| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sadanand Pandey | + 4835 word(s) | 4835 | 2021-02-24 05:26:24 | | | |
| 2 | Rita Xu | -1725 word(s) | 3110 | 2021-02-26 07:13:04 | | |
Video Upload Options
Osteosarcoma (OSA) (also called osteogenic sarcoma) is the most common type of cancer that starts in the bones. It is the most frequent pediatric primary bone tumor. OSA is a rare mesenchymal bone neoplasm derived from mesenchymal stem cells. Genome disorganization, chromosomal modifications, deregulation of tumor suppressor genes, and DNA repair defects are the factors most responsible for OSA development. Recent Progress in nanotechnology platforms in human OSA inspire new ideas to develop more effective therapeutic options.
1. Introduction
Osteosarcoma (OSA) is the most common primary metastatic bone cancer in children, young adults, and sometimes in elderlies [1]. Generally, OSA occurs in the proximal tibia, distal femur, proximal humerus, around the knee, and axial skeleton [2][3]. Although the axial skeleton is rarely affected, the aggressive axial skeleton of OSA has been associated with substantially high morbidity compared with other primary tumors within the appendicular skeleton [4][5]. The annual mortality rate of OSA was estimated to be 4.4 per million for individuals <25 years old and 3.1 per million for all ages [6]. Genome disorganization, aneuploidy with chromosomal modifications, deregulation of tumor suppressor genes, and DNA repair defects are the most common characteristics of OSA [7].
At the time of diagnosis, a minority of patients present with metastatic OSA, mainly involving the lungs [3]. Fortunately, an average of 35% of patients with localized OSA will encounter distant recurrence [8]. Studies have shown that patients living in less affluent communities experienced a higher risk of metastatic OSA at the time of diagnosis [9]. Clinicians routinely confirm OSA by the appearance of mixed radiodense and lytic lesions of the metaphyseal bone [10]. Different imaging techniques, including X-ray computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET), are widely used for detecting primary and secondary OSA tumors [10][11]. Among these techniques, CT is mostly preferred for skeletal system diseases, since MRI is not sensitive to calcium-enriched bone tissues, and PET scanning has low spatial resolution [12]. In conventional CT, either CT contrast agents or bones generate a similar attenuation of X-ray. Therefore, it is hard to differentiate bones from the surrounding OSA site accumulated with contrast agents [13]. This minimizes the efficacy of CT as the recommended diagnostic approach for OSA [14]. Current strategies of treating OSA patients include preoperative chemotherapy, complete surgical resection combined with a high-dose chemotherapy regimen [15][16]. The success rate of surgical resection against localized OSA is stated to be less than 20%, although when accompanied by chemotherapy, it increases dramatically to about 70% [17]. Chemotherapy has become the patients’ choice for OSA treatment; however, systematic chemotherapeutics induce considerable cardiac and nephron-toxicity [18]. Thus, the use of conventional chemotherapeutics for treating OSA patients is limited by their unfavorable side effects [19]. In addition, poor response to chemotherapeutic regimens might occur, due to the heterogeneity and the genomic complexity of OSA [20].
Nanotechnology is a burgeoning research field that has offered groundbreaking solutions for the diagnosis and treatment of OSA [21]. In this regard, a wide range of nanomaterials has been designed for the targeted treatment of OSA with the least cytotoxicity towards normal human cells [22][23]. As engineered nanomaterials, nanoparticles (NPs) have wide-spread applications in OSA diagnosis and treatment [24][25]. This is primarily due to their specialized structure, desirable efficiency of drug encapsulation, and good bioavailability [26][27][28]. As promising nanocarriers, NPs can deliver various chemicals, drugs, small molecules, peptides, nucleic acids, and even vaccines to the target locations [29][30][31].
