
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yongqing Liu | + 1869 word(s) | 1869 | 2021-04-21 08:18:38 | | | |
| 2 | Vivi Li | Meta information modification | 1869 | 2021-04-22 04:00:20 | | |
Video Upload Options
ZEB1 is an important transcription factor for epithelial to mesenchymal transition (EMT) and in the regulation of cell differentiation and transformation. In the cornea, ZEB1 presents in all three layers: the epithelium, the stroma and the endothelium. Mutations of ZEB1 have been linked to multiple corneal genetic defects, particularly to the corneal dystrophies including keratoconus (KD), Fuchs endothelial corneal dystrophy (FECD), and posterior polymorphous corneal dystrophy (PPCD).
1. ZEB1 and Epithelial to Mesenchymal Transition (EMT)
The zinc-finger E homeobox-binding (ZEB) protein family of transcription factors (TFs) are composed of two members: ZEB1 and ZEB2, and best known for their role in driving epithelial to mesenchymal transition (EMT) by inhibiting the expression of the epithelial anchor protein E-cadherin (CDH1), a prominent tight junction protein for connecting epithelial cells. In recent years, our understanding of these transcription factors has been expanded, and it is clear that they are expressed in a variety of neural cells, immune cells, mesenchymal cells and endothelial cells. In these cells, ZEBs play important roles in regulation of cell differentiation, stemness, survival, and proliferation [1].
In 1993, when ZEB1, also termed TCF8 and δEF1, was first identified specifically expressing in the nervous system and the lens of the mesoderm tissue of chicken embryos, as a repressor of δ1-crystallin inhibitor and was considered to be involved in embryogenesis [2]. Later, a mouse model with Zeb1 deletion revealed the role of ZEB1 in development as ZEB1 is a master regulator of EMT in controlling the activation of EMT [3][4]. Its dysregulation has been observed in various cancer types and other pathological processes [5]. ZEB1 is characterized by an amino-terminal (NZF) and a carboxy-terminal (CZF) zinc finger cluster, and a homeodomain (HD) in the center. Other protein binding domains include SMAD binding domain (SBD), p300-P/CAF binding domain (CBD) and CtBP interacting domain (CID) [6]. The predominant mechanism by which ZEB1 represses gene expression is through an active transcriptional repression. ZEB1 can inhibit gene transcription by directly targeting the 5′-CANNTG-3′ E-box sequence located in gene promoters. It is worth noting that the two repressor domains of ZEB1 target different transcription factors and regulate the differentiation of specific tissues [7][8]. In addition, ZEB1 expression is regulated by multiple signaling pathways and components, including TGFβ, WNT, miRNAs and other factors [9].
EMT was initially observed during embryogenesis and plays an important role in early development, including neural and heart valve development, mesoderm and secondary palate formation [10][11][12]. It is recognized that the EMT and its reverse process can be resurrected in adult tissues under a variety of disease conditions including wound healing, fibrosis and cancer [13]. In many epithelial-derived malignancies, after receiving microenvironmental stimuli such as WNT, tumor necrosis factor-α (TNFα) and transforming growth factor beta (TGFβ), epithelial cells lose their ability to adhere to each other, acquire mesenchymal cell characteristics, and become more mobilizable [14]. In addition, EMT is also a key feature of pathological fibrotic responses to injuries, e.g., wound healing and fibrotic organ diseases [15]. During embryogenesis and carcinogenesis, epithelial cells convert from their differentiated state to undifferentiated mesenchymal cell-like state. But, knockdown of ZEB1 can completely reverse this EMT [16].
The growing literature shows that ZEB1 also plays a role in regulation of many physiological and pathological processes of the cornea. In the next section, we will summarize the roles of ZEB1 in regulation of corneal epithelial, stromal and endothelial diseases, and related pathogenic conditions such as corneal wound healing, inflammation and neovascularization.
