M.; Rimoldi, D.; Schroter, M.; Romero, P.; Schreier, M.; French, L.E.; Schneider, P.; Bornand, T.; Fontana, A.; Lienard, D.; et al. Melanoma Cell Expression of Fas(Apo-1/CD95) Ligand: Implications for Tumor Immune Escape. Science 1996, 274, 1363–1366.
- Xiao, W.; Ibrahim, M.L.; Redd, P.S.; Klement, J.D.; Lu, C.; Yang, D.; Savage, N.M.; Liu, K. Loss of Fas Expression and Function Is Coupled with Colon Cancer Resistance to Immune Checkpoint Inhibitor Immunotherapy. Mol. Cancer Res. 2019, 17, 420–430.
- Shibakita, M.; Tachibana, M.; Dhar, D.K.; Kotoh, T.; Kinugasa, S.; Kubota, H.; Nagasue, N. Prognostic Significance of Fas and Fas Ligand Expressions in Human Esophageal Cancer. Clin. Cancer Res. 1999, 5, 9.
- Pinto, E.M.; Rodriguez-Galindo, C.; Choi, J.K.; Pounds, S.; Liu, Z.; Neale, G.; Finkelstein, D.; Hicks, J.M.; Pappo, A.S.; Figueiredo, B.C.; et al. Prognostic Significance of Major Histocompatibility Complex Class II Expression in Pediatric Adrenocortical Tumors: A St. Jude and Children’s Oncology Group Study. Clin. Cancer Res. 2016, 22, 6247–6255.
- Shcheblyakov, D.; Logunov, D.; Tukhvatulin, A.; Shmarov, M.; Naroditsky, B.; Ginzburg, A.L. Toll-Like Receptors (TLRs): The Role in Tumor Progression. Acta Naturae 2010, 2, 21–29.
- Urban-Wojciuk, Z.; Khan, M.M.; Oyler, B.L.; Fåhraeus, R.; Marek-Trzonkowska, N.; Nita-Lazar, A.; Hupp, T.R.; Goodlett, D.R. The Role of TLRs in Anti-cancer Immunity and Tumor Rejection. Front. Immunol. 2019, 10, 2388.
- Matsumoto, M.; Takeda, Y.; Tatematsu, M.; Seya, T. Toll-Like Receptor 3 Signal in Dendritic Cells Benefits Cancer Immunotherapy. Front. Immunol. 2017, 8, 1897.
- Kanczkowski, W.; Tymoszuk, P.; Ehrhart-Bornstein, M.; Wirth, M.P.; Zacharowski, K.; Bornstein, S.R. Abrogation of TLR4 and CD14 Expression and Signaling in Human Adrenocortical Tumors. J. Clin. Endocrinol. Metab. 2010, 95, 421–429.
- Dong, P.; Xiong, Y.; Yue, J.; Hanley, S.J.B.; Watari, H. B7H3 As a Promoter of Metastasis and Promising Therapeutic Target. Front. Oncol. 2018, 8, 264.
- Picarda, E.; Ohaegbulam, K.C.; Zang, X. Molecular Pathways: Targeting B7-H3 (CD276) for Human Cancer Immunotherapy. Clin. Cancer Res. 2016, 22, 3425–3431.
- Liang, J.; Liu, Z.; Pei, T.; Xiao, Y.; Zhou, L.; Tang, Y.; Zhou, C.; Wu, K.; Zhang, F.; Zhang, F.; et al. Clinicopathological and Prognostic Characteristics of CD276 (B7-H3) Expression in Adrenocortical Carcinoma. Dis. Markers 2020, 2020, 1–10.
- Song, Y.H.; Shon, S.H.; Shan, M.; Stroock, A.D.; Fischbachtreschl, C. Adipose-derived stem cells increase angiogenesis through matrix metalloproteinase-dependent collagen remodeling. Integr. Biol. 2016, 8, 205–215.
- Scioli, M.G.; Storti, G.; D’Amico, F.; Gentile, P.; Kim, B.-S.; Cervelli, V.; Orlandi, A. Adipose-Derived Stem Cells in Cancer Progression: New Perspectives and Opportunities. Int. J. Mol. Sci. 2019, 20, 3296.
- Armignacco, R.; Cantini, G.; Poli, G.; Guasti, D.; Nesi, G.; Romagnoli, P.; Mannelli, M.; Luconi, M. The Adipose Stem Cell as a Novel Metabolic Actor in Adrenocortical Carcinoma Progression: Evidence from an In Vitro Tumor Microenvironment Crosstalk Model. Cancers 2019, 11, 1931.
