| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nebojsa Nick Knezevic | + 3029 word(s) | 3029 | 2021-03-01 07:27:10 | | | |
| 2 | Rita Xu | -1448 word(s) | 1581 | 2021-03-09 04:09:58 | | |
Video Upload Options
Complementary and alternative medicines such as herbal medicines are not currently part of the conventional medical system. As the popularity of and global market for herbal medicine grows among all age groups, with supporting scientific data and clinical trials, specific alternative treatments such as herbal medicine can be reclassified as a practice of conventional medicine. One of the most common conditions for which adults use herbal medicine is pain.
1. Introduction
Complementary and alternative medicine (CAM) incorporates a wide range of practices, interventions, therapies, applications, professions, theories, and claims that are not currently part of the conventional medical system. Over time and with supporting scientific data and clinical trials, a specific CAM treatment such as herbal medicine can be reclassified as a practice of conventional medicine. With the present positive social perspective on herbal medicine, its popularity is growing among all age groups [1]. It is a common belief that CAM enables individuals to be more involved with their care, control or offset the adverse events of conventional medicine, and/or find harmony with their culture or philosophies [2]. Patients often seek every possible option for receiving the benefits of medical care while avoiding adverse events [3].
One of the most common conditions in the United States for which adults use CAM is pain. This includes musculoskeletal pain such as cervical, lumbar, or joint pain as well as specific conditions such as arthritis or migraine. Although pain is a physiological and vital response to potential or actual tissue injury, in some cases (such as musculoskeletal pain or special conditions like arthritis) it can become chronic and cause biological changes to the central nervous system or peripheral tissues. Chronic pain can be debilitating and constitutes a high social and economic burden on the health system [4]. In some cases, due to adverse drug reactions, lack of efficacy, or high risk for serious complications, traditional treatments such as opioids or non-steroidal anti-inflammatory drugs (NSAIDs) must be discontinued. Such patients, particularly the elderly, have little option but to suffer from chronic pain or seek nontraditional treatment modalities.
Herbal medicine is one of the most commonly sought forms of CAM. In the United States, herbal medicine is currently used by nearly twenty million Americans [5], with an annual turnover of more than 1.5 billion dollars and growth of approximately 25% each year [6]. According to Hexa Research, the global herbal medicine market was valued at USD 71.19 billion in 2016 [7]. It has been estimated that at least 60% of individuals with arthritis pain or other musculoskeletal pain have tried CAM [8].
According to the World Health Organization (WHO) 1996 guidelines, herbal medicine comprises active end products that contain underground or aerial parts of either plants or plant materials or a combination of both. Most herbal medicines affect the eicosanoid metabolism by inhibiting either both or one of the lipoxygenase and cyclooxygenase (COX) pathways [6]. Their use is generally based on traditional methods, and the ideal extract dose and treatment duration for most herbal medicines have yet to be determined.
Many consumers of herbal medicine believe these treatments are natural and safe, yet herbal medicines contain pharmacological active ingredients that can be associated with numerous and diverse adverse events [6]. Herbal medicines are frequently taken alongside synthetic drugs, which can lead to harmful herb–drug interaction. In many countries, herbal medicines are largely unregulated and have suboptimal product quality; in some Asian herbal mixtures, toxic amounts of heavy metals or mixed synthetic prescription drugs have been reported [6]. Such concerns represent serious safety issues and suggest the possibility of adverse health events for users.
Herbal medicines are usually not the most potent analgesic treatments available. However, they can be highly beneficial for mild to moderate pain [6]. To translate complementary and integrative medicine using herbs into clinical practice and to enable acceptance into treatment guidelines, further rigorous studies are required to confirm the effectiveness and safety of these medicines.