2. The Cornea and ZEB1
The cornea is a transparent avascular tissue that acts as a barrier and protects structures inside the eye. The mammalian cornea is composed of five layers including the epithelium, Bowman’s membrane, the stroma, Descemet’s membrane, and the endothelium (Figure 1). The epithelium is a five-to-six cell layer structure with three epithelial cell types: superficial, wing, and basal cells. The superficial cells are two to three layers of flat polygonal cells. The microvilli on the superficial surface increase corneal surface area that would more efficiently allow oxygen and other nutrients from tears diffusing into the cornea. The wing cells are two to three layers of wing-like shape cells and derived from the basal cells. The basal cells are single layer of cuboidal or columnar cells that have abundant organelles and are active mitotically, also known as transient amplifying (TA) cells. The corneal epithelium is the outmost tissue of the eye that is often bombarded by environmental physical, chemical, and pathogenic insults, sheared and restored constantly. Its cellular homeostasis is maintained by a group of stem cells located at the limbus, a narrow area between the cornea and sclera. These so-called limbal epithelial stem cells (LESC) not only renew themselves locally at the limbus, but also generate TA cells that can move along the Bowman’s membrane towards the corneal center and at the same time move up to the superficial layer to differentiate into epithelial cells simultaneously [17]. The corneal stromal fibroblasts, known as keratocytes, can also be regenerated though at a relatively slower pace [18]. The keratocyte regeneration process however, is not as clear and its reproduction is supposed to be either locally and/or come from the progenitor at the limbus region [19].The corneal endothelium is not regenerable, corneal dystrophies are therefore mostly due to the endothelial defects or damages [20]. Corneal transplantation is the sole solution for severe corneal wound that lead to LESC diminishing and unreversible corneal dystrophies. In addition, the cornea is an avascular tissue to benefit its transparency. The nearest blood vessels to the cornea are also located in the limbus where an angiogenesis can be initiated by severe corneal damages leading to a pathogenesis called corneal neovascularization. As ZEB1 is required for cell proliferation in the alkali-induced mouse corneal neovascularization model, we show that Zeb1 is highly expressed in corneal epithelial basal cells, vascular endothelial cells, and infiltrated immune cells (Figure 2) [21].
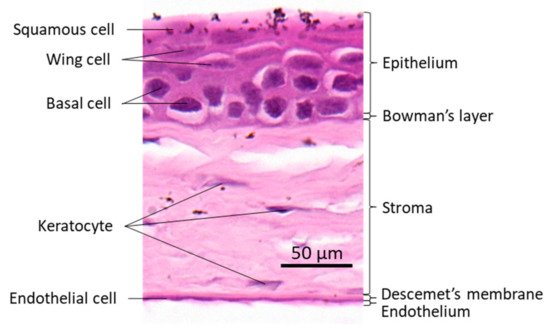
Figure 1. Mouse corneal structure and cell types.
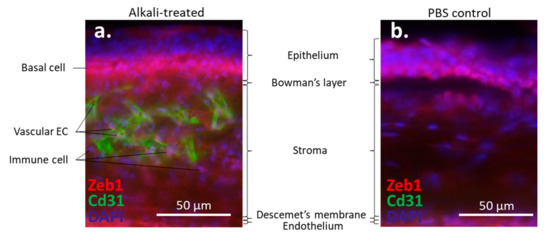
Figure 2. Alkali-induced mouse corneal neovascularization (NV). (a) In the alkali-treated cornea, Zeb1 is highly expressed in epithelial basal cells (BC), Cd31-expressing vascular endothelial cells (EC), and infiltrated immune cells (IC). (b) In the PBS control cornea, no Cd31-positive EC and IC are detected.
3. ZEB1 and Corneal Epithelium
Although the presence of ZEB1 in corneal epithelial cells is evident [22], studies suggest that another zinc finger protein basonuclin (BNC), similar to ZEB1, is a key regulator of the proliferation potential (stemness) of keratinocytes [22]. Tiwari et al. performed a spatiotemporal expression profile of Klf4 in Klf4∆/∆CE corneal epithelium-specific ablation mice. They found that Klf4∆/∆CE cells migrated faster than wild-type cells and showed abnormal stratification, so unable to restore epithelial characteristics. Zeb1 and Zeb2 were up-regulated in Klf4∆/∆CE cornea, which may be related to the role of Klf4 in inhibiting EMT in corneal epithelial homeostasis [23].