Previous studies have indicated that NPs enhance the delivery of chemotherapeutic drugs to OSA cells overexpressing specific antigens or surface receptors [21][32]. PEGylated gold NPs modified with doxorubicin were also more effective than doxorubicin alone for OSA treatment [33]. Newly synthesized NPs loaded with multiple anti-cancer drugs have shown a great advantage in systemic OSA therapy [34]. In addition, NPs possess excellent spectral CT performance have emerged as alternative CT contrasting agents for OSA diagnosis [14][25]. Other nanomaterial-based delivery systems, such as metal nanocages [35], nanocomposites [36], nanocapsules [37], nanoliposomes [38], nanohydrogels [39], and nanomicelles [40], have also provided several advantages over routine therapies for OSA.
Despite significant advances in the diagnosis and treatment of OSA, the overall survival of patients has been stagnant for over two decades [10]. Therefore, recent studies have increasingly focused on improving therapeutic strategies for enhancing the diagnostic accuracy of OSA and combating its progression [41][42].
2. Diagnosis of Human Osteosarcoma
2.1. Current Approaches for Diagnosis of OSA
Clinicians to identify OSA use several examinations. Some examinations are often conducted to learn whether cancer has spread from, where it originated to another part of the body or not, known as metastasis (metastases is the plural). As cancer cells break away from the main tumor and join the bloodstream or lymphatic system, metastases most generally grow. Fluids are transported across the body by these systems. This means that the cancer cells can travel far from the original tumor and form new tumors when they settle and grow in a different part of the body. When cancer cells from the main tumor, usually in the abdomen or abdominal cavity, break off and expand in surrounding areas, such as the liver, lungs, or bones, metastases may often also develop. Imaging tests use X-rays, magnetic fields, or radioactive substances to create pictures of the inside of the body. Imaging tests are performed for a number of reasons, such as: (i) to help determine whether cancer may be a suspicious area; (ii) to help determine whether cancer may have started in another part of the body; (iii) to find out how far cancer has spread; (iv) to help determine whether treatment is working; and (v) to look for signs that cancer may have returned [48][49]. Today, the most trusted OSA diagnostic tools are imaging tests. The most widely used tool for OSA diagnosis is bone X-ray, chest X-ray, computed tomography (CT) scan, MRI scan, positron emission tomography (PET) scan or PET-CT scan, bone scan, and biopsy which include core needle biopsy and surgical (open) biopsy [50][51][52][53]. For osteosarcoma, the guaranteed option for a specialist to determine whether a body region has cancer is the biopsy method. In this approach, a small sample of tissue taken for examination in a laboratory. A bone scanning process with a radioactive tracer may also be done enough to see through the bones [54]. An MRI method can use with magnetic fields to achieve precise pictures of the tissue and determine the size of the tumor. On the other hand, using X-rays obtained from various angles, a CT scan takes photographs of the inside of the body. These images can be combined by a computer into a detailed, 3D image showing any anomalies or cancers [55][56][57].
Existing approaches to OSA diagnosis such as CT, X-ray, and MRI are limited by the signal intensity and do not detect a small mass of tumors. Detection of tumors largely depends on the visual resolution of the imaging method. Tiny tumors below 1 mm3 are unlikely to be identified by CT and MRI [58][59][60]. Scientists have investigated nano-agents for contrast-enhancing imaging methodologies in past years. Due to their targeting capacity and tumor aggregation, functional nanostructures can improve X-ray contrast and detection sensitivity.
