
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Igor Yu. Dolmatov | + 4278 word(s) | 4278 | 2021-02-19 10:51:41 | | | |
| 2 | Vivi Li | + 16 word(s) | 4294 | 2021-03-02 03:35:38 | | |
Video Upload Options
Holothurians, or sea cucumbers, belong to the phylum Echinodermata. They show good regenerative abilities. This entry provides an analysis of available data on the molecular aspects of regeneration mechanisms in holothurians. The genes and signaling pathways activated during the asexual reproduction and the formation of the anterior and posterior parts of the body, as well as the molecular mechanisms that provide regeneration of the nervous and digestive systems, are considered here. Damage causes a strong stress response, the signs of which are recorded even at late regeneration stages. In holothurian tissues, the concentrations of reactive oxygen species and antioxidant enzymes increase. Furthermore, the cellular and humoral components of the immune system are activated. Extracellular matrix remodeling and Wnt signaling play a major role in the regeneration in holothurians. All available morphological and molecular data show that the dedifferentiation of specialized cells in the remnant of the organ and the epithelial morphogenesis constitute the basis of regeneration in holothurians.
1. Introduction
Holothurians, or sea cucumbers, are members of the class Holothuroidea, phylum Echinodermata. They have an elongated, often worm-like, body bearing various outgrowths. Like all echinoderms, holothurians are exclusively marine animals. They live in a wide range of depths, from shallow, intertidal zones to a depth of 5000 m or more, in all regions of the world’s oceans. Most holothurians are benthic organisms, but there are also swimming species [1][2], and, possibly, fully pelagic ones [3]. These animals are of great importance as focus species of fisheries and aquaculture in South-East Asia and Australia, where about 47 holothurian species are used for various purposes [4][5].
Holothurians have good regenerative abilities [6][7]. They can heal cutaneous wounds and regenerate small appendages of the body such as tentacles and tube feet. Holothurians are able to restore all internal organs, including the gonad [7][8][9][10][11][12]. Moreover, they can regenerate large regions of the body after transverse fission or cutting into two or three parts [8][13][14][15][16][17].
Investigations into regeneration in holothurians are most frequently associated with studies on restoration of their internal organs, since these animals possess a unique type of autotomy referred to as evisceration. Holothurians can eject a part of internal organs (viscera) in case of deterioration of environmental conditions (increase in water temperature, freshening, or pollution) or due to various artificial impacts (weak electric current, injection of distilled water, KCl, or dilute ammonia solution into the body cavity) [13]. In a number of holothurian species, evisceration is seasonal [18][19][20]. The function and causes of evisceration still remain poorly understood. The ejection of viscera can be provoked by a predator’s attack, invasion and reproduction of parasites, or accumulation of waste products in tissues [19]. Moreover, evisceration can be a way to survive adverse conditions. For example, holothurians often eviscerate in response to deteriorating environmental conditions such as reduction in oxygen concentration, water pollution, and temperature rise [21][22].
Viscera can be removed in two different ways: Through the anterior or posterior ends of the body. During evisceration through the anal orifice (posterior evisceration), the middle part of the digestive tube located between the esophagus and the cloaca, the respiratory organs (respiratory trees), and part of the reproductive system (gonadal tubules) are removed [13][22]. This way is typical mainly of holothurians from the orders Holothuriida and Synallactida, as well as of a number of species from the order Dendrochirotida [8][22][23]. Evisceration through the anterior end of the body (anterior evisceration) occurs only in members of the order Dendrochirotida [8][13][22]. In this case, the entire digestive tract is removed, except the cloaca, the oral complex of organs (aquapharyngeal bulb, AB), and part of the gonadal tubules.
When considering regeneration in holothurians, one characteristic feature of echinoderms deserves particular mentioning. To date, no reliable evidence for the presence of stem cells in these animals have been found [24][25]. The exceptions are primordial germ cells and, probably, coelomocyte stem cells [26][27]. All attempts to detect stem cells using various mammalian stem cell markers such as Yamanaka factors or mammalian intestinal stem cell markers have failed so far [28][29][30]. The fact that pluripotency factors may be involved not only in maintaining the undifferentiated state of stem cells, but also in dedifferentiation and transdifferentiation of specialized cells also hampers the search [31][32][33].
On the other hand, numerous morphological studies show that regeneration in echinoderms occur due to differentiated cells of organ remnant [7][9][10][12][34][35][36][37][38][39][40][41]. The good regenerative abilities in echinoderms are explained by the ease of dedifferentiation and transdifferentiation of specialized cells [9][36][42][43][44][45].
Coelomic epithelium is a special tissue system of echinoderms that plays an important role in regeneration. It has been shown that coelomic epithelial cells in holothurians and crinoids can be involved in the formation of luminal epithelium of the gut [46][47][48]; in holothurians and sea urchins, it is involved in muscle development and regeneration [9][34][49][50][51]. Its cells are first to respond to damage, undergoing dedifferentiation and beginning to migrate and proliferate [42][43][52][53][54]. Thus, coelomic epithelium in echinoderms is a system with quite substantial histoblastic potencies [7]. Coelomic epithelium in vertebrates performs similar functions. It is a main source of many cell types, mainly fibroblasts and smooth muscle, but also organ-specific cells with very specialized physiological functions [55].
Coelomic epithelium is a derivative of mesoderm and is formed by the growth of the third pair of coelomes, somatocoels [56][57]. In holothurians, this process causes the formation of a large body cavity filled with coelomic fluid. The somatocoel wall forms coelomic epithelium (mesothelium) that covers all the internal organs. Thus, coelomic epithelia of different organs have a common origin. However, mesothelium is modified in the process of organogenesis and is represented in various organs by different morphological varieties. The latter differ both in the structure of epithelial cells (peritoneocytes and myoepithelial cells) and in the function performed [56]. The mesothelium also includes nerve cells and axon bundles forming the basiepithelial nerve plexus [56]. The final stage of the coelomic epithelium development is the formation of myocytes and muscle bundles that make up large muscles (longitudinal muscle bands, retractor muscles, etc.) [49][50][51].
Another derivative of somatocoels is gut mesentery. It is formed through merger of adjacent regions of the walls of the left and right somatocoels on the dorsal and ventral sides of holothurians [56][57]. A layer of connective tissue is formed between the walls of the coelomes, which leads to the formation of gut mesentery supporting the gut in the body cavity. Subsequently, the ventral mesentery is destroyed.
To date, regeneration has been described in sufficient detail from four holothurian species belonging to three orders: Synallactida (Apostichopus japonicus (Selenka, 1867)), Holothuriida (Holothuria (Selenkothuria) glaberrima Selenka, 1867), and Dendrochirotida (Eupentacta fraudatrix (D’yakonov and Baranova in D’yakonov, Baranova and Savel’eva, 1958) and Cladolabes schmeltzii (Ludwig, 1875)). Morphological events of regeneration in them were studied in detail, morphogenesis was described on the cellular level, and sources of regeneration of various organs were identified, which helps interpret molecular genetic data. Furthermore, each of the holothurian species above has its own characteristics of regeneration, which allows a more comprehensive study of the regeneration phenomenon [58]. C. schmeltzii has the capability of asexual reproduction by transverse fission and, accordingly, regeneration of body fragments [23][45][59][60]. A. japonicus and H. glaberrima eviscerate the internal organs through the anal orifice, and both species lose the same organs [37][61]. After this type of damage, two gut anlagen are formed. Luminal epithelium of the gut regenerated from the epithelia of cloaca (posterior anlage) and esophagus remnant (anterior anlage). Studies have shown that gut regeneration is also very similar on the cellular level in A. japonicus and H. glaberrima [37][41][61][62]. The holothurian E. fraudatrix is capable of anterior evisceration, while the gut regeneration in this species is radically different from that in the other above-listed species [42][61]. The major distinguishing feature of the digestive system regeneration in E. fraudatrix is the formation of enterocytes through the transdifferentiation of coelomic epithelial cells [46].