Li et al. discovered that SPRR1B was up-regulated by the pro-inflammatory cytokines IL-1 and IFN through the p38 MAPK-mediated signaling pathway, which lead to the activation of the transcription factors CREB and ZEB1, respectively [16]. The SPRR1B induction of p38 MAPK pathway by IFN depends on the ZEB1 response element on the SPRR1B promoter. They proposed a p38-dependent mechanism in which IFN promotes squamous metaplasia through ZEB1-mediated up-regulation of the keratinizing envelope protein SPRR1B. These results revealed that key intracellular signal transduction intermediates participate in the immune-mediated process of ocular surface squamous epithelial metaplasia. This study plows a new avenue for further understanding the significance of ZEB1 in diseases involving corneal differentiation and inflammation, and for potential targeted therapies to prevent abnormal differentiation during chronic corneal inflammation [16].
Ortiz-Melo et al. utilized the rabbit corneal epithelial cell line RCE1 (5T5) as a 3D-culture model, three stages of differentiation were determined according to the growth state of the cultured cells, namely proliferative non-differentiated cells, committed cells, and cells that constitute a stratified epithelium with a limbal epithelial-like structure. They found that the proliferative non-differentiated cells shared characteristics with corneal epithelial stem cells. During this stage, these stem-like cells showed an Oct4+, Klf4+, Myc+, ΔNp63α+, Abcg2+, Vimentin+, Zeb1+, Vangl1+, Krt3-, Krt12- phenotype. They further indicated that Zeb1+ may be related to the difference between corneal epithelium and epidermis and underlying the regulatory mechanism of corneal epithelial cell differentiation [24].
JI-AE is expressed in human corneal epithelial cells, its knockout (KO) increases the expression of ZEB1 in corneal fibroblasts, and this effect in the epithelial cells is mediated by IGF1. Knockdown (KD) of IGF1 by siRNA in the cells further proves that IGF1 secreted by the corneal epithelial cells induces the expression of N-Cadherin in cultured corneal fibroblasts, which is regulated by ZEB1 [25].
4. ZEB1 and Corneal Wound Healing
Cell proliferation and fibrosis play important roles in a wide variety of physiological processes such as wound healing. In corneal endothelial wound healing, severely affected endothelial cells undergo an endothelial to mesenchymal transition (EnMT), lose some endothelial properties and gain some mesenchymal abnormalities. These cells exhibit proliferative, mobile, and fibrotic properties, which can lead to a reduction in the known reflectivity of the endothelium—loss of corneal transparency.
Lee et al. demonstrated that FGF2 induced the expression of SNAI1, which further activated the expression of ZEB1 and CDK2/cyclin E1 [26]. ZEB1 thereafter induced the mesenchymal phenotype through vimentin, fibronectin, and type I collagen expression. They summarized that FGF2 initiates the EnMT through SNAI1 activation of ZEB1 and CDK2, thereby inducing the production of the matrix proteins COL1A1 and COL1A2 [26]. Lee et al. observed such an EnMT in the mouse corneal endothelium after surgical injury in vivo [26]. They found that a surgical injury induced the expression of Fgf2 and a group of EnMT-related genes like Snai1, Zeb1, Col1a1, Col1a2, Fn1, Vim, Cdk2 and Ccne1 in the mouse corneal endothelium. Knockdown of Fgf2 in mouse corneal endothelial cells by siRNA inhibited the injury-dependent expression of Fgf2, Snai1, Zeb1, and Cdh1, indicating that corneal EnMT is under the control of the Fgf2-activated EMT factor Zeb1 [27]. Transient FGF2 stimulation could increase the expression of SP1 and SP3 in human corneal endothelial cells whereas knockdown of ZEB1 by siRNA only reduced the expression of the FGF2-induced SP1 mRNA and protein, but not SP3 [28]. The expression of the FGF2-induced EnMT genes such as FN1, VIM and COLI was reduced by siRNA knockdown of SP1 and SP3. Compared with SP3, inhibition of SP1 had a greater inhibitory effect. Although SP1 and SP3 proteins were found to interact each other, SP1 and SP3 could bind to the same promoter binding site of EnMT-related genes individually. In addition, siRNA knockdown of Zeb1 inhibited the formation of mouse corneal endothelial injury-dependent fibrosis in vivo. They concluded that ZEB1, through activation of SP1, plays an important role in corneal endothelial fibrosis induced by mesenchymal transition [28].