2.2. Nanomaterials for Diagnosis of Human Osteosarcoma
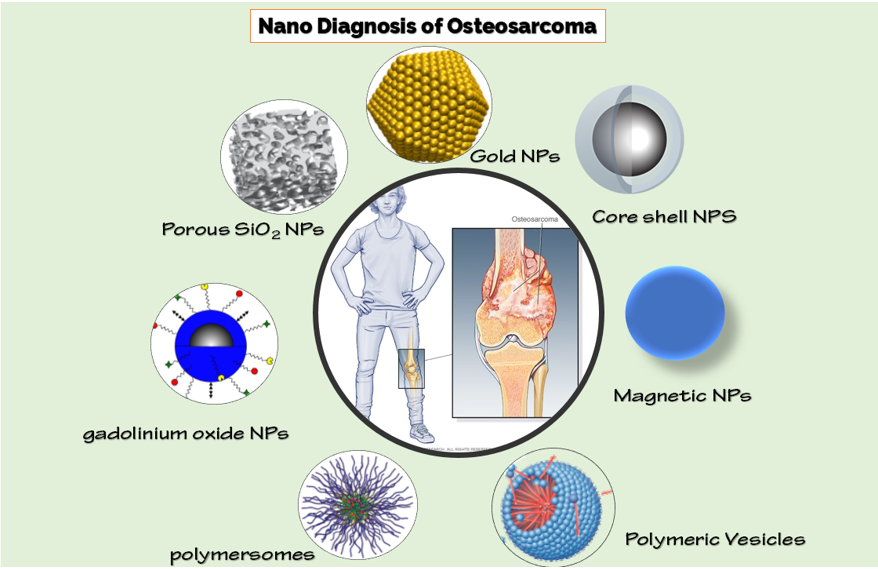
The nanotechnology approach can improve the diagnostic sensitivity of OSA. Thousands of nanocarriers have reached the clinic, and there are hundreds of nanomaterials proposals being tested by the Food and Drug Administration (FDA) [61][62]. The small size of nanoparticles enables them to overcome biological barriers and reach greater therapeutic effectiveness [63][64][65]. Nanotechnology has combined with the above-mentioned imaging approaches for targeted imaging and can provide a clinical need for high sensitivity and specificity (Figure 1). We have studied the latest state widely of the art of nanomaterials for OSA diagnosis in the following paragraphs.
Figure 1. Different nanoparticles for diagnosis of osteosarcoma (OSA).
2.2.1. Single-Photon Emission Computed Tomography (SPECT)/CT Imaging
SPECT has been a cornerstone of the science of nuclear medicine. More recently, in many clinical cases, the combination of the functional imaging available with SPECT and the anatomical imaging of CT has gained more acceptance and been proved useful. SPECT/CT imaging has shown outstanding penetration capacity and is more applicable for deep tissue imaging and especially for OSA imaging [66][67]. A perfect option for the therapeutic management and evaluation of malignant osteolysis could be the imaging and therapy role of versatile nanomaterials [68]. Different forms of nanomedicines based on albumin have been investigated for cancer therapy, guided imaging, and biosensors [69][70][71]. For example, an alternative to therapeutic navigation and monitoring of malignant osteolysis could be to leverage the imaging and therapy role of flexible nanomedicine. Chen et al. reported the development of albumin-based gadolinium oxide nanoparticles loaded with doxorubicin and conjugated with bone-seeking alendronate for targeted delivery and therapeutic monitoring [68]. After radio labeling with 125I and SPECT imaging, the authors observed a good distribution of NPs in the body. SPECT imaging also showed the enhanced bone tumor accumulation and prolonged retention of NPs in bone cancer. On the other hand, CT imaging and pathological examination showed that the combination therapy in this study was effective. The finding of this study indicated that albumin-based nanomaterials would provide a system for bone imaging and evaluation.
Developing alternative medical strategies that allow real-time monitoring of drugs and also imaging of tissue is an important nanomedicine approach. A high level of control is provided by real-time magnetic resonance (MR) guidance of laser-induced thermal therapy (LITT). Therefore, this method allows a minimally invasive alternative for resistant focal metastatic intracranial tumors to be killed and treated [72]. In this light, Zhou et al. demonstrated an integrated system for dual diagnosis and treatment of bone tumors. The platform was based on bone-responsive polymeric vesicles with exceptional SPECT/CT imaging ability and good antitumor efficiency [73]. The polymer vesicle is self-assembled from poly(ε-caprolactone)67-b-poly((L-glutamic acid)6-stat-(L-glutamic acid-alendronic acid)16) (PCL67-b-P(Glu6-stat-(Glu-ADA)16)) in water and without a co-solvent. A combination of SPECT/CT and NPs perfectly monitored the distribution of the drug in the bone cancer of in vivo model (rabbits). The study clearly shows the ability of polymer vesicles for simultaneous imaging and successful treatment of malignant bone tumors, offering an optimistic approach for imaging-guided cancer treatment.