2. Regeneration after Fission
2.1. Morphological Aspects of Fission and Regeneration
Echinoderms exhibit different ways of asexual reproduction, of which the most common one is fission [63]. To date, 16 species of fissiparous holothurians are known that can be model objects for studying the mechanisms of fission with subsequent regeneration [59]. Most holothurian species have a thick body wall composed of connective tissue [56]. Accordingly, ECM remodeling is an important component of the transverse fission mechanism [63][64][65]. It has been shown that connective tissue in holothurians possesses a unique ability to alter its mechanical properties under effects of various factors [66][67]. Thus it is called mutable collagenous tissue (MCT) [68] or catch connective tissue [69]. The body-wall connective tissue in holothurians can occur in three states: Stiff, standard, and soft [65]. The mechanical stretching results in the decreased stiffness of the connective tissue and promotes its transition into the soft state [65].
Furthermore, stiffness of MCT depends on the interaction of three protein groups: Matrix metalloproteinases (MMPs), tissue inhibitors of metalloproteinases (TIMPs), and cross-link complexes connecting collagen fibrils to one another [70]. As the activity of TIMPs increases, MMPs are blocked. As a result, cross-links are formed between collagen fibrils, and MCT strengthens. Conversely, an increase in the MMPs concentration or activity in connective tissue leads to the destruction of cross-link complexes. This destruction enables collagen fibrils to slide along one another, which brings MCT into a compliant state. Thus, a local change in properties of the body-wall connective tissue allows holothurian to divide into two parts.
After division the anterior fragment of holothurian contains the AB, the gonad, and the anterior half of the intestinal tube (Figure 1a,b). The posterior part of the gut, the cloaca, and the respiratory organs (respiratory trees) remain in the posterior fragment (Figure 1a,e).
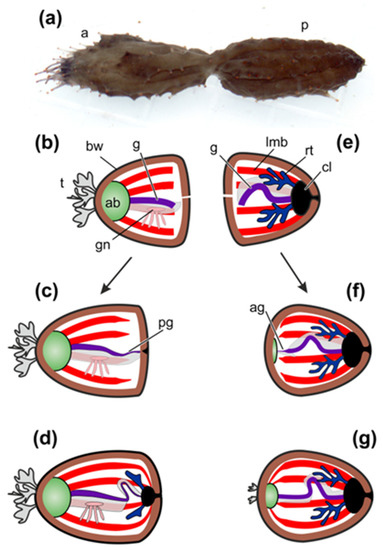
Figure 1. Scheme of regeneration of internal organs after fission in Cladolabes schmeltzii. (a) C. schmeltzii during fission. (b) Anterior fragment just after fission. (c) Formation of gut and cloaca in anterior fragment. (d) Formation of respiratory trees in anterior fragment. (e) Posterior fragment just after fission. (f) Formation of aquapharyngeal bulb (AB) and gut anlage in posterior fragment. (g) Posterior fragment with regenerated internal organs. a, anterior part; ab, aquapharyngeal bulb; ag, anterior anlage of gut; bw, body wall; cl, cloaca; g, gut; gn, gonad; lmb, longitudinal muscle band; p, posterior part; pg, posterior anlage of gut; rt, respiratory tree; t, tentacles.
The first morphological signs of regeneration in both fragments are detected after 5 days post-fission [45][60]. In the anterior fragment of holothurians, the remaining part of the gut becomes slightly shortened, probably due to the destruction of a portion of cells, and its end region is narrowed. A connective-tissue thickening grows out from the gut along the edge of the gut mesentery and gradually extends posteriorly [45]. Simultaneously with this process, enterocytes in the wound zone begin to dedifferentiate and divide mitotically. Intercellular junctions between them are not broken. The luminal epithelium of the gut grows into the connective-tissue thickening (Figure 1c). Subsequently, the anlage gradually extends backwards and reaches the posterior wall of the body, where the cloaca and respiratory trees have already formed by this time (Figure 1d) [45].
In the posterior fragment, regeneration begins with the formation of AB, which develops at the anterior end of the animal [60]. The gut regeneration in the posterior fragment occurs through the transformation of the anterior part of the digestive tube. The anterior end of the gut remnant becomes thinner. It gives rise to a connective-tissue thickening, which grows anteriorly along the mesentery edge and connects to AB (Figure 1f). Enterocytes are dedifferentiated and divide mitotically, but intercellular junctions between them are not destroyed. As a result, the luminal epithelium of the gut grows into the connective-tissue thickening and then into AB. Subsequently, tentacles and organs of the water–vascular system are formed (Figure 1g). Thus, the main regeneration processes in holothurians after fission are ECM remodeling, cell dedifferentiation, cell proliferation, and epithelial morphogenesis.
In regeneration, the significance of ECM remodeling is associated with the fact that most organs, both in holothurians and in all echinoderms, are epithelial formations (mostly tubular), containing a large amount of connective tissue. Accordingly, when organs regenerate after fission, the connective-tissue base (connective-tissue thickening) is formed first, and then certain cells or epithelia migrate into it [45][60]. This pattern of organ formation is typical of regeneration not only after fission, but also after evisceration (see below). For this reason, major attention was paid to the structure of connective tissue and the mechanisms of its modification during asexual reproduction in holothurians [71].
2.2. Structural Components of Connective Tissue
Transcripts of genes of many ECM components that are characteristic of most multicellular animals—collagens, proteoglycans, and glycoproteins—have been found in holothurians [71][72][73][74][75]. On the other hand, the differences in connective tissue of echinoderms and vertebrates have also been identified. In particular, one of the major ECM components in vertebrates is elastin, whose fibers are formed through the polymerization of tropoelastin [76]. Echinoderms lack the tropoelastin gene [71].
Another distinguishing feature of holothurian ECM is the absence of tenascins and fibronectins [77]. These proteins play an important role in the structural integrity of connective tissues in vertebrates [78][79]. In some of holothurians, transcripts have been found that are blasted as tenascin-like proteins [71][72][80]. These contigs encode the domains characteristic of tenascins, EGF, FBG, and TILa. Nevertheless, according to Hynes [81], all these domains are ancient in origin and are found in many animals; however, in the combination characteristic of tenascins, they occur only in chordates. No transcripts of the fibronectin gene were found in C. schmeltzii [71]. Thus, the lack of such proteins as elastin, fibronectin, and tenascin indicates significant differences in the connective tissue organization between echinoderms and chordates.
Due to the lack of elastin, its function in echinoderms is probably performed by fibrillin. In ECM, it forms a network consisting of microfibrils 10–14 nm in diameter that surrounds and penetrates bundles of collagen fibrils [82]. It is suggested that fibrillin microfibrils may be involved in ligament contraction in sea urchins [83]. As has been shown recently, fibrillin microfibrils play an important role in ECM functioning [84]. They are involved in the distribution, accumulation, and modulation of signals from transforming growth factor-β (TGF-β) and bone morphogenetic protein (BMP) that regulate various aspects of cell activity, including ECM formation and remodeling [85]. In addition, fibrillins can bind to integrin receptors and a number of other molecules and, as a result, signals about changes in the extracellular microenvironment are transmitted to cell. In fact, fibrillin microfibrils form niches accumulating various factors [86].
Holothurians have laminins, nidogens, fibulins, agrin, dystroglycan, perlecan, and thrombospondins. The proteins encoded by these genes, along with collagens and fibrillins, are included in the basic set of “basement membrane ECM toolkit”, typical of all Bilateria [87]. The genes are involved in the construction and functioning of connective tissue. For example, fibulins can bind to many ECM components such as fibrillin, and play an important role in stabilizing supramolecular complexes of connective-tissue [88]. Fibulin 1 has been shown to accelerate the disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS)-mediated aggrecan proteolysis and, thus, participate in tissue renewal [89].
Thrombospondins (TSPs) play certain role in the organization of ECM, since they can serve as molecular bridges between various components of connective tissue [90]. They have been shown to interact with MMPs, fibrillar collagen, and TGF-β. TSP-1 and TSP-2 can inhibit the activity of MMP2 [91] and regulate its level in extracellular matrix [92][93]. Thus, TSPs interacts with the molecules that in echinoderms may be involved in mechanisms responsible for modifying ECM during fission and regeneration.
