
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marta Carvalho | + 2106 word(s) | 2106 | 2021-04-06 10:05:30 | | | |
| 2 | Bruce Ren | -21 word(s) | 2085 | 2021-04-14 03:36:31 | | |
Video Upload Options
Engineering biomaterials that mimic the extracellular matrix (ECM) of bone is of significant importance since most of the outstanding properties of the bone are due to matrix constitution. Bone ECM is composed of a mineral part comprising hydroxyapatite and of an organic part of primarily collagen with the rest consisting on non-collagenous proteins.
1. Introduction
New promising solutions for bone tissue engineering have been developed over the last years following the dramatic increase of the number of bone-related medical conditions that require clinical interventions. In fact, each year, more than one million non-union fractures are treated in the United States [1]. Moreover, 5–10% of the bone fractures that occur worldwide do not heal [1]. Besides bone fractures, bone tissue can also be damaged by traumas, tumors, infections, or bone diseases. Furthermore, new strategies to engineer bone tissue are required as an alternative to the use of bone grafts, addressing the increasing worldwide incidence of bone disorders in an aging society severely impacted by obesity, lack of exercise, and with reducing healing capacity [2]. Even though bone tissue engineering appears as a promising alternative, to date the gold-standard treatment for bone regeneration still relies on bone grafts, autologous, allogenic, and xenogeneic grafts [1] (Figure 1). These approaches have some limitations and are not ideal for bone regeneration. Autografts have been applied since they can provide a matrix with osteogenic cells and osteoinductive factors to support new bone growth, without having immunological rejection and promote better osseointegration. Nonetheless, the availability of quality graft material is limited and possible complications may occur, such as pain, infections, scarring, and weakening of the donor bone. Moreover, there is a high morbidity associated with this procedure, since more than one surgery is needed [1][3]. Allografts, usually harvested from cadavers, have also some limitations, namely the higher risk of immunologic rejection and infection though it requires less procedures than autografts, minimizing the surgical time and accelerating the patient recovery [3]. As an alternative to allografts, xenografts consist of transplantation of bone tissue across species. The most common xenograft used in orthopedic surgery is bovine derived. Xenografts have some advantages compared to other grafts, such as being readily available due to the abundance of donor bone tissue and being less expensive than allografts [2][3]. In fact, commercially-available xenografts are approximately one-tenth the price of commercially-available allografts. Also, because of the extensive sterilization processes, their shelf life is generally long. However, xenografts present several challenges such as the risk of disease transmission and a higher risk of immune response of the host tissue compared to allografts [3]. Moreover, xenografts require intensive sterile processing, which can decrease their osteoinductive properties.
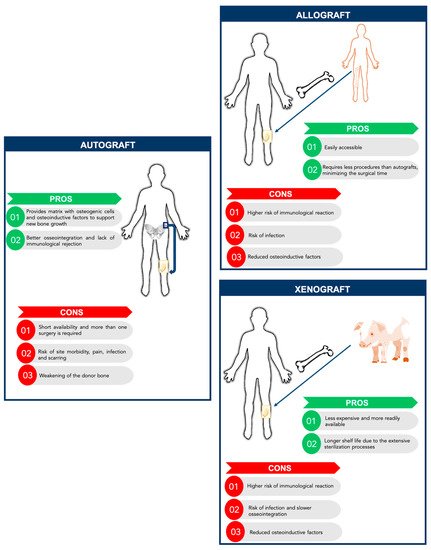
Figure 1. Comparison between autografts, allografts, and xenografts: Advantages and disadvantages.
Bone tissue engineering has the potential for solving these problems by combining different elements such as cells, molecules, and scaffolds. The standard tissue engineering approach uses a combination of growth factors, scaffolds and osteogenic cells (triangular concept). However, Giannoudis and colleagues developed and discussed a new concept, the diamond concept, in which a fourth element, vascularization, should be also considered as a contributor to bone healing. Thus, the diamond concept in bone tissue engineering combines four basic elements [4]: (i) A biomaterial with osteogenic ability for bone formation that acts as a scaffold for the other elements; (ii) osteogenic cells capable to creating or inducing new bone formation at the defect site; (iii) osteoinductive molecules that trigger cells and recruit resident cells to form new functional bone tissue; and (iv) vascularization to support the viability of the defect site thus allowing the diffusion of oxygen and nutrients to the defect region (Figure 2).