In another study, Lu et al. reported enhanced OSA killing and CT imaging using ultrahigh drug loading and NIR-responsive bismuth sulfide@mesoporous silica NPs. Here, authors have prepared a core-shell of bismuth sulfide NPs and mesoporous silica (Bi2 S3 @MSN NPs) and then attached covalently to arginine-glycine-aspartic acid (RGD) peptide (c(RGDyC)) [25]. The nanoplatform had a perfect sensitivity for OSA and accumulated in cancer cells (10-fold more than peri-tumoral tissue) for better monitoring with CT imaging.
The current positive CT contrast agents (CTCAs) provide a good CT density value (CT-DV) but accurate diagnosis of some diseases such as OSA is limited in this approach [74]. To solve this problem, Meng et al. developed an innovative strategy based on negative CT contrast agents (NCTCAs) to reduce the CT-DV of OSA [74]. They synthesized ammonia borane loaded-hollow mesoporous silica NPs modified with PEG for satisfactory diagnosis of OSA. By reacting to the acidic medium of OSA, nanostructures can generate in situ H2 in OSA regions. This result showed a nearly 20-fold reduction in CT density in OSA.
Recently, a progressive type of CT called gemstone spectral CT (GSCT), has obtained great attention. GSCT has a high capacity for material decomposition and monochromatic images to overcome the drawbacks of traditional CT [75][76]. Jin et al. prepared lutecium (Lu)-based up-conversion nanoparticles (UCNPs, PEG–NaLuF4: Yb/Er). Lu-based UCNPs showed higher spectral CT performance than iohexol (contrast agent) and can be a better spectral CT contrast agent for the diagnosis of OSA [14]. In vitro and in vivo GSCT demonstrate nanoplatform experiments can provide greater diagnostic elucidation and separate the OSA from the surrounding bones. Owing to the various X-ray amplification properties of UCNPs and iohexol under different energy, iohexol failed to distinguish between cancer bone and healthy bone. The findings thus indicate that the excellent biocompatibility of Lu-based UCNPs has great potential for further clinical diagnosis of skeletal system diseases.
2.2.2. Fluorescence Imaging
Fluorescence imaging is a form of a non-invasive imaging tool that can help to visualize biological processes in a living organism. Images can be created using a variety of techniques, including microscopy, spectroscopy, and imaging probes. The fluorescence imaging approach measures emitted photons by laser-excited fluorescent probes. NPs are usually attached to fluorescent dyes to imagine bone tissues by fluorescence imaging [77][78][79]. A significant prognostic factor for bone tumor growth is lymph sarcoma [80]. Therefore, it is important to develop novel probes for non-invasive and early stages detection of metastatic lymph nodes (MLNs). To address this issue, Yin et al. developed a novel matrix metalloproteinase-2 (MMP-2)-activatable probe constructed with a near-infrared dye (Cy5), a quencher (QSY21), and a tumor-targeting peptide cRGD covalently linked through a radionuclide (125I)-labeled peptide substrate for accurate detection of MLNs [81]. The probe produced MMP-2 concentration-dependent fluorescence near-infrared (NIR) upon vicinity with activated MMP-2. The fluorescence radiation provided sensitive and precise imaging of MLNs through optical and SPECT imaging techniques. Zhou et al. designed and synthesized the two homologous forms of fluorescent probes CH1055-PEG-PT and CH1055-PEG-Affibody that show extremely promising results for targeting imaging of OSA and its lung organ metastasis, respectively. it’s found that the close to NIR-II imaging quality of CH1055-PEG-PT is way superior thereto of CT for the first in vivo 143B tumor imaging, and this probe-guided surgery for accurate surgery of 143B tumor [82].