- Strong, A.L.; Ohlstein, J.F.; Biagas, B.A.; Rhodes, L.V.; Pei, D.T.; Tucker, H.A.; Llamas, C.; Bowles, A.C.; DuTreil, M.F.; Zhang, S.; et al. Leptin produced by obese adipose stromal/stem cells enhances proliferation and metastasis of estrogen receptor positive breast cancers. Breast Cancer Res. 2015, 17, 1–16.
- Chen, C.; Chang, Y.-C.; Lan, M.S.; Breslin, M. Leptin stimulates ovarian cancer cell growth and inhibits apoptosis by increasing cyclin D1 and Mcl-1 expression via the activation of the MEK/ERK1/2 and PI3K/Akt signaling pathways. Int. J. Oncol. 2013, 42, 1113–1119.
- Fiorentini, C.; Grisanti, S.; Cosentini, D.; Abate, A.; Rossini, E.; Berruti, A.; Sigala, S. Molecular Drivers of Potential Immunotherapy Failure in Adrenocortical Carcinoma. J. Oncol. 2019, 2019, 1–7.
- Zheng, S.; Cherniack, A.D.; Dewal, N.; Moffitt, R.A.; Danilova, L.; Murray, B.A.; Lerario, A.M.; Else, T.; Knijnenburg, T.A.; Ciriello, G.; et al. Comprehensive Pan-Genomic Characterization of Adrenocortical Carcinoma. Cancer Cell 2016, 29, 723–736.
- Coutinho, A.E.; Chapman, K.E. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol. Cell. Endocrinol. 2011, 335, 2–13.
- Guendisch, S.; Boeckeler, E.; Behrends, U.; Amtmann, E.; Ehrhardt, H.; Jeremias, I. Gluco-corticoids Augment Survival and Proliferation of Tumor Cells. Anticancer Res. 2012, 32, 10.
- Celada, A.; McKercher, S.; Maki, R. Repression of major histocompatibility complex IA expression by glucocorticoids: The glucocorticoid receptor inhibits the DNA binding of the X box DNA binding protein. J. Exp. Med. 1993, 177, 691–698.
- Curtale, G.; Renzi, T.A.; Drufuca, L.; Rubino, M.; Locati, M. Glucocorticoids downregulate TLR4 signaling activity via its direct targeting by miR-511-5p. Eur. J. Immunol. 2017, 47, 2080–2089.
- Casey, R.; Giger, O.; Seetho, I.; Marker, A.; Pitfield, D.; Boyle, L.; Gurnell, M.; Shaw, A.; Tischkowitz, M.; Maher, E.; et al. Rapid disease progression in a patient with mismatch repair-deficient and cortisol secreting adrenocortical carcinoma treated with pembrolizumab. Semin. Oncol. 2018, 45, 151–155.
- Caccese, M.; Barbot, M.; Ceccato, F.; Padovan, M.; Gardiman, M.P.; Fassan, M.; Denaro, L.; Emanuelli, E.; D’Avella, D.; Scaroni, C.; et al. Rapid disease progression in patient with mismatch-repair deficiency pituitary ACTH-secreting adenoma treated with checkpoint inhibitor pembrolizumab. Anti-Cancer Drugs 2020, 31, 199–204.
- Daniel, E.; Aylwin, S.; Mustafa, O.; Ball, S.; Munir, A.; Boelaert, K.; Chortis, V.; Cuthbertson, D.J.; Daousi, C.; Rajeev, S.P.; et al. Effectiveness of Metyrapone in Treating Cushing’s Syndrome: A Retrospective Multicenter Study in 195 Patients. J. Clin. Endocrinol. Metab. 2015, 100, 4146–4154.
- Fleseriu, M.; Biller, B.M.K.; Findling, J.W.; Molitch, M.E.; Schteingart, D.E.; Gross, C.; Auchus, R.; Bailey, T.; Carroll, T.; Colleran, K.; et al. Mifepristone, a Glucocorticoid Receptor Antagonist, Produces Clinical and Metabolic Benefits in Patients with Cushing’s Syndrome. J. Clin. Endocrinol. Metab. 2012, 97, 2039–2049.
- Castinetti, F.; Fassnacht, M.; Johanssen, S.; Terzolo, M.; Bouchard, P.; Chanson, P.; Cao, C.D.; Morange, I.; Pico, A.; Ouzounian, S.; et al. Merits and pitfalls of mifepristone in Cushing’s syndrome. Eur. J. Endocrinol. 2009, 160, 1003–1010.