2.3. Proteins Modifying ECM
In addition to the genes of connective-tissue structural components, holothurians have a wide variety of genes encoding ECM-modifying proteins. Many different serine, cysteine, aspartyl, and metal peptidases and their inhibitors have been identified [71][94][95][96][97][98]. In the study of regeneration in holothurians, much attention is paid to MMPs that can degrade collagen [96][97][99][100][101]. In the transcriptomes of holothurians, products of MMPs genes and genes of their inhibitors (TIMPs) have been found [71][72][75][102]. In some of holothurian species, the number of TIMPs can reach 45 [103]. The increase in the number of TIMPs occurred, apparently, due to the increased role of connective tissue in the vital activities of echinoderms such as asexual reproduction, autotomy, and regeneration [71][103].
One of the factors that have an effect on the connective tissue strength in echinoderms is assumed to be tensilin [67][104]. Tensilins are TIMP-like proteins [67][71]. It is worth mentioning that the tensilin gene is found only in species belonging to members of relatively young groups of holothurians and, consequently, have formed within the class Holothuroidea [71]. No similar proteins are found in Apodida (the most ancient order of holothurians [105]), as well as in other echinoderms. This may indicate evolutionary changes in the mechanisms of functioning of ECM that occurred in the phylogeny of echinoderms. Depending on needs, the properties and functions of TIMP-like proteins varied in different classes of Echinodermata. Holothurians differ from other echinoderms by their more developed body wall and the almost complete reduction of skeleton [56]. The increased role of connective tissue was probably accompanied by the modification of ECM remodeling mechanisms and the emergence of a separate group of TIMP-like genes, tensilins.
3. Regeneration after Anterior Evisceration
3.1. Morphological Aspects of Regeneration
The morphological aspects of gut regeneration after anterior evisceration have been described in sufficient detail only from one holothurian species, E. fraudatrix [24][42][43][46][61]. The early post-evisceration stage is approximately the same as in A. japonicus and H. glaberrima (Figure 4a–f). Within the first 3 dpe, the wound at the anterior end of the animal is healed [42], immunity is activated, the cell composition of the coelomic fluid is restored [26][27], and connective-tissue thickenings are formed along the mesentery edge (Figure 4d–f) [42][61]. The main difference from A. japonicus and H. glaberrima is that E. fraudatrix ejects not only the gut, but also the aquapharyngeal bulb (AB) during evisceration. As a result, regeneration begins with the formation of the AB anlage, and then, after 3 dpe, the anterior connective-tissue thickening begins to grow from it along the mesentery edge [42].
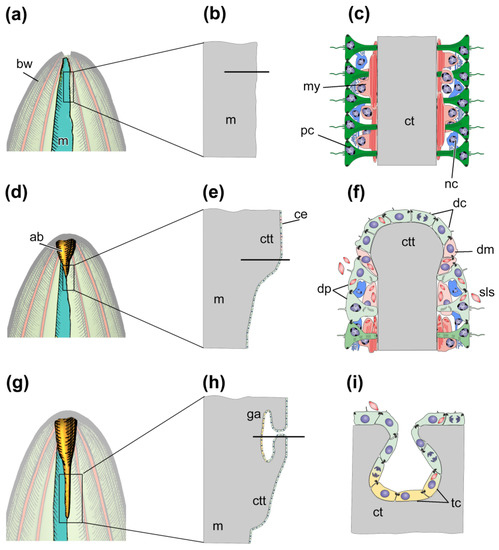
Figure 4. Scheme of regeneration of anterior part of digestive system after anterior evisceration. (a) Anterior part of holothurian just after evisceration; (b) Longitudinal section of the region of the mesentery, which is marked by the rectangle in (a). (c) Transverse section of the region of mesentery which is marked by solid line in (b). (d) Anterior part of holothurian on third day post-evisceration; (e) Longitudinal section of the region of the tissue thickening, which is marked by the rectangle in (d). (f) Transverse section of the region of connective tissue thickening which is marked by solid line in (e). (g) Anterior part of holothurian on seventh day post-evisceration; (h) Longitudinal section of the region of gut anlage, which is marked by the rectangle in (g). (i) Transverse section of the site of embedding of coelomic epithelial cells into connective tissue thickening which is marked by solid line in (h). ab, aquapharyngeal bulb; bw, body wall; ce, coelomic epithelium; ct, connective tissue; ctt, connective tissue thickening; dc, de-differentiated coelomic epithelial cell; dm, de-differentiating myoepithelial cell; dp, de-differentiating peritoneal cell; ga, anterior gut anlage; m, mesentery; my, myoepithelial cell; nc, nerve cell; pc, peritoneal cell; sls, myofilaments grouped into spindle-like structures, tc, coelomic epithelial cell during transdifferentiation.
After 5–7 dpe, the coelomic epithelial cells are embedded into the connective-tissue thickening and begin transdifferentiation (Figure 4g–i) [46]. The embedding occurs in a quite limited area on the ventral side of mesentery, near the AB anlage. The embedded cells divide and gradually migrate anteriorly and posteriorly inside the thickening, forming the luminal epithelium of the anterior gut anlage. The cells do not lose relationships with each other, and intercellular junctions are maintained. Transdifferentiation proceeds quite quickly, and the signs of specialization of enterocytes in luminal epithelium appear after 10 dpe [46]. The posterior anlage forms in the same way as in A. japonicus and H. glaberrima. Subsequently, both anlagen of the gut grow along the mesentery edge towards each other and merge into a single digestive tube within 15–20 dpe.
3.2. Transcriptomic Data
A total of 11 genes of TFs were identified and characterized in E. fraudatrix that showed higher transcriptional activity during transdifferentiation (5–7 dpe) than in the previous and subsequent stages (3 dpe and 10 dpe, respectively) [102]. They belong to 6 TF classes: Tryptophan cluster (Ef-elf), C2H2 zinc finger (Ef-prdm9, Ef-egr1, Ef-klf1/2/4 (klf2), Ef-snai2), bHLH (Ef-tcf24, Ef-msc, Ef-id2), C4 zinc finger (Ef-gata3), polycomb group ring finger (Ef-pcgf2), and T-box (Ef-tbx20). During this period, in addition to the embedding and transdifferentiation of a part of coelomic epithelial cells, the dedifferentiation and proliferation of coelomic epithelial cells on the surface of mesentery and anlage, and also the ECM synthesis and modification continue in the anterior gut anlage of E. fraudatrix. It can be assumed that genes whose expression increases during this period are involved in the regulation of one or more of the above mentioned processes.
The dedifferentiation, proliferation, and maintenance of the undifferentiated state of coelomic epithelial cells can involve Ef-elf, Ef-prdm9, Ef-klf1/2/4, and Ef-egr1. Ef-ELF belongs to the Ets family, whose members are powerful regulators of cell proliferation, angiogenesis, hematopoiesis, tumor transformation, and differentiation [106][107]. Proteins of the PRDM family are important epigenetic regulators of development, cell differentiation, and pluripotency in mice [108]. The KLF proteins play diverse roles in cell proliferation, differentiation, and development [109]. EGR1 is involved in the cell cycle progression of various tumor types, and also in hepatic regeneration in mammals [110][111].
The Ef-gata3, Ef-egr1, and Ef-klf1/2/4 genes may be involved in the regulation of transdifferentiation. The Ef-gata3 gene is of particular interest as its homolog in Caenorhabditis elegans is involved in transdifferentiation [112]. Moreover, it regulates the mesoderm and endoderm specification in echinoderms and mammals [113][114]. In planarians, EGR1 is a “putative pioneer factor to directly activate wound-induced genes” in whole-body regeneration [115]. KLF2 and KLF4 are key TFs that maintain a stem cell-like state and somatic cell reprogramming [116]. It is worth noting that in the holothurians H. glaberrima and A. japonicus the expression of klf1/2/4 does not change during gut regeneration [28][75][117]. The different dynamics of klf1/2/4 expression between these two species and E. fraudatrix confirms presence of different gut regeneration mechanisms in holothurians [58].