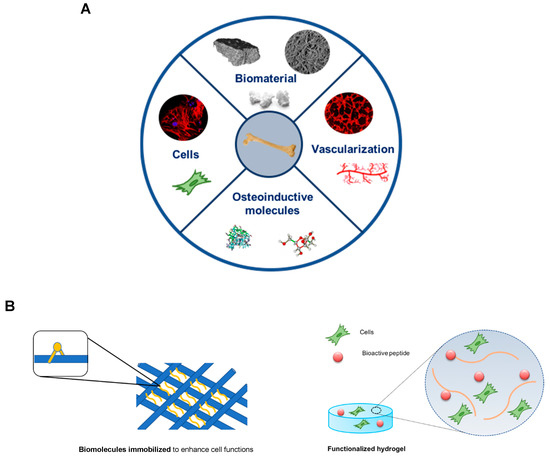
Figure 2. Bone tissue engineering strategies. (A) The bone tissue engineering paradigm highlights (1) Biomimetic scaffold, (2) osteogenic cells, (3) osteoinductive molecules, and (4) vascularization. (B) Schematic representation of biomolecules immobilized into a porous scaffold (left) and a functionalized hydrogel with bioactive peptides and cells incorporated (right).
Several scaffolds have been developed for bone tissue engineering, including natural biomaterials (such as collagen, gelatin, and chitosan), ceramic implants (such as hydroxyapatite), polymeric synthetic materials (such as polylactic acid (PLA) and polyglycolic acid (PGA)) and composite scaffolds [5]. Even though materials science technology has resulted in clear improvements for bone regeneration, challenges to achieve functional and mechanically competent bone growth still remain. One approach in the design of bone scaffolds involves the production of biomimetic bone matrices, in which the bone replacement material should interact with the surrounding tissues by biomolecular recognitions [6]. Among the molecular signals used for bone tissue engineering applications, the number of alternatives is yet smaller compared to the number of different scaffolds materials that can be used. Bone morphogenetic proteins (BMPs), a group of growth factors, play the leading role in the field, being able to promote proliferation and differentiation of osteogenic cells [7]. BMP-derived peptides have been widely used for bone tissue engineering applications, in particular derived from BMP-2 and BMP-7 [6]. BMP-2 peptides have been shown to promote osteogenic differentiation of MSCs [7][8]. Lin and co-workers developed a copolymer membrane loaded with a novel synthetic BMP-2 derived peptide, P24, and observed enhanced osteogenic differentiation of MSCs in vitro and bone regeneration in vivo [8]. Furthermore, BMP-2 and -7 received approval from the FDA and the EMA to be used in combination with type I collagen for the treatment of severe tibial fractures and posterolateral spinal fusions [9][10][11]. Although being widely used, several side effects of BMPs have been reported, such as postoperative inflammation and associated adverse effects, ectopic bone formation, osteoclast-mediated bone resorption, and inappropriate adipogenesis [12]. BMPs have other drawbacks, such as the high costs of production and the high doses required, raising questions about their cost-effectiveness [13]. In addition to above, calcitonin gene-related peptide (CGRP) has been widely applied due to its bone regeneration potential. CGRP might play a crucial role in promoting osteoblast proliferation and differentiation by bonding with functional receptors and transporters on the osteogenic cells and by stimulating growth factors production, such as BMP-2 [14]. In fact, Mi and colleagues demonstrated that CGRP administration increased new bone formation by promoting MSCs migration and differentiation [14]. A recent study from Lai and co-workers presented a new strategy to immobilize CGRP onto TiO2 nanotubes through polydopamine [15]
Recently, the field has shifted towards investigating the interaction between extracellular matrix (ECM) proteins and cell membrane receptors [5]. This approach avoids the use of growth factors and better mimics the bone ECM, reducing the side effects and increasing the efficiency of bone healing process. ECM proteins can be used intact or reduced to peptides with specific sequences that will trigger the action required. Therefore, these new osteoinductive peptides are easy and less expensive to manufacture, more unlikely to elicit immune responses due to their small size and stable in physiological conditions [16]. An attractive strategy consists of combining different ECM peptides to enhance cellular processes, such as adhesion and proliferation but also to promote osteogenic differentiation and angiogenesis. Combining peptides with important functions/properties increases the effectiveness and versatility of the final product to be used in clinical applications. In spite of the great improvements on incorporating ECM peptides on biomimetic materials, the influence of non-collagenous bone ECM proteins on osteogenic differentiation remains to be evaluated. This review aims to present a summary of the different non-collagenous proteins found in bone ECM and their important functions in context of bone tissue engineering applications, specifically on their role in cell adhesion, proliferation, osteogenic differentiation, and angiogenic capacity.