- Tagawa, N.; Minamitani, E.; Yamaguchi, Y.; Kobayashi, Y. Alternative mechanism for anti-obesity effect of dehydroepiandrosterone: Possible contribution of 11β-hydroxysteroid dehydrogenase type 1 inhibition in rodent adipose tissue. Steroids 2011, 76, 1546–1553.
- Khorram, O.; Vu, L.; Yen, S.S.C. Activation of Immune Function by Dehydroepiandrosterone (DHEA) in Age-Advanced Men. Journals Gerontol. Ser. A Boil. Sci. Med Sci. 1997, 52, M1–M7.
- Opposing Effects of Dehydroepiandrosterone and Dexamethasone on the Generation of Monocyte-Derived Dendritic Cells. Eur. J. Endocrinol. 2000, 143. Available online: (accessed on 8 February 2021).
- Straub, R.H.; Konečná, L.; Hrach, S.; Rothe, G.; Kreutz, M.; Scholmerich, J.; Falk, W.; Lang, B. Serum Dehydroepiandrosterone (DHEA) and DHEA Sulfate Are Negatively Correlated with Serum Interleukin-6 (IL-6), and DHEA Inhibits IL-6 Secretion from Mononuclear Cells in Manin Vitro: Possible Link between Endocrinosenescence and Immunosenescence. J. Clin. Endocrinol. Metab. 1998, 83, 2012–2017.
- Spranger, S.; Gajewski, T.F. Mechanisms of Tumor Cell–Intrinsic Immune Evasion. Annu. Rev. Cancer Biol. 2018, 2, 213–228.
- Jiménez-Sánchez, A.; Memon, D.; Pourpe, S.; Veeraraghavan, H.; Li, Y.; Vargas, H.A.; Gill, M.B.; Park, K.J.; Zivanovic, O.; Konner, J.; et al. Heterogeneous Tumor-Immune Microenvironments among Differentially Growing Metastases in an Ovarian Cancer Patient. Cell 2017, 170, 927–938.e20.
- Luke, J.J.; Bao, R.; Sweis, R.F.; Spranger, S.; Gajewski, T.F. WNT/β-catenin Pathway Activation Correlates with Immune Exclusion across Human Cancers. Clin. Cancer Res. 2019, 25, 3074–3083.
- Liu, Y.; Patel, L.; Mills, G.B.; Lu, K.H.; Sood, A.K.; Ding, L.; Kucherlapati, R.; Mardis, E.R.; Levine, D.A.; Shmulevich, I.; et al. Clinical Significance of CTNNB1 Mutation and Wnt Pathway Activation in Endometrioid Endometrial Carcinoma. J. Natl. Cancer Inst. 2014, 106.
- Krishnamurthy, N.; Kurzrock, R. Targeting the Wnt/beta-catenin pathway in cancer: Update on effectors and inhibitors. Cancer Treat. Rev. 2018, 62, 50–60.
- Diamond, J.R.; Becerra, C.; Richards, D.; Mita, A.; Osborne, C.; O’Shaughnessy, J.; Zhang, C.; Henner, R.; Kapoun, A.M.; Xu, L.; et al. Phase Ib clinical trial of the anti-frizzled antibody vantictumab (OMP-18R5) plus paclitaxel in patients with locally advanced or metastatic HER2-negative breast cancer. Breast Cancer Res. Treat. 2020, 184, 53–62.
- Davis, S.L.; Cardin, D.B.; Shahda, S.; Lenz, H.-J.; Dotan, E.; O’Neil, B.H.; Kapoun, A.M.; Stagg, R.J.; Berlin, J.; Messersmith, W.A.; et al. A phase 1b dose escalation study of Wnt pathway inhibitor vantictumab in combination with nab-paclitaxel and gemcitabine in patients with previously untreated metastatic pancreatic cancer. Investig. New Drugs 2020, 38, 821–830.
- Ko, A.H.; Chiorean, E.G.; Kwak, E.L.; Lenz, H.-J.; Nadler, P.I.; Wood, D.L.; Fujimori, M.; Inada, T.; Kouji, H.; McWilliams, R.R. Final results of a phase Ib dose-escalation study of PRI-724, a CBP/beta-catenin modulator, plus gemcitabine (GEM) in patients with advanced pancreatic adenocarcinoma (APC) as second-line therapy after FOLFIRINOX or FOLFOX. J. Clin. Oncol. 2016, 34, e15721.
- Gaujoux, S.; Hantel, C.; Launay, P.; Bonnet, S.; Perlemoine, K.; Lefèvre, L.; Guillaud-Bataille, M.; Beuschlein, F.; Tissier, F.; Bertherat, J.; et al. Silencing Mutated β-Catenin Inhibits Cell Proliferation and Stimulates Apoptosis in the Adrenocortical Cancer Cell Line H295R. PLoS ONE 2013, 8, e55743.