Ef-elf and Ef-id2 can be involved in processes associated with the digestive system development and enterocyte differentiation. The expression of sea urchin elf is detected everywhere in late gastrula, being more concentrated in the gut [118]. The id2 gene encodes a protein that, strictly speaking, is not a TF, since it lacks the basic DNA-binding domain. Nevertheless, it plays an important role in the digestive system development in vertebrates by preventing the precocious differentiation of the embryonic intestinal epithelium [119].
As is mentioned above, in the period when transdifferentiation occurs, the dedifferentiation of myoepithelial cells continues on the surface of the gut anlage. Among the identified TFs, Ef-msc and Ef-tbx20 may probably be involved in the regulation of this process. In mammals, MSC (musculin) is a lineage-restricted repressor of embryonic skeletal muscle development [120]. TBX20 is a crucial cardiogenic TF in mammals [121]. The tbx20 gene is also expressed in the nervous system and embryonic lateral mesoderm in mice [121][122].
Homologs of three TFs, Ef-pcgf, Ef-snai2, and Ef-id2, in vertebrates are involved in the mechanisms of the epithelial–mesenchymal transition (EMT). PCGF2 is a chromatin-modifying protein that inhibits EMT and negatively regulates stem cell-like properties [123][124]. SNAI proteins play a crucial role in EMT and in repressing the mesenchymal-epithelial transition, being important in embryonic and tumor development [125][126][127]. On the other hand, a complex of SNAI and ID2 inhibits EMT [128][129]. Co-expression of Ef-snai2 and Ef-id2 may indicate a partial involvement of the EMT mechanisms during the gut regeneration in E. fraudatrix. It is probable that, while embedding in the connective-tissue thickening, coelomic epithelial cells acquire some mesenchymal features [130][131][132].
Gene tcf24 was first described in 2002 from humans as a paralog of the tcf23 [133]. Since then, no information about its functions has been obtained. Activation of Ef-tcf24 during regeneration in holothurians is the first description of its involvement in regeneration. The increase in the Ef-tcf24 expression after 5-7 dpe in E. fraudatrix suggests participation of this gene in gut regeneration in holothurians.
Moreover, genes of Cyclins, Cdcs, Retinoic acid receptors and some other proteins participating in retinoic acid anabolism, are up-regulated during regeneration of anterior gut anlage in E. fraudatrix [102].
3.3. Wnt Signaling Pathway
The Wnt signaling pathway is activated in the regeneration of the organs of the anterior part of the body in E. fraudatrix (AB and the anterior part of the gut). Gene transcripts of four ligands (wntA, wnt4, wnt6, and wnt16) and three receptors (frizzled1/2/7, frizzled4, and frizzled5/8) have been identified in the AB and gut anlagen [134]. All of them exhibit different expression dynamics. According to preliminary data, transcripts of the studied genes are localized in coelomic epithelium and organs of the water–vascular system of forming AB [135].
The increase in the wnt16 expression occurs earliest of all, after 3 dpe. In vertebrates, the wnt16 expression is often associated with the development or regeneration of connective-tissue structures, the transformation of the extracellular matrix, and the activation of metalloproteinases [136][137][138]. In E. fraudatrix, an increase in the activity of MMPs and ECM remodeling is observed in this period [97].
After 5-7 dpe, the expression of wntA, wnt4, wnt6, frizzled1/2/7, and frizzled4 increases abruptly and reaches its maximum. At this time, the main structures of AB and luminal epithelium are formed [42][46]. It is probable that Wnt signaling, performed through these ligands and receptors, influences the regeneration of the canals of the AB water–vascular system (water–vascular ring and radial water–vascular canals), and is also involved in the regulation of transdifferentiation.
The following regeneration period (10 dpe) is characterized by either reduced or insignificantly changed expression of most of the studied genes. The exception is frizzled5/8, whose number of transcripts reaches its maximum. Subsequently, after 14 and 20 dpe, changes in the expression of all Wnt and frizzled genes are minimal. It is worth mentioning that the dynamics of frizzled1/2/7 differ between E. fraudatrix and A. japonicus [139][134]. This may be explained by different functions of pathways which activated through the receptor Frizzled1/2/7.
Furthermore, the Wnt5 expression is revealed during the regeneration of the anterior structures in E. fraudatrix [140]. Unlike other ligands, Wnt5-positive cells were localized mainly in the connective tissue of the AB anlage and regenerating nerve cords. The number of such cells gradually increases after evisceration, reaching a maximum after 10-14 dpe, and then drops to the control values. The probable function of Wnt5 is the involvement in the regulation of ECM remodeling and nervous system regeneration. Wnt5 is known to be a regulator of neuronal organization and growth in planarians [141]. In mammals, Wnt5A is involved in the activation of MMPs during the development of skeletal elements [142].
References
- Bluhm, H.; Gebruk, A. Holothuroidea (Echinodermata) of the Peru Basin—Ecological and taxonomic remarks based on underwater images. Mar. Ecol. 1999, 20, 167–195.
- Solan, M.; Germano, J.D.; Rhoads, D.C.; Smith, C.; Michaud, E.; Parry, D.; Wenzhofer, F.; Kennedy, B.; Henriques, C.; Battle, E.; et al. Towards a greater understanding of pattern, scale and process in marine benthic systems: A picture is worth a thousand worms. J. Exp. Mar. Biol. Ecol. 2003, 285, 313–338.
- Ohta, S. Photographic observations of the deep sea pelagothuriid holothurian Enypniastes (Elasipoda, Holothurioidea). J. Oceanogr. Soc. Jpn. 1985, 41, 121–133.
- Conand, C. Population status, fisheries and trade of sea cucumbers in Africa and the Indian Ocean. In Sea Cucumbers. A Global Review of Fisheries and Trade. FAO Fisheries and Aquaculture Technical Paper. No. 516; Toral-Granda, V., Lovatelli, A., Vasconcellos, M., Eds.; FAO: Rome, Italy, 2008; pp. 143–193.
- Uthicke, S.; Byrne, M.; Conand, C. Genetic barcoding of commercial Bêche-de-mer species (Echinodermata: Holothuroidea). Mol. Ecol. Resour. 2010, 10, 634–646.
- Candia Carnevali, M.D. Regeneration in echinoderms: Repair, regrowth, cloning. Invertebr. Surv. J. 2006, 3, 64–76.
- Dolmatov, I.Y. Regeneration in echinoderms. Russ. J. Mar. Biol. 1999, 25, 225–233.
- Dolmatov, I.Y. New data on asexual reproduction, autotomy, and regeneration in holothurians of the order Dendrochirotida. Russ. J. Mar. Biol. 2014, 40, 228–232.
- Dolmatov, I.Y.; Ginanova, T.T. Muscle regeneration in holothurians. Microsc. Res. Tech. 2001, 55, 452–463.
- Dolmatov, I.Y.; Ginanova, T.T. Post-autotomy regeneration of the respiratory trees in the holothurian Apostichopus japonicus (Holothurioidea, Aspidochirotida). Cell Tissue Res. 2009, 336, 41–58.
- Kille, F.R. Regeneration of the reproductive system following binary fission in the sea cucumber Holothuria parvula. Biol. Bull. 1942, 83, 55–66.
- Mashanov, V.S.; García-Arrarás, J.E. Gut regeneration in holothurians: A snapshot of recent developments. Biol. Bull. 2011, 221, 93–109.
- Hyman, L.H. The Invertebrates: Echinodermata. The Coelome Bilateria; McGraw-Hill Book Co.: New York, NY, USA, 1955; p. 763.
- Monticelli, F.S. Sull’ autotomia delle Cucumaria planci (Br.). Atti Accad. Naz. Lincei Cl. Sci. Fis. Mat. Nat. Rendi. 1896, 5, 231–239.
- Torelle, E. Regeneration in holothuria. Zool. Anz. 1910, 35, 15–22.