2. St John’s Wort
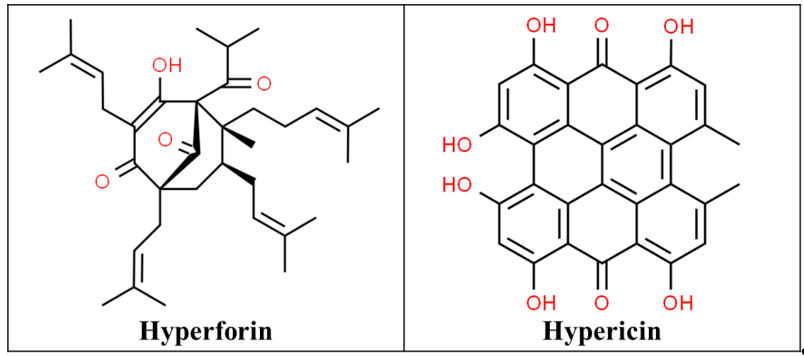
St John’s Wort (SJW) is extracted from the flowers and leaves of the plant Hypericum perforatum native to Asia and Europe, which was later introduced to North America by the Europeans. It includes at least 10 active constituents but the two principal pharmacological components are hyperforin and hypericin (Figure 1), which are responsible for their beneficial effects [9][10]. Hypericin can inhibit serotonin, norepinephrine, and dopamine reuptake, weakly inhibits monoamine oxidases (MAOIs) A and B and the crude extracts have a high affinity for gamma-aminobutyric acid (GABA) receptors. This has led to its role as an anxiolytic, sedative, antidepressant, and analgesic [11].
Among the herbs reviewed, SJW has the most potential for drug interaction. Adverse events are mostly due to its drug interaction with other selective serotonin reuptake inhibitors (SSRIs, such as paroxetine), MAOIs, opiates, tricyclic antidepressants, cold and flu medications which can lead to serotonin syndrome [14][15]. It has also been shown to be uterotonic in in vitro studies and rarely causes photosensitivity [10]. Its flavonoid components especially quercetin have been shown to have analgesic activity [16]. SJW has also been found to cause nausea, vomiting and anxiety when taken with sertraline, an antidepressant used in the treatment of chronic pain [17].
Hyperforin activates a regulator of the cytochrome P450 (CYP450) 3A4 transcription and results in the expression of 3A4 in the hepatocytes. St. John’s wort can also induce 2C9, 2D6, 2C19, 2E1, and 1A2. Concomitant use of opioids such as fentanyl, hydrocodone, codeine, tramadol, oxycodone and methadone with SJW may decrease the opioid concentrations and lead to withdrawal symptoms. Conversely, discontinuing the SJW may also cause increased opiate concentration causing toxicity [18][19][20][21]. The likely mechanism of this interaction between SJW and codeine is by inducing CYP3A4 metabolism, codeine conversion to the inactive norcodeine increases, resulting in less codeine being available for CYP2D6 to form its active metabolite morphine. It has also been reported that SJW active agents can inhibit MAOI A and B, but at concentrations up to 10 μM it may not be clinically relevant [22]. SJW’s analgesic effect plus its interaction with other analgesics such as fentanyl, morphine, ketamine, oxycodone has been studied in clinical trials.
3. Ginger
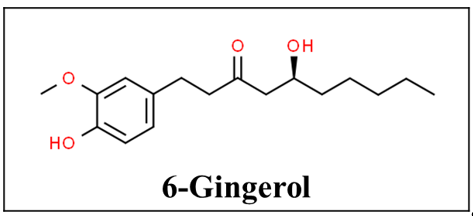
Ginger, also known as Zingiber officinale, has beautiful flowers but its tuberous rhizome has been used as a spice and medicine by herbalists mostly in India and China for the past 2500 years. The plant is found in most tropical countries [23][24]. It has uses for muscle pain and swelling, arthritis, headaches, digestive and appetite problems, prevention of motion sickness, postoperative nausea and vomiting, hyperemesis gravidarum, and also cold and bacterial infections due to its anti-oxidant mechanism [11][25]. Gingerols, especially 6-gingerol (Figure 2), are the active components of ginger. Ginger’s anti-emetic activity is not well understood but it is proposed to be caused by direct stimulation of the gastrointestinal tract or by antagonizing serotonin in the gut or central nervous system [26][27][28]. Its anti-inflammatory effects come from inhibiting arachidonic acid metabolism [27][28]. Based on the available data there is a delayed therapeutic action and therefore it does not help treat acute pain conditions such as exercise-induced muscle pain [29][30][31][32].
Figure 2. Chemical structure of 6-gingerol [33].
The adverse effects of ginger include drowsiness, excessive sedation, and arrhythmia [11], and perioperative physicians should be aware of its potent thromboxane-synthetase inhibition, which interferes with platelet aggregation and increases bleeding time based on in-vitro studies [34][35][36]. However, it has not been proven in in vivo human studies [37]. The concomitant use of ginger with other herbs or drugs with similar pharmacologic potential such as naproxen may increase the risk of bleeding and therapy modification should be considered [22]. If needed platelet functions status testing should be done before neuraxial or regional anesthetic procedures in any patient with bleeding or bruising history.