2. Bone Extracellular Matrix: Characterization, Properties, and Quality
Most of the outstanding properties of the bone are related to its matrix constitution [17]. Bone ECM has two components: A mineral part comprising of hydroxyapatite (70–90%) and an organic part (10–30%) composed primarily of collagen (approx. 90% of organic matrix) with the rest being non-collagenous proteins (~10%) [17][18]. The organic matrix of bone is mainly composed of collagen, however, Herring and co-workers identified the presence of other non-collagenous proteins [19] (Figure 3). Type I collagen is the most prevalent protein in the body and can be found not only in mineralized but also in non-mineralized tissues, playing a critical role in the structure and function of different skeletal tissues [18]. However, type I collagen is not the only protein involved in mineralization. Improved technologies have led to the isolation of a large number of non-collagenous matrix proteins. It is known that some matrix proteins bind to collagen forming fibrils. Thus, collagen serves as a scaffold upon which nucleators of hydroxyapatite, such as non-collagenous proteins, are present (Figure 4) [18][19][20]. Although some studies have already described the potential role of these non-collagenous proteins, their contributions and role in bone tissue engineering applications remain to be well investigated. Moreover, bone ECM quality may be determined not only by the nature of collagen type I, but also by mineral and non-collagenous proteins composition [21][22]. Using different characterization techniques and diseased mice models, it has been demonstrated that the nano-structural organization influences bone properties. In fact, several diseases related with deregulation of type I collagen and mineralization showed impairment of bone quality and other bone properties, such as bone fragility and strength. Mice with osteogenesis imperfecta, a condition derived from mutation in type I collagen, presented bone fragility and reduction in strength [23][24]. Osteopetrosis is a condition responsible for hypermineralization of bone that increases bone fragility [25] and involves altered interactions between collagen and mineral component that modify the nature of organization in bone at the nanometer scale. Non-collagenous proteins have also been suggested to influence the mechanical quality of bone matrix. Studies on osteopontin (OPN) showed that it behaves like “glue” in bone [26]. In the presence of calcium ions, OPN is capable of sacrificial bonding, a nanoscale mechanism that dissipates energy and inhibits crack growth. Osteocalcin (OC), the most abundant bone specific non-collagenous protein, complexes with OPN [27] and regulates bone mineralization through its strong affinity to hydroxyapatite. Previous works from our group found that fracture in bone initiates as dilatational bands that form as a result of OC-OPN interaction. In the absence of either protein, the complex is disrupted, resulting in a dramatic loss of toughness [28].
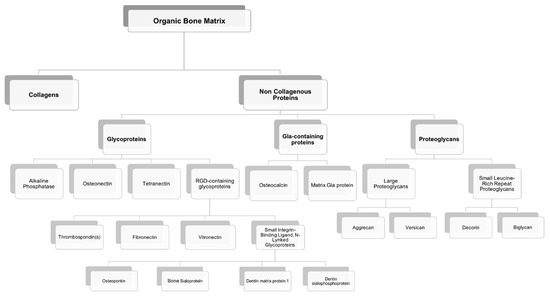
Figure 3. Organic components of the bone extracellular matrix.