- Reichenbach, N.; Holloway, S. Potential for asexual propagation of several commercially important species of tropical sea cucumbers (Echinodermata). J. World Aquac. Soc. 1995, 26, 272–278.
- Reichenbach, N.; Nishar, Y.; Saeed, A. Species and size-related trends in asexual propagation of commercially important species of tropical sea cucumbers (Holothuroidea). J. World Aquac. Soc. 1996, 27, 475–482.
- Bertolini, F. Rigenerazione dell’apparato digerente nello Holothuria. Pubbl. Staz. Zool. Napoli 1932, 12, 432–443.
- Byrne, M. Evisceration behavior and the seasonal incidence of evisceration in the holothurian Eupentacta quinquesemita (Selenka). Ophelia 1985, 24, 75–90.
- Swan, E.F. Seasonal Evisceration in the sea cucumber, Parastichopus californicus (Stimpson). Science 1961, 133, 1078–1079.
- Emson, R.H.; Mladenov, P.V. Studies of the fissiparous holothurian Holothuria parvula (Selenka) (Echinodermata, Holothuroidea). J. Exp. Mar. Biol. Ecol. 1987, 111, 195–211.
- Emson, R.H.; Wilkie, I.C. Fission and autotomy in echinoderms. Oceanogr. Mar. Biol. Annu. Rev. 1980, 18, 155–250.
- Dolmatov, I.Y.; Nguyen, A.K.; Kamenev, Y.O. Asexual reproduction, evisceration, and regeneration in holothurians (Holothuroidea) from Nha Trang Bay of the South China Sea. Russ. J. Mar. Biol. 2012, 38, 243–252.
- Dolmatov, I.Y.; Mashanov, V.S. Regeneration in Holothurians; Dalnauka: Vladivostok, Russia, 2007; p. 212.
- Vogt, G. Hidden treasures in stem cells of indeterminately growing bilaterian invertebrates. Stem Cell Rev. Rep. 2012, 8, 305–317.
- Eliseikina, M.G.; Magarlamov, T.Y.; Dolmatov, I.Y. Stem cells of holothuroid coelomocytes. In Echinoderms: Durham; Harris, L.G., Bottger, S.A., Walker, C.W., Lesser, M.P., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 163–166.
- Zavalnaya, E.G.; Shamshurina, E.V.; Eliseikina, M.G. The Immunocytochemical Identification of PIWI-positive cells during the recovery of a coelomocyte population after evisceration in the holothurian Eupentacta fraudatrix (Djakonov et Baranova, 1958) (Holothuroidea: Dendrochirota). Russ. J. Mar. Biol. 2020, 46, 97–104.
- Mashanov, V.S.; Zueva, O.R.; Garcia-Arraras, J.E. Expression of pluripotency factors in echinoderm regeneration. Cell Tissue Res. 2015, 359, 521–536.
- Mashanov, V.; Zueva, O.; Mashanova, D.; García-Arrarás, J.E. Expression of stem cell factors in the adult sea cucumber digestive tube. Cell Tissue Res. 2017, 370, 427–440.
- Mashanov, V.S.; Zueva, O.R.; García-Arrarás, J.E. Transcriptomic changes during regeneration of the central nervous system in an echinoderm. BMC Genom. 2014, 15, 357.
- Maki, N.; Suetsugu-Maki, R.; Sano, S.; Nakamura, K.; Nishimura, O.; Tarui, H.; Del Rio-Tsonis, K.; Ohsumi, K.; Agata, K.; Tsonis, P.A. Oocyte-type linker histone B4 is required for transdifferentiation of somatic cells in vivo. FASEB J. 2010, 24, 3462–3467.
- Maki, N.; Suetsugu-Maki, R.; Tarui, H.; Agata, K.; Del Rio-Tsonis, K.; Tsonis, P.A. Expression of stem cell pluripotency factors during regeneration in newts. Dev. Dyn. 2009, 238, 1613–1616.
- Zhu, W.; Pao, G.M.; Satoh, A.; Cummings, G.; Monaghan, J.R.; Harkins, T.T.; Bryant, S.V.; Randal Voss, S.; Gardiner, D.M.; Hunter, T. Activation of germline-specific genes is required for limb regeneration in the Mexican axolotl. Dev. Biol. 2012, 370, 42–51.
- Dolmatov, I.Y.; Eliseikina, M.G.; Bulgakov, A.A.; Ginanova, T.T.; Lamash, N.E.; Korchagin, V.P. Muscle regeneration in the holothurian Stichopus japonicus. Roux Arch. Dev. Biol. 1996, 205, 486–493.
- Frolova, L.T.; Dolmatov, I.Y. Microscopic anatomy of the digestive system in normal and regenerating specimens of the brittlestar Amphipholis kochii. Biol. Bull. 2010, 218, 303–316.
- García-Arrarás, J.E.; Dolmatov, I.Y. Echinoderms: Potential model systems for studies on muscle regeneration. Curr. Pharm. Des. 2010, 16, 942–955.
- García-Arrarás, J.E.; Estrada-Rodgers, L.; Santiago, R.; Torres, I.I.; Díaz-Miranda, L.; Torres-Avillán, I. Cellular mechanisms in the regeneration of the intestine of the sea cucumber, Holothuria glaberrima Selenka (Holothuroidea: Echinodermata). J. Exp. Zool. 1998, 281, 288–304.
- Mladenov, P.V.; Bisgrove, B.; Asotra, S.; Burke, R.D. Mechanisms of arm tip regeneration in the sea star, Leptasterias hexactis. Roux Arch. Dev. Biol. 1989, 198, 19–28.
- Mashanov, V.S.; Zueva, O.R.; Heinzeller, T. Regeneration of the radial nerve cord in a holothurian: A promising new model system for studying post-traumatic recovery in the adult nervous system. Tissue Cell 2008, 40, 351–372.
- Mashanov, V.S.; Frolova, L.T.; Dolmatov, I.Y. Structure of the digestive tube in the holothurian Eupentacta fraudatrix (Holothuroidea, Dendrochirota). Russ. J. Mar. Biol. 2004, 30, 314–322.
- Shukalyuk, A.I.; Dolmatov, I.Y. Regeneration of the digestive tube in the holothurian Apostichopus japonicus after evisceration. Russ. J. Mar. Biol. 2001, 27, 168–173.
- Dolmatov, I.Y. Regeneration of the aquapharyngeal complex in the holothurian Eupentacta fraudatrix (Holothuroidea, Dendrochirota). In Keys for Regeneration. Monographs in Developmental Biology; Taban, C.H., Boilly, B., Eds.; Karger: Basel, Switzerland, 1992; Volume 23, pp. 40–50.
- Dolmatov, I.Y. Proliferation of tissues of regenerating aquapharyngeal complex in holothurians. Russ. J. Dev. Biol. 1993, 24, 72–81.
- Kalacheva, N.V.; Eliseikina, M.G.; Frolova, L.T.; Dolmatov, I.Y. Regeneration of the digestive system in the crinoid Himerometra robustipinna occurs by transdifferentiation of neurosecretory-like cells. PLoS ONE 2017, 12, e0182001.
- Kamenev, Y.O.; Dolmatov, I.Y. Posterior regeneration following fission in the holothurian Cladolabes schmeltzii (Dendrochirotida: Holothuroidea). Microsc. Res. Tech. 2015, 78, 540–552.
- Mashanov, V.S.; Dolmatov, I.Y.; Heinzeller, T. Transdifferentiation in holothurian gut regeneration. Biol. Bull. 2005, 209, 184–193.
- Kalacheva, N.V.; Dolmatov, I.Y. Cellular source of digestive system regeneration in Lamprometra palmata and Anneissia bennetti. In Proceedings of the Abstracts of 10th European Conference on Echinoderms, Borissiak Paleontological Institute RAS, Moscow, Russia, 16‒19 September 2019; p. 42.
- Mozzi, D.; Dolmatov, I.Y.; Bonasoro, F.; Candia Carnevali, M.D. Visceral regeneration in the crinoid Antedon mediterranea: Basic mechanisms, tissues and cells involved in gut regrowth. Centr. Eur. J. Biol. 2006, 1, 609–635.