2.2.3. Magnetic Resonance Imaging (MRI)
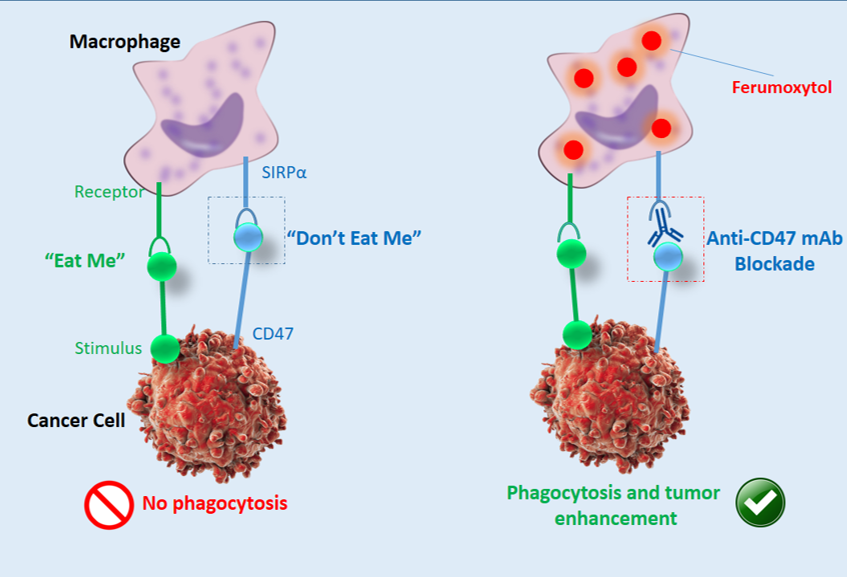
MRI is a technology for non-invasive imaging that generates accurate anatomical images in three dimensions (3D). It is also used to track the identification, diagnosis, and treatment of diseases. Due to the high sensitivity of MRI to the reflection of fluid in the body, it provides image data and physiological information simultaneously. Generally, MRI is used in conjunction with contrast agents [83][84][85]. In order to increase the speed at which protons realign with the magnetic field, contrast agents (often containing the element Gadolinium) can be given to a patient intravenously before or during the MRI. The quicker the realignment of the protons, the brighter the picture. Mohanty et al. claimed that in OSA, ferumoxytol NPs can increase MRI and monitor macrophage reaction to CD47 mAb (Figure 2) [86]. Their result showed that tumor-associated macrophages (TAMs) in sarcomas are triggered by CD47 monoclonal antibodies (mAbs) and can kill cancer cells.
Figure 2. Schematic illustration of ferumoxytol-MRI as an imaging approach for CD47 immunotherapy, reproduced from [86].
As new MRI contrast agents were developed by Pourtau et al. for bone metastasis imaging, multi-functional maghemite NP-encapsulated polymersomes attached to an antibody directed against human endothelial receptor 2 [87]. MRI displayed targeting and improved retention of antibody-attached polymersomes at the tumor tissue after administration in mice carrying bone cancer.
2.2.4. Photo-Acoustic Imaging (PAI)
For the development of successful treatment strategies, detection of different types of tumor remains is important. Ma et al. developed peptide-based probes for photo-acoustic imaging (PAI) and targeted diagnosis of OSA [88]. Using phage display-based monitoring on an OSA cell line (UMR-106), PT6 and PT7 (tumor-specific oligopeptides) were identified. On tissue microarrays, the defined oligopeptides were capable of detecting clinical OSA specimens and pegylated Au nanorods-oligopeptides (PGNR) were explicitly prepared to target UMR-106 cells. More significantly, PAI showed that after systemic administration, both PGNR-PT6 and PGNR-PT7 could specifically attach to subcutaneous UMR-106 xenografts and increase the contrast of OSA pictures by 170% and 230%, respectively in tumor-bearing mice.