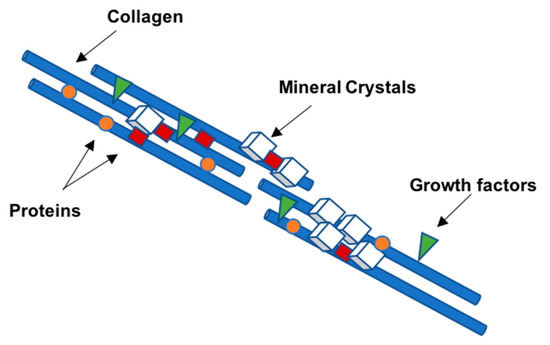
Figure 4. Schematic diagram representing the organization of the collagen molecules reinforced with calcium phosphate nanocrystals, proteins, and growth factors arranged in a semi-regular pattern.
3. Non-Collagenous Bone Matrix Proteins
Non-collagenous proteins have been isolated from bone and have been found to be biologically active, even though their functions are not yet completely understood. Based on their localization patterns, each of these proteins may perform different functions. Therefore, it is extremely important to better understand the properties and functions of these proteins, aiming to design innovative strategies for bone tissue engineering applications. It has been speculated that non-collagenous proteins might have an important role in cell attachment, cell differentiation, and regulation of hydroxyapatite minerals deposition [29]. Some of these proteins may be multifunctional, playing different roles in the bone, thus defining a single function may not be sufficient. Also, some of these proteins might act together, having a synergistic effect on cellular behavior and mechanical properties of bone, or they can compensate some effects resulting from deregulation of the levels of other non-collagenous proteins present in bone matrix (Figure 5). However, non-collagenous proteins may also present some drawbacks such as limited information about their mechanism of action, high-water solubility, and limited availability.
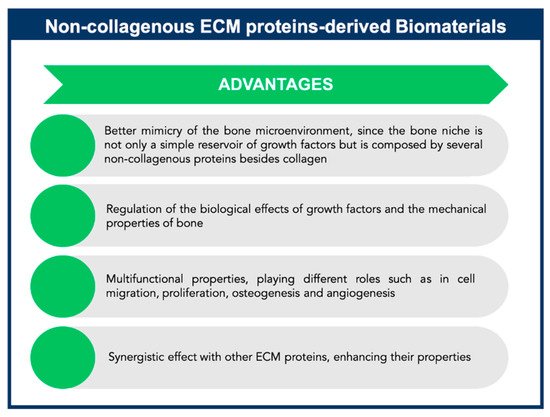
Figure 5. Advantages of non-collagenous extracellular matrix (ECM) proteins-derived biomaterials for Bone Tissue Engineering applications.
Interestingly, not all types of bones contain the same amount of non-collagenous bone proteins. In humans, for example, cortical bone contains 30× more OC than trabecular bone, but trabecular bone contains 21× more osteonectin (ON) [30]. Moreover, it is possible to find non-collagenous proteins in some other tissues besides bone, specifically OPN and ON present a general tissue distribution. Bone sialoprotein (BSP) and OC are also found in other mineralizing tissues, such as dentin. Therefore, importance of these proteins in bone physiology cannot be underestimated. Indeed some studies have reported that mutations in some of these proteins may result in abnormal bone [29].
The multifunctional properties of these non-collagenous proteins make them attractive agents for incorporation within an appropriate scaffold to enable stem cell-based bone tissue engineering (Figure 5). These proteins can be used successfully as signaling molecules to direct stem cell recruitment, attachment, and differentiation and create a mature and mineralized extracellular matrix.
In bone, non-collagenous proteins are mainly composed of two major types: Glycoproteins and gamma-carboxyglutamic acid (Gla)-containing proteins; however some proteoglycans can also be found in smaller content [31][32]. The most relevant and abundant glycoproteins are represented by alkaline phosphatase (ALP), ON, and the Arginine-Glycine-Aspartic acid (RGD)-containing proteins, which include, but are not limited to, OPN and sialoproteins. Of the Gla-containing proteins, OC is the major component.