- Dolmatov, I.Y. Development and evolution of the muscle system in the Echinodermata. In Echinoderms: Durham; Harris, L.G., Bottger, S.A., Walker, C.W., Lesser, M.P., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 163–166.
- Dolmatov, I.Y.; Ivantey, V.A. Histogenesis of longitudinal muscle bands in holothurians. Russ. J. Dev. Biol. 1993, 24, 67–72.
- Dolmatov, I.Y.; Mashanov, V.S.; Zueva, O.R. Derivation of muscles of the Aristotle’s lantern from coelomic epithelia. Cell Tissue Res. 2007, 327, 371–384.
- Candelaria, A.G.; Murray, G.; File, S.K.; García-Arrarás, J.E. Contribution of mesenterial muscle dedifferentiation to intestine regeneration in the sea cucumber Holothuria glaberrima. Cell Tissue Res. 2006, 325, 55–65.
- García-Arrarás, J.E.; Bello, S.A.; Malavez, S. The mesentery as the epicenter for intestinal regeneration. Semin. Cell Dev. Biol. 2019, 92, 45–54.
- Murray, G.; García-Arrarás, J.E. Myogenesis during holothurian intestinal regeneration. Cell Tissue Res. 2004, 318, 515–524.
- Ariza, L.; Carmona, R.; Cañete Sánchez, A.; Cano, E.; Muñoz-Chápuli, R. Coelomic epithelium-derived cells in visceral morphogenesis. Dev. Dyn. 2015, 245, 307–322.
- Smiley, S. Holothuroidea. In Microscopic Anatomy of Invertebrates; Harrison, F.W., Chia, F.S., Eds.; Wiley-Liss Inc.: New York, NY, USA, 1994; Volume 14, pp. 401–471.
- Smiley, S.; McEuen, F.S.; Chaffee, C.; Krishnan, S. Echinodermata: Holothuroidea. In Reproduction of Marine Invertebrates; Giese, A.C., Pearse, J.S., Pearse, V.B., Eds.; Boxwood: Pacific Grove, CA, USA, 1991; Volume 6, pp. 633–750.
- Dolmatov, I.Y. Variability of regeneration mechanisms in echinoderms. Russ. J. Mar. Biol. 2020, 46, 391–404.
- Dolmatov, I.Y. Asexual reproduction in holothurians. Sci. World J. 2014, 2014, 13.
- Kamenev, Y.O.; Dolmatov, I.Y. Anterior regeneration after fission in the holothurian Cladolabes schmeltzii (Dendrochirotida: Holothuroidea). Microsc. Res. Tech. 2017, 80, 183–194.
- Leibson, N.L. Regeneration of digestive tube in holothurians Stichopus japonicus and Eupentacta fraudatrix. In Keys for Regeneration. Monographs in Developmental Biology; Taban, C.H., Boilly, B., Eds.; Karger: Basel, Switzerland, 1992; Volume 23, pp. 51–61.
- Wang, X.; Li, X. The morphological and histological observation of regeneration of alimentary tract in sea cucumber Apostichopus japonicus. J. Dalian Fish. Univ. 2007, 22, 340–346.
- Mladenov, P.V.; Burke, R.D. Echinodermata: Asexual propagation. In Reproductive Biology of Invertebrates. Asexual Propagation and Reproductive Strategies; Adiyodi, K.G., Adiyodi, R.G., Eds.; Oxford and IBH Publishing Co. PVT. LTD.: New Delhi, India; Bombay, India; Calcutta, India, 1994; Volume 6, pp. 339–383.
- Motokawa, T.; Sato, E.; Umeyama, K. Energy expenditure associated with softening and stiffening of echinoderm connective tissue. Biol. Bull. 2012, 222, 150–157.
- Motokawa, T.; Tsuchi, A. Dynamic mechanical properties of body-wall dermis in various mechanical states and their implications for the behavior of sea cucumbers. Biol. Bull. 2003, 205, 261–275.
- Wilkie, I.C. Autotomy as a prelude to regeneration in echinoderms. Microsc. Res. Tech. 2001, 55, 369–396.
- Wilkie, I.C. Mutable collagenous tissue: Overview and biotechnological perspective. In Progress in Molecular and Subcellular Biology. Subseries Marine Molecular Biotechnology; Matranga, V., Ed.; Springer: Heidelberg, Germany, 2005; pp. 221–250.
- Wilkie, I.C. Variable tensility in echinoderm collagenous tissues: A review. Mar. Behav. Physiol. 1984, 11, 1–34.
- Motokawa, T. Connective tissue catch in echinoderms. Biol. Rev. 1984, 59, 255–270.
- Ribeiro, A.R.; Barbaglio, A.; Oliveira, M.J.; Ribeiro, C.C.; Wilkie, I.C.; Candia Carnevali, M.D.; Barbosa, M.A. Matrix metalloproteinases in a sea urchin ligament with adaptable mechanical properties. PLoS ONE 2012, 7, e49016.
- Dolmatov, I.Y.; Afanasyev, S.V.; Boyko, A.V. Molecular mechanisms of fission in echinoderms: Transcriptome analysis. PLoS ONE 2018, 13, e0195836.
- Ortiz-Pineda, P.A.; Ramírez-Gómez, F.; Pérez-Ortiz, J.; González-Díaz, S.; Jesús, F.S.; Hernández-Pasos, J.; Valle-Avila, C.D.; Rojas-Cartagena, C.; Suárez-Castillo, E.C.; Tossas, K.; et al. Gene expression profiling of intestinal regeneration in the sea cucumber. BMC Genom. 2009, 10, 262.
- Rojas-Cartagena, C.; Ortiz-Pineda, P.; Ramirez-Gomez, F.; Suarez-Castillo, E.C.; Matos-Cruz, V.; Rodriguez, C.; Ortiz-Zuazaga, H.; Garcia-Arraras, J.E. Distinct profiles of expressed sequence tags during intestinal regeneration in the sea cucumber Holothuria glaberrima. Physiol. Genom. 2007, 31, 203–215.
- Sun, L.; Chen, M.; Yang, H.; Wang, T.; Liu, B.; Shu, C.; Gardiner, D.M. Large scale gene expression profiling during intestine and body wall regeneration in the sea cucumber Apostichopus japonicus. Comp. Biochem. Physiol. Part D Genom. Proteom. 2011, 6, 195–205.
- Sun, L.; Yang, H.; Chen, M.; Ma, D.; Lin, C. RNA-Seq reveals dynamic changes of gene expression in key stages of intestine regeneration in the sea cucumber Apostichopus japonicus. PLoS ONE 2013, 8, e69441.
- Hynes, R.O.; Naba, A. Overview of the matrisome—An inventory of extracellular matrix constituents and functions. Cold Spring Harb. Perspect. Biol. 2012, 4, a004903.
- Tucker, R.P.; Chiquet-Ehrismann, R. Evidence for the evolution of tenascin and fibronectin early in the chordate lineage. Int. J. Biochem. Cell Biol. 2009, 41, 424–434.
- Chiquet-Ehrismann, R.; Tucker, R.P. Tenascins and the importance of adhesion modulation. Cold Spring Harb. Perspect. Biol. 2011, 3.
- Schwarzbauer, J.E.; DeSimone, D.W. Fibronectins, their fibrillogenesis, and in vivo functions. Cold Spring Harb. Perspect. Biol. 2011, 3.
- Ba, H.; Yao, F.; Yang, L.; Qin, T.; Luan, H.; Li, Z.; Zou, X.; Hou, L. Identification and expression patterns of extracellular matrix-associated genes fibropellin-ia and tenascin involved in regeneration of sea cucumber Apostichopus japonicus. Gene 2015, 565, 96–105.
- Hynes, R.O. The evolution of metazoan extracellular matrix. J. Cell Biol. 2012, 196, 671–679.
- Thurmond, F.A.; Koob, T.J.; Bowness, J.M.; Trotter, J.A. Partial biochemical and immunologic characterization of fibrillin microfibrils from sea cucumber dermis. Connect. Tissue Res. 1997, 36, 211–222.