2.2.5. Multimodal Imaging
A successful effort to increase the efficiency of diagnosis is the mixture of different imaging techniques [89][90]. A key measure for tumor evaluation and treatment is the invasion stage of tumor-draining lymph nodes (LNs) [91][92]. For more than one imaging technique, multimodal imaging or multiplexed imaging refers to simultaneous signal generation. For example, the use of optical, magnetic, and radioactive reporters to be detected by SPECT, MRI, and PET could be combined. For accurate tumor imaging of OSA, each approach can be combined to create multimodal imaging. In this background, Xu et al. proposed an integrative MRI/NIR/SPECT approach based on 99mTc-labeled gadolinium oxide NPs for improved OSA and tumor-draining lymph node (LN) identification [93]. Nanoplatform with complementary strengths of each modality correctly located OSA tumors. They showed that nanoprobe could be applied in an OSA model with perfect resolution and good sensitivity imaging of lymphatic drainage. In addition, in clinical practice, the nanoprobe can increase the efficacy of the system for nodal resection and tumor staging.
Wang et al. developed a practical dual-modality MR/CT probe for in vivo imaging of OSA [42]. The protein-directed synthesis approach provided an effective alternative to the chemistry-based method. Bovine serum albumin (BSA) is attached to gadolinium NPs (GdNPs) and then iodinated using the chloramine-T procedure. The iodinated BSA-GdNPs (I-BSA-GdNPs) showed a strong coefficient of X-ray attenuation and great drive for MRI. The I-BSA-GdNPs were intravenously injected into orthotopic OSA-bearing rats. Aggregation and retention of NPs in tumors enabled dual-modality and non-invasive imaging. The dual-model, long-circulating I-BSA-GdNPs nanoprobe scan be applied for image-guided surgery and drug delivery application.
3. Conclusions, Challenges, and Perspectives
Over the past few years, a significant number of targeted nanomaterials have been established for the diagnosis and treatment of malignant bone tumors such as OSA. It is imperative to provide a better understanding of the fundamental concepts involved in the design and application of nanoparticles for diagnosis, treatment, or the combination of imaging and therapeutics in various clinical circumstances, following this remarkable progress in the advancement of nanotherapeutic and imaging methods for cancer detection and treatment. OSA has rapidly metastasizing ability and proves challenging for the treatment rationales. Nanotherapeutics being developed for the OSA include metallic, lipid, polymeric, magnetic, and stimuli-sensitive drug delivery systems. Nanotherapeutics improve the safety and compatibility profile in the diseases by minimizing off-target accumulation. However, tumor biology itself plays a critical role and needs to be studied extensively for the outcomes in the case of nanoparticles therapy. The majority of the nanostructured approaches are in the cellular stages of drug delivery and need to be translated into clinical trials after extensive research. While these targeted NPs showed satisfactory benefits in OSA diagnosis and therapy, there are still difficult problems to solve in the future. For instance, in vivo verification of nanoparticles, and especially subsequent toxic evaluation and bone tissue targeted delivery for either cancer bone metastasis or other bone diseases still require further and extensive experiments to accelerate their potential clinical implementation. Some nano polymeric materials are not very strongly cytotoxic and it can also be expected that they will be offered to humans in the coming years. Nanotechnology is expected to play a pivotal role in future OSA diagnostics. With the development of technology, more powerful diagnostic techniques such as multimodal imaging can be seen in the coming days. Physicists, chemists, engineers, biologists, and clinicians, motivated by the rapid and encouraging developments in nanotechnology, will continue to challenge themselves to design innovative and efficient nanosystems for cancer diagnosis and treatment.
References
- Longhi, A., Errani, C., De Paolis, M., Mercuri, M., Bacci, G. Primary bone osteosarcoma in the pediatricage: State of the art. Cancer Treat. Rev. 2006,32,423–436.
- Fletcher,C.D.;Unni,K.K.;Mertens,F. Pathology and genetics of tumours of soft tissue and bone.:IARC Press, Lyon,France,2002;Volume4.
- Luetke, A., Meyers, P.A., Lewis, I., Juergens, H. Osteosarcoma treatment – Where do we stand? A state of the art review. Cancer Treat. Rev. 2014,40,523–532.