References
- Yaszemski, M.J.; Payne, R.G.; Hayes, W.C.; Langer, R.; Mikos, A.G. Evolution of bone transplantation: Molecular, cellular and tissue strategies to engineer human bone. Biomaterials 1996, 17, 175–185.
- Koond, G.L.; Diba, M.; Mikos, A.G. Materials design for bone-tissue engineering. Nat. Rev. Mater. 2020, 5, 584–603.
- Stock, U.A.; Vacanti, J.P. Tissue engineering: Current state and prospects. Annu. Rev. Med. 2001, 52, 443–451.
- Giannoudis, P.V.; Einhorn, T.A.; Marsh, D. Fracture healing: The diamond concept. Injury 2007, 38, S3–S6.
- Fernandez-Yague, M.A.; Abbah, S.A.; McNamara, L.; Zeugolis, D.I.; Pandit, A.; Biggs, M.J. Biomimetic approaches in bone tissue engineering: Integrating biological and physicomechanical strategies. Adv. Drug Deliv. Rev. 2015, 84, 1–29.
- Lutolf, M.P.; Weber, F.E.; Schmoekel, H.G.; Schense, J.C.; Kohler, T.; Müller, R.; Hubbell, J.A. Repair of bone defects using synthetic mimetics of collagenous extracellular matrices. Nat. Biotechnol. 2003, 21, 513–518.
- Zhou, X.; Feng, W.; Qiu, K.; Chen, L.; Wang, W.; Nie, W.; Mo, X.; He, C. BMP-2-derived peptide and dexamethasone incorporated mesoporous silica nanoparticles for enhanced osteogenic differentiation of bone mesenchymal stem cells. ACS Appl. Mater. Interfaces 2015, 7, 15777–15789.
- Lin, Z.Y.; Duan, Z.X.; Guo, X.D.; Li, J.F.; Lu, H.W.; Zheng, Q.X.; Quan, D.P.; Yang, S.H. Bone induction by biomimetic PLGA-(PEG-ASP)n copolymer loaded with a novel synthetic BMP-2-related peptide in vitro and in vivo. J. Control. Release 2010, 144, 190–195.
- Wang, E.A.; Rosen, V.; D’Alessandro, J.S.; Bauduy, M.; Cordes, P.; Harada, T.; Israel, D.I.; Hewick, R.M.; Kerns, K.M.; LaPan, P.; et al. Recombinant human bone morphogenetic protein induces bone formation. Proc. Natl. Acad. Sci. USA 1990, 87, 2220–2224.
- Friedlaender, G.E.; Perry, C.R.; Cole, J.D.; Cook, S.D.; Cierny, G.; Muschler, G.F.; Zych, G.A.; Calhoun, J.H.; LaForte, A.J.; Yin, S. Osteogenic protein-1 (bone morphogenetic protein-7) in the treatment of tibial nonunions. J. Bone Joint Surg. Am. 2001, 83, S151–S158.
- Boden, S.D.; Kang, J.; Sandhu, H.; Heller, J.G. Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans: A prospective, randomized clinical pilot trial: 2002 Volvo Award in clinical studies. Spine 2002, 27, 2662–2673.
- James, A.W.; LaChaud, G.; Shen, J.; Asatrian, G.; Nguyen, V.; Zhang, X.; Ting, K.; Soo, C. A review of the clinical side effects of bone morphogenetic protein-2. Tissue Eng. Part. B Rev. 2016, 22, 284–297.
- Woo, E.J. Adverse events after recombinant human BMP2 in nonspinal orthopaedic procedures. Clin. Orthop. Relat. Res. 2013, 471, 1707–1711.
- Mi, J.; Xu, J.; Yao, H.; Li, X.; Tong, W.; Li, Y.; Dai, B.; He, X.; Chow, D.H.K.; Li, G.; et al. Calcitonin gene-related peptide enhances distraction osteogenesis by increasing angiogenesis. Tissue Eng. Part. A 2021, 27, 87–102.