4. Turmeric
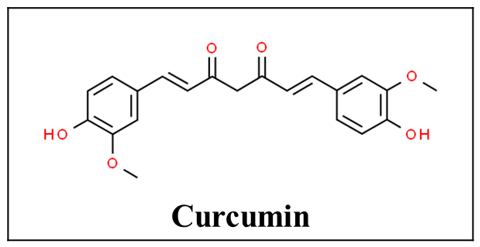
The rhizome of the turmeric plant also known as Curcuma longa contains an active polyphenolic compound called curcumin (Figure 3). It has traditionally been used as an antiseptic, anti-inflammatory agent for wound healing as well as an antioxidant and analgesic agent [38][39]. Curcumin can regulate inflammatory cytokines such as interleukin (IL)-1 beta, IL-6, IL-12, Tumor necrosis factor (TNF)-alpha, interferon (IFN) gamma, and associated AP-1, NF-kappa B, and JAK-STAT signaling pathways. With its anti-inflammatory effects, it has been used in autoimmune diseases such as rheumatoid arthritis, inflammatory bowel disease, and multiple sclerosis [40]. In terms of drug interactions, turmeric supplementation of paclitaxel chemotherapy was found to improve the quality of life and pain scores in breast cancer patients [41].
Figure 3. Chemical structure of curcumin [42].
Oral turmeric is well tolerated and safe for general use. Due to its poor bioavailability, higher doses are often used to achieve a systemic effect [43]. Studies have shown curcumin can inhibit platelet-activating factor and arachidonic acid platelet aggregation [44]. Due to its anti-thrombotic effects, concomitant use of turmeric with other drugs with similar pharmacologic potential such as naproxen may increase the risk of bleeding, and therapy modification is recommended.
Several pre-clinical studies have investigated interactions between curcumin and drugs. One such study in mouse models of acute nociceptive pain demonstrated a synergistic interaction in combination with pregabalin [45]. Curcumin was found to downregulate opioid-related nociceptin receptor 1 gene expression, which codes for nociceptin opioid peptide receptor (NOP), one of the four opioid receptors. This suggests an inhibitory effect on morphine-induced activation of the same gene, possibly decreasing tolerance and addiction to morphine and other analgesic opioids [46]. A synergistic anti-nociceptive effect was also noted in the combination of curcumin and diclofenac, an NSAID, in rats. Although curcumin did not produce significant alteration in oral diclofenac bioavailability, this interaction may have therapeutic advantages [47]. Another study on rats also noted that curcumin exhibited a synergistic interaction with a sub-analgesic dose of diclofenac [48].
References
- Eisenberg, D.M.; Davis, R.B.; Ettner, S.L.; Appel, S.; Wilkey, S.; van Rompay, M.; Kessler, R.C. Trends in Alternative Medicine Use in the United States, 1990–1997: Results of a Follow-up National Survey. JAMA 1998, 280, 1569–1575.
- Gentz, B.A. Alternative Therapies for the Management of Pain in Labor and Delivery. Clin. Obstet. Gynecol. 2001, 44, 704–732.
- Ernst, E.; Willoughby, M.; Weihmayr, T.H. Nine Possible Reasons for Choosing Complementary Medicine. Perfusion 1995, 11, 356–359.
- NCCIH. Nonpharmacologic Management of Pain; NCCIH: Bethesda, MD, USA, 2016.
- Nahin, R.L.; Barnes, P.M.; Stussman, B.J.; Bloom, B. Costs of Complementary and Alternative Medicine (Cam) and Frequency of Visits to Cam Practitioners: United States, 2007. Natl. Health Stat. Rep. 2009, 18, 1–14.
- Weiner, D.K.; Ernst, E. Complementary and Alternative Approaches to the Treatment of Persistent Musculoskeletal Pain. Clin. J. Pain 2004, 20, 244–255.
- Hexaresearch. Global Herbal Medicine Market Size, Value, 2014–2024; Hexaresearch: Pune, MH, India, 2017.
- Rao, J.K.; Mihaliak, K.; Kroenke, K.; Bradley, J.; Tierney, W.M.; Weinberger, M. Use of Complementary Therapies for Arthritis among Patients of Rheumatologists. Ann. Intern. Med. 1999, 131, 409–416.