- Ribeiro, A.R.; Barbaglio, A.; Benedetto, C.D.; Ribeiro, C.C.; Wilkie, I.C.; Carnevali, M.D.C.; Barbosa, M.A. New insights into mutable collagenous tissue: Correlations between the microstructure and mechanical state of a sea-urchin ligament. PLoS ONE 2011, 6, e24822.
- Giusti, B.; Pepe, G. Fibrillins in tendon. Front. Aging Neurosci. 2016, 8, 237.
- Olivieri, J.; Smaldone, S.; Ramirez, F. Fibrillin assemblies: Extracellular determinants of tissue formation and fibrosis. Fibrogenesis Tissue Repair. 2010, 3, 24.
- Sengle, G.; Sakai, L.Y. The fibrillin microfibril scaffold: A niche for growth factors and mechanosensation? Matrix Biol. 2015, 47, 3–12.
- Whittaker, C.A.; Bergeron, K.-F.; Whittle, J.; Brandhorst, B.P.; Burke, R.D.; Hynes, R.O. The echinoderm adhesome. Dev. Biol. 2006, 300, 252–266.
- de Vega, S.; Iwamoto, T.; Yamada, Y. Fibulins: Multiple roles in matrix structures and tissue functions. Cell. Mol. Life Sci. 2009, 66, 1890–1902.
- Lee, N.V.; Rodriguez-Manzaneque, J.C.; Thai, S.N.-M.; Twal, W.O.; Luque, A.; Lyons, K.M.; Argraves, W.S.; Iruela-Arispe, M.L. Fibulin-1 acts as a cofactor for the matrix metalloprotease ADAMTS-1. J. Biol. Chem. 2005, 280, 34796–34804.
- Adams, J.C.; Lawler, J. The thrombospondins. Cold Spring Harb. Perspect. Biol. 2011, 3, a009712.
- Bein, K.; Simons, M. Thrombospondin type 1 repeats interact with matrix metalloproteinase 2: Regulation of metalloproteinase activity. J. Biol. Chem. 2000, 275, 32167–32173.
- Yang, Z.; Kyriakides, T.R.; Bornstein, P. Matricellular proteins as modulators of cell-matrix interactions: Adhesive defect in thrombospondin 2-null fibroblasts is a consequence of increased levels of matrix metalloproteinase-2. Mol. Biol. Cell. 2000, 11, 3353–3364.
- Yang, Z.; Strickland, D.K.; Bornstein, P. Extracellular matrix metalloproteinase 2 levels are regulated by the low density lipoprotein-related scavenger receptor and Thrombospondin 2. J. Biol. Chem. 2001, 276, 8403–8408.
- Pasten, C.; Rosa, R.; Ortiz, S.; Gonzalez, S.; García-Arrarás, J.E. Characterization of proteolytic activities during intestinal regeneration of the sea cucumber, Holothuria glaberrima. Int. J. Dev. Biol. 2012, 56, 681–691.
- Yuan, Z.; Dahms, H.U.; Han, L.L.; Li, Q.Y.; Zhang, Q.Z.; Wu, R.J.; Tan, J.; Zou, X.Y.; Hou, L. Cloning and characterization of a trypsin-like serine protease gene, a novel regeneration-related gene from Apostichopus japonicus. Gene 2012, 502, 46–52.
- Miao, T.; Wan, Z.; Sun, L.; Li, X.; Xing, L.; Bai, Y.; Wang, F.; Yang, H. Extracellular matrix remodeling and matrix metalloproteinases (ajMMP-2 like and ajMMP-16 like) characterization during intestine regeneration of sea cucumber Apostichopus japonicus. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2017, 212, 12–23.
- Lamash, N.E.; Dolmatov, I.Y. Proteases from the regenerating gut of the holothurian Eupentacta fraudatrix. PLoS ONE 2013, 8, e58433.
- Shulga, A.P.; Lamash, N.E. Proteinases with gelatinase activity and their role in ambulacrum regeneration in holothurians Eupentacta fraudatrix (D’yakonov and Baranova, 1958) and Cucumaria japonica (Semper, 1868) (Echinodermata: Holothuroidea). Russ. J. Mar. Biol. 2020, 46, 461–471.
- Dolmatov, I.Y.; Shulga, A.P.; Ginanova, T.T.; Eliseikina, M.G.; Lamash, N.E. Metalloproteinase inhibitor GM6001 delays regeneration in holothurians. Tissue Cell 2019, 59, 1–9.
- Quiñones, J.L.; Rosa, R.; Ruiz, D.L.; García-Arrarás, J.E. Extracellular matrix remodeling and metalloproteinase involvement during intestine regeneration in the sea cucumber Holothuria glaberrima. Dev. Biol. 2002, 250, 181–197.
- Dolmatov, I.Y.; Kalacheva, N.V.; Shulga, A.P.; Tkacheva, E.S.; Boyko, A.V.; Girich, A.S. Expression of MMP, TIMP, and Sox genes during regeneration in holothurian Eupentacta fraudatrix. Genes 2021, in press.
- Boyko, A.V.; Girich, A.S.; Tkacheva, E.S.; Dolmatov, I.Y. The Eupentacta fraudatrix transcriptome provides insights into regulation of cell transdifferentiation. Sci. Rep. 2020, 10, 1522.
- Clouse, R.M.; Linchangco, G.V.; Kerr, A.M.; Reid, R.W.; Janies, D.A. Phylotranscriptomic analysis uncovers a wealth of tissue inhibitor of metalloproteinases variants in echinoderms. R. Soc. Open Sci. 2015, 2, 150377.
- Tipper, J.P.; Lyons-Levy, G.; Atkinson, M.A.; Trotter, J.A. Purification, characterization and cloning of tensilin, the collagen-fibril binding and tissue-stiffening factor from Cucumaria frondosa dermis. Matrix Biol. 2002, 21, 625–635.
- Miller, A.K.; Kerr, A.M.; Paulay, G.; Reich, M.; Wilson, N.G.; Carvajal, J.I.; Rouse, G.W. Molecular phylogeny of extant Holothuroidea (Echinodermata). Mol. Phylogenet. Evol. 2017, 111, 110–131.
- Hsu, T.; Trojanowska, M.; Watson, D.K. Ets proteins in biological control and cancer. J. Cell. Biochem. 2004, 91, 896–903.
- Oikawa, T.; Yamada, T. Molecular biology of the Ets family of transcription factors. Gene 2003, 303, 11–34.
- Vervoort, M.; Meulemeester, D.; Béhague, J.; Kerner, P. Evolution of Prdm genes in animals: Insights from comparative. Genom. Mol. Biol. Evol. 2016, 33, 679–696.
- Ilsley, M.D.; Gillinder, K.R.; Magor, G.W.; Huang, S.; Bailey, T.L.; Crossley, M.; Perkins, A.C. Krüppel-like factors compete for promoters and enhancers to fine-tune transcription. Nucleic Acids Res. 2017, 45, 6572–6588.
- Lai, S.; Yuan, J.; Zhao, D.; Shen, N.; Chen, W.; Ding, Y.; Yu, D.; Li, J.; Pan, F.; Zhu, M.; et al. Regulation of mice liver regeneration by early growth response-1 through the GGPPS/RAS/MAPK pathway. Int. J. Biochem. Cell Biol. 2015, 64, 147–154.
- Yan, L.; Wang, Y.; Liang, J.; Liu, Z.; Sun, X.; Cai, K. MiR-301b promotes the proliferation, mobility, and epithelial-to-mesenchymal transition of bladder cancer cells by targeting EGR1. Biochem. Cell Biol. 2017, 95, 571–577.
- Riddle, M.R.; Weintraub, A.; Nguyen, K.C.; Hall, D.H.; Rothman, J.H. Transdifferentiation and remodeling of post-embryonic C. elegans cells by a single transcription factor. Development 2013, 140, 4844–4849.