- Lai, M.; Yan, X.; Shen, K.; Tang, Q.; Fang, X.; Zhang, C.; Zhu, Z.; Hou, Y. The effect of calcitonin gene-related peptide functionalized TiO2 nanotubes on osteoblast and osteoclast differentiation in vitro. Colloids Surf. A 2020, 600, 124899.
- Karadag, A.; Iqbai, H.; Yazici, H. Peptide-mediated bone tissue engineering. In Racing of the Surface; Li, B., Moriarty, T., Webster, T., Xing, M., Eds.; Springer: Cham, Switzerland, 2020.
- Sroga, G.E.; Karim, L.; Colon, W.; Vashishth, D. Biochemical Characterization of Major Bone-Matrix Proteins Using Nanoscale-Size Bone Samples and Proteomics Methodology. Mol. Cell. Proteomics 2011, 10, M110.006718.
- Vashishth, D. The role of the collagen matrix in skeletal fragility. Curr. Osteoporos. Rep. 2007, 5, 62–66.
- Herring, G.M.; Ashton, B.A. The isolation of soluble proteins, glycoproteins, and proteoglycans from bone. Prep. Biochem. 1974, 4, 179–200.
- Roach, H.I. Why does bone matrix contain non-collagenous proteins? The possible roles of osteocalcin, osteonectin, osteopontin and bone sialoprotein in bone mineralisation and resorption. Cell Biol. Int. 1994, 18, 617–628.
- Viguet-Carrin, S.; Garnero, P.; Delmas, P.D. The role of collagen in bone strength. Osteoporos. Int. 2006, 17, 319–336.
- Yerramshetty, J.S.; Akkus, O. The associations between mineral crystallinity and the mechanical properties of human cortical bone. Bone 2008, 42, 476–482.
- Fratzl, P.; Paris, O.; Klaushofer, K.; Landis, W.J. Bone mineralization in an osteogenesis imperfect mouse model studied by small-angle x-ray scattering. J. Clin. Invest. 2006, 97, 396–402.
- Dong, X.N.; Zoghi, M.; Ran, Q.; Wang, X. Collagen mutation causes changes of the microdamage morphology in bone of an OI mouse model. Bone 2002, 47, 1071–1075.
- Jämsä, T.; Rho, J.Y.; Fan, Z.; MacKay, C.A.; Marks, S.C., Jr.; Tuukkanen, J. Mechanical properties in long bones of rat osteopetrotic mutations. J. Biomech. 2002, 35, 161–165.
- Fantner, G.E.; Adams, J.; Turner, P.; Thurner, P.J.; Fisher, L.W.; Hansma, P.K. Nanoscale ion mediated networks in bone: Osteopontin can repeatedly dissipate large amounts of energy. Nano Letters 2007, 7, 2491–2498.
- Ritter, N.M.; Farach-Carson, M.C.; Butler, W.T. Evidence for the formation of a complex between osteopontin and osteocalcin. J. Bone Miner. Res. 1992, 7, 877–885.
- Poundarik, A.A.; Diab, T.; Sroga, G.E.; Ural, A.; Boskey, A.L.; Gundberg, C.M.; Vashishth, D. Dilatational band formation in bone. Proc. Natl. Acad. Sci. USA 2012, 109, 19178–19183.
- Boskey, A.L. Noncollagenous matrix proteins and their role in mineralization. Bone Miner. 1989, 6, 111–123.
- Ninomiya, J.T.; Tracy, R.P.; Calore, J.D.; Gendreau, M.A.; Kelm, R.J.; Mann, K.G. Heterogeneity of human bone. J. Bone Min. Res. 1990, 5, 933–938.
- Marcus, R.; Dempster, D.; Cauley, J.; Feldman, D. Osteoporosis, 4th ed.; Academic Press: Cambridge, MA, USA, 2013.
- Bilezikian, J.P.; Raisz, L.G.; Martin, T.J. Principles of Bone Biology, 3rd ed.; Academic Press: Cambridge, MA, USA, 2008.