- Budavari, S. The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals, 11th ed.; Merck: Rahway, NJ, USA, 1989; p. 11.
- Hodges, P.J.; Kam, P.C. The Peri-Operative Implications of Herbal Medicines. Anaesthesia 2002, 57, 889–899.
- Fetrow, C.W.; Avila, J.R. The Complete Guide to Herbal Medicines; Simon and Schuster: New York, NY, USA, 2000.
- CSID:16736597. Hyperforin. Available online: (accessed on 27 January 2021).
- CSID:4444511. Hypericin. Available online: (accessed on 27 January 2021).
- Gordon, J.B. Ssris and St.John’s Wort: Possible Toxicity? Am. Fam. Phys. 1998, 57, 950.
- Gillman, P.K. Monoamine Oxidase Inhibitors, Opioid Analgesics and Serotonin Toxicity. Br. J. Anaesth 2005, 95, 434–441.
- Vasilchenko, E.A. Analgesic Action of Flavonoids of Rhododendron Luteum Sweet, Hypericum Perforatum L.; Lespedeza Bicolor Turcz, and L. Hedysaroids (Pall.). Kitag Rastit. Resur. 1986, 22, 12–21.
- Wirth, J.H.; Hudgins, J.C.; Paice, J.A. Use of Herbal Therapies to Relieve Pain: A Review of Efficacy and Adverse Effects. Pain Manag. Nurs. 2005, 6, 145–167.
- Janssen Pharmaceuticals Inc. Duragesic (Fentanyl) Prescribing Information; Janssen Pharmaceuticals Inc.: Titusville, NJ, USA, 2018.
- Sandoz Canada Inc. Codeine Phosphate Injection [Product Monograph]; Sandoz Canada Inc.: Boucherville, QC, Canada, 2019.
- Zogenix Inc. Zohydro Er (Hydrocodone Bitartrate) [Prescribing Information]; Zogenix Inc.: San Diego, CA, USA, 2014.
- Nieminen, T.H.; Hagelberg, N.M.; Saari, T.I.; Neuvonen, M.; Laine, K.; Neuvonen, P.J.; Olkkola, K.T. St John’s Wort Greatly Reduces the Concentrations of Oral Oxycodone. Eur. J. Pain 2010, 14, 854–859.
- Ulbricht, C.; Chao, W.; Costa, D.; Rusie-Seamon, E.; Weissner, W.; Woods, J. Clinical Evidence of Herb-Drug Interactions: A Systematic Review by the Natural Standard Research Collaboration. Curr. Drug. Metab 2008, 9, 1063–1120.
- Barrett, B.; Kiefer, D.; Rabago, D. Assessing the Risks and Benefits of Herbal Medicine: An Overview of Scientific Evidence. Altern. Ther. Health Med. 1999, 5, 40–49.
- Langner, E.; Greifenberg, S.; Gruenwald, J. Ginger: History and Use. Adv. Ther. 1998, 15, 25–44.
- Fischer-Rasmussen, W.; Kjaer, S.K.; Dahl, C.; Asping, U. Ginger Treatment of Hyperemesis Gravidarum. Eur. J. Obstet. Gynecol. Reprod. Biol. 1991, 38, 19–24.
- Ernst, E.; Pittler, M.H. Efficacy of Ginger for Nausea and Vomiting: A Systematic Review of Randomized Clinical Trials. Br. J. Anaesth. 2000, 84, 367–371.
- Koo, K.L.; Ammit, A.J.; Tran, V.H.; Duke, C.C.; Roufogalis, B.D. Gingerols and Related Analogues Inhibit Arachidonic Acid-Induced Human Platelet Serotonin Release and Aggregation. Thromb. Res. 2001, 103, 387–397.
- Grant, K.L.; Lutz, R.B. Alternative therapies: Ginger. Am. J. Health Syst. Pharm. 2000, 57, 945–947.
- Black, C.D.; Oconnor, P.J. Acute Effects of Dietary Ginger on Quadriceps Muscle Pain During Moderate-Intensity Cycling Exercise. Int. J. Sport Nutr. Exerc. Metab. 2008, 18, 653–664.
- Black, C.D.; O’Connor, P.J. Acute Effects of Dietary Ginger on Muscle Pain Induced by Eccentric Exercise. Phytother. Res. 2010, 24, 1620–1626.