- Leal, L.F.; Bueno, A.C.; Gomes, D.C.; Abduch, R.; De Castro, M.; Antonini, S.R. Inhibition of the Tcf/beta-catenin complex increases apoptosis and impairs adrenocortical tumor cell proliferation and adrenal steroidogenesis. Oncotarget 2015, 6, 43016–43032.
- Ross, J.S.; Wang, K.; Rand, J.V.; Gay, L.; Presta, M.J.; Sheehan, C.E.; Ali, S.M.; Elvin, J.A.; Labrecque, E.; Hiemstra, C.; et al. Next-generation sequencing of adrenocortical carcinoma reveals new routes to targeted therapies. J. Clin. Pathol. 2014, 67, 968–973.
- Vatrano, S.; Volante, M.; Duregon, E.; Giorcelli, J.; Izzo, S.; Rapa, I.; Votta, A.; Germano, A.; Scagliotti, G.; Berruti, A.; et al. Detailed genomic characterization identifies high heterogeneity and histotype-specific genomic profiles in adrenocortical carcinomas. Mod. Pathol. 2018, 31, 1257–1269.
- Mantovani, F.; Walerych, D.; Del Sal, G. Targeting mutant p53 in cancer: A long road to precision therapy. FEBS J. 2016, 284, 837–850.
- Wasserman, J.D.; Zambetti, G.P.; Malkin, D. Towards an understanding of the role of p53 in adrenocortical carcinogenesis. Mol. Cell. Endocrinol. 2012, 351, 101–110.
- Blagih, J.; Zani, F.; Chakravarty, P.; Hennequart, M.; Pilley, S.; Hobor, S.; Hock, A.K.; Walton, J.B.; Morton, J.P.; Gronroos, E.; et al. Cancer-Specific Loss of p53 Leads to a Modulation of Myeloid and T Cell Responses. Cell Rep. 2020, 30, 481–496.e6.
- Long, J.; Wang, A.; Bai, Y.; Lin, J.; Yang, X.; Wang, D.; Yang, X.; Jiang, Y.; Zhao, H. Development and validation of a TP53-associated immune prognostic model for hepatocellular carcinoma. EBioMedicine 2019, 42, 363–374.
- Guo, G.; Cui, Y. New perspective on targeting the tumor suppressor p53 pathway in the tumor microenvironment to enhance the efficacy of immunotherapy. J. Immunother. Cancer 2015, 3, 9.
- Altieri, B.; Ronchi, C.L.; Kroiss, M.; Fassnacht, M. Next-generation therapies for adrenocortical carcinoma. Best Pr. Res. Clin. Endocrinol. Metab. 2020, 34, 101434.
- Jin, Y.; Tymen, S.D.; Chen, D.; Fang, Z.J.; Zhao, Y.; Dragas, D.; Dai, Y.; Marucha, P.T.; Zhou, X. MicroRNA-99 Family Targets AKT/mTOR Signaling Pathway in Dermal Wound Healing. PLoS ONE 2013, 8, e64434.
- Conciatori, F.; Ciuffreda, L.; Bazzichetto, C.; Falcone, I.; Pilotto, S.; Bria, E.; Cognetti, F.; Milella, M. mTOR Cross-Talk in Cancer and Potential for Combination Therapy. Cancers 2018, 10, 23.
- De Martino, M.C.; Van Koetsveld, P.M.; Feelders, R.A.; De Herder, W.W.; Dogan, F.; Janssen, J.A.M.J.L.; Bruinink, D.H.O.; Pivonello, C.; Waaijers, A.M.; Colao, A.; et al. IGF and mTOR pathway expression and in vitro effects of linsitinib and mTOR inhibitors in adrenocortical cancer. Endocrine 2019, 64, 673–684.
- Doghman, M.; El Wakil, A.; Cardinaud, B.; Thomas, E.; Wang, J.; Zhao, W.; Valle, M.H.C.P.-D.; Figueiredo, B.C.; Zambetti, G.P.; Lalli, E. Regulation of Insulin-like Growth Factor–Mammalian Target of Rapamycin Signaling by MicroRNA in Childhood Adrenocortical Tumors. Cancer Res. 2010, 70, 4666–4675.
- Fraenkel, M.; Gueorguiev, M.; Barak, D.; Salmon, A.; Grossman, A.B.; Gross, D.J. Everolimus therapy for progressive adrenocortical cancer. Endocrine 2013, 44, 187–192.
More
Information
Subjects:
Oncology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
720
Revisions:
2 times
(View History)
Update Date:
30 Apr 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