In the early stage of corneal wound healing, a short process is required to restore the homeostasis of the epidermis, which is reminiscent of EMT. In skin wound healing, this process activates and mobilizes local keratinocytes in the skin to move to the wound bed, thereby re-epithelizing it [29]. ZEB1 is a transcriptional repressor of the epithelial gene E-cadherin [15]. Loss of E-cadherin is known to cause EMT [30][31]. However, in the repair of the corneal epithelium, whether the role of ZEB1 is similar to its role in skin wound repair has not yet been studied.
References
- Scott, C.L.; Omilusik, K.D. ZEBs: Novel Players in Immune Cell Development and Function. Trends Immunol. 2019, 40, 431–446.
- Funahashi, J.; Sekido, R.; Murai, K.; Kamachi, Y.; Kondoh, H. Delta-crystallin enhancer binding protein delta EF1 is a zinc finger-homeodomain protein implicated in postgastrulation embryogenesis. Development 1993, 119, 433–446.
- Shirakihara, T.; Saitoh, M.; Miyazono, K. Differential regulation of epithelial and mesenchymal markers by deltaEF1 proteins in epithelial mesenchymal transition induced by TGF-beta. Mol. Biol. Cell 2007, 18, 3533–3544.
- Nishimura, G.; Manabe, I.; Tsushima, K.; Fujiu, K.; Oishi, Y.; Imai, Y.; Maemura, K.; Miyagishi, M.; Higashi, Y.; Kondoh, H.; et al. DeltaEF1 mediates TGF-beta signaling in vascular smooth muscle cell differentiation. Dev. Cell 2006, 11, 93–104.
- Zhang, Y.; Xu, L.; Li, A.; Han, X. The roles of ZEB1 in tumorigenic progression and epigenetic modifications. Biomed. Pharm. 2019, 110, 400–408.
- Perez-Moreno, M.A.; Locascio, A.; Rodrigo, I.; Dhondt, G.; Portillo, F.; Nieto, M.A.; Cano, A. A new role for E12/E47 in the repression of E-cadherin expression and epithelial-mesenchymal transitions. J. Biol. Chem. 2001, 276, 27424–27431.
- Yang, S.; Zhao, L.; Yang, J.; Chai, D.; Zhang, M.; Zhang, J.; Ji, X.; Zhu, T. δEF1 represses BMP-2-induced differentiation of C2C12 myoblasts into the osteoblast lineage. J. Biomed. Sci. 2007, 14, 663–679.
- Sanchez-Tillo, E.; Siles, L.; de Barrios, O.; Cuatrecasas, M.; Vaquero, E.C.; Castells, A.; Postigo, A. Expanding roles of ZEB factors in tumorigenesis and tumor progression. Am. J. Cancer Res. 2011, 1, 897–912.
- Wu, H.T.; Zhong, H.T.; Li, G.W.; Shen, J.X.; Ye, Q.Q.; Zhang, M.L.; Liu, J. Oncogenic functions of the EMT-related transcription factor ZEB1 in breast cancer. J. Transl. Med. 2020, 18, 51.
- Seelan, R.S.; Mukhopadhyay, P.; Pisano, M.M.; Greene, R.M. Developmental epigenetics of the murine secondary palate. ILAR J. 2012, 53, 240–252.
- Lencinas, A.; Chhun, D.C.; Dan, K.P.; Ross, K.D.; Hoover, E.A.; Antin, P.B.; Runyan, R.B. Olfactomedin-1 activity identifies a cell invasion checkpoint during epithelial-mesenchymal transition in the chick embryonic heart. Dis. Models Mech. 2013, 6, 632–642.
- Yen, G.; Croci, A.; Dowling, A.; Zhang, S.; Zoeller, R.T.; Darling, D.S. Developmental and functional evidence of a role for Zfhep in neural cell development. Brain Res. Mol. Brain Res. 2001, 96, 59–67.
- Zhang, P.; Sun, Y.; Ma, L. ZEB1: At the crossroads of epithelial-mesenchymal transition, metastasis and therapy resistance. Cell Cycle 2015, 14, 481–487.
- Jung, H.Y.; Fattet, L.; Yang, J. Molecular pathways: Linking tumor microenvironment to epithelial-mesenchymal transition in metastasis. Clin. Cancer Res. 2015, 21, 962–968.