- Black, C.D.; Herring, M.P.; Hurley, D.J.; O’Connor, P.J. Ginger (Zingiber Officinale) Reduces Muscle Pain Caused by Eccentric Exercise. J. Pain 2010, 11, 894–903.
- Terry, R.; Posadzki, P.; Watson, L.K.; Ernst, E. The Use of Ginger (Zingiber Officinale) for the Treatment of Pain: A Systematic Review of Clinical Trials. Pain Med. 2011, 12, 1808–1818.
- CSID:391126. (+)-[6]-Gingerol. Available online: (accessed on 27 January 2021).
- Backon, J. Ginger as an Antiemetic: Possible Side Effects Due to Its Thromboxane Synthetase Activity. Anaesthesia 1991, 46, 705–706.
- Srivastava, K.C. Aqueous Extracts of Onion, Garlic and Ginger Inhibit Platelet Aggregation and Alter Arachidonic Acid Metabolism. Biomed. Biochim. Acta 1984, 43, S335–S346.
- Backon, J. Ginger: Inhibition of Thromboxane Synthetase and Stimulation of Prostacyclin: Relevance for Medicine and Psychiatry. Med. Hypotheses 1986, 20, 271–278.
- Vaes, L.P.; Chyka, P.A. Interactions of Warfarin with Garlic, Ginger, Ginkgo, or Ginseng: Nature of the Evidence. Ann. Pharm. 2000, 34, 1478–1482.
- Sahbaie, P.; Sun, Y.; Liang, D.Y.; Shi, X.Y.; Clark, J.D. Curcumin Treatment Attenuates Pain and Enhances Functional Recovery after Incision. Anesth. Analg. 2014, 118, 1336–1344.
- Lee, J.Y.; Shin, T.J.; Choi, J.M.; Seo, K.S.; Kim, H.J.; Yoon, T.G.; Lee, Y.S.; Han, H.; Chung, H.J.; Oh, Y.; et al. Antinociceptive Curcuminoid, Kms4034, Effects on Inflammatory and Neuropathic Pain Likely Via Modulating Trpv1 in Mice. Br. J. Anaesth. 2013, 111, 667–672.
- Bright, J.J. Curcumin and Autoimmune Disease. Adv. Exp. Med. Biol. 2007, 595, 425–451.
- Kalluru, H.; Kondaveeti, S.S.; Telapolu, S.; Kalachaveedu, M. Turmeric Supplementation Improves the Quality of Life and Hematological Parameters in Breast Cancer Patients on Paclitaxel Chemotherapy: A Case Series. Complement Ther. Clin. Pract. 2020, 41, 101247.
- CSID:839564. Curcumin. Available online: (accessed on 27 January 2021).
- Asher, G.N.; Spelman, K. Clinical Utility of Curcumin Extract. Altern. Ther. Health Med. 2013, 19, 20–22.
- Keihanian, F.; Saeidinia, A.; Bagheri, R.K.; Johnston, T.P.; Sahebkar, A. Curcumin, Hemostasis, Thrombosis, and Coagulation. J. Cell Physiol. 2018, 233, 4497–4511.
- Leksiri, S.; Hasriadi, P.; Wasana, W.D.; Vajragupta, O.; Rojsitthisak, P.; Towiwat, P. Co-Administration of Pregabalin and Curcumin Synergistically Decreases Pain-Like Behaviors in Acute Nociceptive Pain Murine Models. Molecules 2020, 25, 18.
- Seo, E.-J.; Efferth, T.; Panossian, A. Curcumin Downregulates Expression of Opioid-Related Nociceptin Receptor Gene (Oprl1) in Isolated Neuroglia Cells. Phytomedicine 2018, 50, 285–299.
- De Paz-Campos, M.A.; Ortiz, M.I.; Pina, A.E.C.; Zazueta-Beltran, L.; Castaneda-Hernandez, G. Synergistic Effect of the Interaction between Curcumin and Diclofenac on the Formalin Test in Rats. Phytomedicine 2014, 21, 1543–1548.
- Mittal, N.; Joshi, R.; Hota, D.; Chakrabarti, A. Evaluation of Antihyperalgesic Effect of Curcumin on Formalin-Induced Orofacial Pain in Rat. Phytother. Res. 2009, 23, 507–512.