- Materna, S.C.; Ransick, A.; Li, E.; Davidson, E.H. Diversification of oral and aboral mesodermal regulatory states in pregastrular sea urchin embryos. Dev. Biol. 2013, 375, 92–104.
- Lentjes, M.H.; Niessen, H.E.; Akiyama, Y.; de Bruïne, A.P.; Melotte, V.; van Engeland, M. The emerging role of GATA transcription factors in development and disease. Expert Rev. Mol. Med. 2016, 18, e3.
- Gehrke, A.R.; Neverett, E.; Luo, Y.-J.; Brandt, A.; Ricci, L.; Hulett, R.E.; Gompers, A.; Ruby, J.G.; Rokhsar, D.S.; Reddien, P.W.; et al. Acoel genome reveals the regulatory landscape of whole-body regeneration. Science 2019, 363, eaau6173.
- Yamane, M.; Ohtsuka, S.; Matsuura, K.; Nakamura, A.; Niwa, H. Overlapping functions of Krüppel-like factor family members: Targeting multiple transcription factors to maintain the naïve pluripotency of mouse embryonic stem cells. Development 2018, 145, dev162404.
- Quispe-Parra, D.J.; Medina-Feliciano, J.G.; Cruz-González, S.; Ortiz-Zuazaga, H.; García-Arrarás, J.E. Transcriptomic analysis of early stages of intestinal regeneration in Holothuria glaberrima. Sci. Rep. 2021, 11, 346.
- Rizzo, F.; Fernandez-Serra, M.; Squarzoni, P.; Archimandritis, A.; Arnone, M.I. Identification and developmental expression of the ets gene family in the sea urchin (Strongylocentrotus purpuratus). Dev. Biol. 2006, 300, 35–48.
- Nigmatullina, L.; Norkin, M.; Dzama, M.M.; Messner, B.; Sayols, S.; Soshnikova, N. Id2 controls specification of Lgr5+ intestinal stem cell progenitors during gut development. EMBO J. 2017, 36, 869–885.
- Hishikawa, K.; Marumo, T.; Miura, S.; Nakanishi, A.; Matsuzaki, Y.; Shibata, K.; Ichiyanagi, T.; Kohike, H.; Komori, T.; Takahashi, I.; et al. Musculin/MyoR is expressed in kidney side population cells and can regulate their function. J. Cell Biol. 2005, 169, 921–928.
- DeBenedittis, P.; Jiao, K. Alternative splicing of T-box transcription factor genes. Biochem. Biophys. Res. Commun. 2011, 412, 513–517.
- Takashima, Y.; Suzuki, A. Regulation of organogenesis and stem cell properties by T-box transcription factors. Cell. Mol. Life Sci. 2013, 70, 3929–3945.
- Lee, J.Y.; Park, M.K.; Park, J.H.; Lee, H.J.; Shin, D.H.; Kang, Y.; Lee, C.H.; Kong, G. Loss of the polycomb protein Mel-18 enhances the epithelial–mesenchymal transition by ZEB1 and ZEB2 expression through the downregulation of miR-205 in breast cancer. Oncogene 2014, 33, 1325–1335.
- Wang, X.F.; Zhang, X.W.; Hua, R.X.; Du, Y.Q.; Huang, M.Z.; Liu, Y.; Cheng, Y.F.; Guo, W.J. Mel-18 negatively regulates stem cell-like properties through downregulation of miR-21 in gastric cancer. Oncotarget 2016, 7, 63352–63361.
- Kalinkova, L.; Zmetakova, I.; Smolkova, B.; Minarik, G.; Sedlackova, T.; Horvathova Kajabova, V.; Cierna, Z.; Mego, M.; Fridrichova, I. Decreased methylation in the SNAI2 and ADAM23 genes associated with de-differentiation and haematogenous dissemination in breast cancers. BMC Cancer 2018, 18, 875.
- Zhou, Y.; Liu, Q.; Dai, X.; Yan, Y.; Yang, Y.; Li, H.; Zhou, X.; Gao, W.; Li, X.; Xi, Z. Transdifferentiation of type II alveolar epithelial cells induces reactivation of dormant tumor cells by enhancing TGF-β1/SNAI2 signaling. Onco. Rep. 2018, 39, 1874–1882.
- Chen, Y.; Wang, K.; Qian, C.N.; Leach, R. DNA methylation is associated with transcription of Snail and Slug genes. Biochem. Biophys. Res. Commun. 2013, 430, 1083–1090.
- Kamata, Y.U.; Sumida, T.; Kobayashi, Y.; Ishikawa, A.; Kumamaru, W.; Mori, Y. Introduction of ID2 enhances invasiveness in ID2-null oral squamous cell carcinoma cells via the SNAIL axis. Cancer Genom. Proteom. 2016, 13, 493–497.
- Chang, C.; Yang, X.; Pursell, B.; Mercurio, A. Id2 complexes with the SNAG domain of Snai1 inhibiting Snai1-mediated repression of integrin 4. Mol. Cell. Biol. 2013, 33.
- Radice, G.P. The spreading of epithelial cells during wound closure in Xenopus larvae. Dev. Biol. 1980, 76, 26–46.
- Schöck, F.; Perrimon, N. Molecular mechanisms of epithelial morphogenesis. Annu. Rev. Cell Dev. Biol. 2002, 18, 463–493.
- Schöck, F.; Perrimon, N. Cellular processes associated with germ band retraction in Drosophila. Dev. Biol. 2002, 248, 29–39.
- McLellan, A.S.; Langlands, K.; Kealey, T. Exhaustive identification of human class II basic helix–loop–helix proteins by virtual library screening. Mech. Dev. 2002, 119, S285–S291.
- Girich, A.S.; Isaeva, M.P.; Dolmatov, I.Y. Wnt and frizzled expression during regeneration of internal organs in the holothurian Eupentacta fraudatrix. Wound Repair Regen. 2017, 25, 828–835.
- Girich, A.S. (NSCMB FEB RAS, Vladivostok, Russia). Personal communication, 2021.
- Witte, F.; Dokas, J.; Neuendorf, F.; Mundlos, S.; Stricker, S. Comprehensive expression analysis of all Wnt genes and their major secreted antagonists during mouse limb development and cartilage differentiation. Gene Expr. Patterns 2009, 9, 215–223.
- Rai, M.F.; Schmidt, E.J.; McAlinden, A.; Cheverud, J.M.; Sandell, L.J. Molecular insight into the association between cartilage regeneration and ear wound healing in genetic mouse models: Targeting new genes in regeneration. G3 2013, 3, 1881–1891.
- Ozeki, N.; Mogi, M.; Hase, N.; Hiyama, T.; Yamaguchi, H.; Kawai, R.; Kondo, A.; Nakata, K. Wnt16 signaling is required for IL-1beta-induced matrix metalloproteinase-13-regulated proliferation of human stem cell-derived osteoblastic cells. Int. J. Mol. Sci. 2016, 17, 221.
- Yuan, J.; Gao, Y.; Sun, L.; Jin, S.; Zhang, X.; Liu, C.; Li, F.; Xiang, J. Wnt signaling pathway linked to intestinal regeneration via evolutionary patterns and gene expression in the sea cucumber Apostichopus japonicus. Front. Genet. 2019, 10, 10.
- Girich, A.S.; Dolmatov, I.Y.; Lamash, N.E. The distribution of the Wnt5 protein in the tissues of the holothurian Eupentacta fraudatrix (Djakonov et Baranova, 1958) (Holothuroidea: Dendrochirotida) in the norm and during regeneration. Russ. J. Mar. Biol. 2014, 40, 66–70.
- Adell, T.; Salò, E.; Boutros, M.; Bartscherer, K. Smed-Evi/Wntless is required for β-catenin-dependent and -independent processes during planarian regeneration. Development 2009, 136, 905–910.
- Ge, X.; Ma, X.; Meng, J.; Zhang, C.; Ma, K. Role of Wnt-5A in interleukin-1–induced matrix metalloproteinase expression in rabbit temporomandibular joint condylar chondrocytes. Arthritis Rheum. 2009, 60, 2714–2722.