- Liu, Y.; El-Naggar, S.; Darling, D.S.; Higashi, Y.; Dean, D.C. Zeb1 links epithelial-mesenchymal transition and cellular senescence. Development 2008, 135, 579–588.
- Li, S.; Gallup, M.; Chen, Y.T.; McNamara, N.A. Molecular mechanism of proinflammatory cytokine-mediated squamous metaplasia in human corneal epithelial cells. Invest. Ophthalmol. Vis. Sci. 2010, 51, 2466–2475.
- Secker, G.A.; Daniels, J.T. Limbal Epithelial Stem Cells of the Cornea; StemBook: Cambridge, MA, USA, 2008.
- Fini, M.E. Keratocyte and fibroblast phenotypes in the repairing cornea. Prog. Retin. Eye Res. 1999, 18, 529–551.
- Pinnamaneni, N.; Funderburgh, J.L. Concise review: Stem cells in the corneal stroma. Stem Cells 2012, 30, 1059–1063.
- Zavala, J.; Lopez Jaime, G.R.; Rodriguez Barrientos, C.A.; Valdez-Garcia, J. Corneal endothelium: Developmental strategies for regeneration. Eye 2013, 27, 579–588.
- Jin, L.; Zhang, Y.; Liang, W.; Lu, X.; Piri, N.; Wang, W.; Kaplan, H.J.; Dean, D.C.; Zhang, L.; Liu, Y. Zeb1 promotes corneal neovascularization by regulation of vascular endothelial cell proliferation. Commun. Biol. 2020, 3, 349.
- Tseng, H.; Green, H. Basonuclin: A keratinocyte protein with multiple paired zinc fingers. Proc. Natl. Acad. Sci. USA 1992, 89, 10311–10315.
- Tiwari, A.; Loughner, C.L.; Swamynathan, S.; Swamynathan, S.K. KLF4 Plays an Essential Role in Corneal Epithelial Homeostasis by Promoting Epithelial Cell Fate and Suppressing Epithelial-Mesenchymal Transition. Invest. Ophthalmol. Vis. Sci. 2017, 58, 2785–2795.
- Ortiz-Melo, M.T.; Garcia-Murillo, M.J.; Salazar-Rojas, V.M.; Campos, J.E.; Castro-Munozledo, F. Transcriptional profiles along cell programming into corneal epithelial differentiation. Exp. Eye Res. 2020, 202, 108302.
- Ko, J.A.; Yanai, R.; Nishida, T. IGF-1 released by corneal epithelial cells induces up-regulation of N-cadherin in corneal fibroblasts. J. Cell Physiol. 2009, 221, 254–261.
- Lee, J.G.; Jung, E.; Heur, M. Fibroblast growth factor 2 induces proliferation and fibrosis via SNAI1-mediated activation of CDK2 and ZEB1 in corneal endothelium. J. Biol. Chem. 2018, 293, 3758–3769.
- Lee, J.; Jung, E.; Heur, M. Injury induces endothelial to mesenchymal transition in the mouse corneal endothelium in vivo via FGF2. Mol. Vis. 2019, 25, 22–34.
- Lee, J.; Jung, E.; Gestoso, K.; Heur, M. ZEB1 Mediates Fibrosis in Corneal Endothelial Mesenchymal Transition Through SP1 and SP3. Invest. Ophthalmol. Vis. Sci. 2020, 61, 41.
- Singh, K.; Sinha, M.; Pal, D.; Tabasum, S.; Gnyawali, S.C.; Khona, D.; Sarkar, S.; Mohanty, S.K.; Soto-Gonzalez, F.; Khanna, S.; et al. Cutaneous Epithelial to Mesenchymal Transition Activator ZEB1 Regulates Wound Angiogenesis and Closure in a Glycemic Status-Dependent Manner. Diabetes 2019, 68, 2175–2190.
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196.
- Haensel, D.; Sun, P.; MacLean, A.L.; Ma, X.; Zhou, Y.; Stemmler, M.P.; Brabletz, S.; Berx, G.; Plikus, M.V.; Nie, Q.; et al. An Ovol2-Zeb1 transcriptional circuit regulates epithelial directional migration and proliferation. EMBO Rep. 2019, 20, e46273.