- Ozaki,T.;etal. Osteosarcoma of the pelvis: Experience of the Cooperative Osteosarcoma Study Group. Clin. Oncol. 2003,21,334–341.
- Picci,P. Osteosarcoma(osteogenic sarcoma).Orphanet J. Rare Dis. 2007,2,1–4.
- Mirabello,L.,Troisi,R.J.;A,S.Savage. Osteosarcoma incidence and survival rates from 1973 to 2004: Data from theSurveillance, Epidemiology, and End Results Program. Cancer: Interdiscip. Int. J. Am. Cancer Soc. 2009,115,1531–1543.
- Thomas,R.;etal. Influence of genetic background on tumor karyotypes: Evidence for breed-associated cytogenetic aberrations in canine appendicular osteosarcoma. Chromosome Res. 2009,17,365–377.
- Kempf-Bielack,B.;etal.Osteosarcoma relapse after combined modality therapy:Ananalysis of unselected patients in the Cooperative Osteosarcoma Study Group(COSS). Clin. Oncol. 2005,23,559–568.
- Miller,B.J.;etal. Risk factors for metastatic disease at presentation with osteosarcoma: An analysis of the SEER database. Bone Jt. Surg. Am. Vol. 2013,95,doi:10.2106/JBJS.L.01189.
- Geller,D.S.;Gorlick,R. Osteosarcoma:A review of diagnosis, management, and treatment strategies. Adv. Hematol. Onco.2010,8,705–718.
- Hamada,K.;etal.EvaluationofchemotherapyresponseinosteosarcomawithFDG-PET. Nucl. Med. 2009,23,89–95.
- Huang,R.;etal. Development of PET probes for cancerimaging. Top. Med. Chem. 2015,15,795–819.
- Schirra,C.O.;etal. Spectral CT:A technology primer for contrast agent development. Contrast Media Mol. Imaging 2014,9,62–70.
- Jin,Y.;etal. Harness the power of upconversion nanoparticles for spectral computed tomography diagnosis of osteosarcoma. Funct. Mater. 2018,28,1802656.
- Chen,B.;etal. Ifosfamide-loaded poly(lactic-co-glycolicacid) PLGA-dextran polymeric nanoparticles to improve the antitumor efficacy in Osteosarcoma. BMC Cancer 2015,15,752.
- Higuchi,T.;etal. Trabectedin and irinotecan combination regresses a cisplatinum-resistant osteosarcoma in a patient-derived orthotopic xenograft nude-mouse model. Biophys. Res. Commun. 2019,513,326–331.
- Schwartz,C.L.;etal. Multiple Drug Resistance in Osteogenic Sarcoma: INT0133 From the Children's Oncology Group. Clin. Oncol. 2007,25,2057–2062.
- PosthumaDeBoer,J.;vanRoyen,B.;Helder,M. Mechanisms of therapy resistance in osteosarcoma: Areview. Discov. 2013,1,8.
- Li,Y.;etal. Co-delivery of doxorubicin and paclitaxel by reduction/pH dual responsive nanocarriers for osteosarcoma therapy. Drug Deliv. 2020,27,1044–1053.
- Ferrari;S;Serra,M. An update on chemotherapy for osteosarcoma. Expert Opin. On Pharmacother. 2015,16,2727–2736.
- Wang,S.-Y.;etal. Recent advances of drug delivery nanocarriers in osteosarcoma treatment. Cancer 2020,11,69.
- Khan,S.;etal.Catechins-modifiedselenium-dopedhydroxyapatitenanomaterialsforimprovedosteosarcomatherapythroughgenerationofreactiveoxygenspecies. Oncol. 2019,9,499.
- Tang,Z.;etal. Mechanisms of oxidative stress, apoptosis, and autophagy involved in graphene oxide nanomaterials anti-osteosarcoma effect. J. Nanomed. 2018,13,2907.
- Huang,X.;etal. Surface engineering of nanoparticles with ligands for targeted delivery to osteosarcoma. Colloids Surf. B Biointerfaces2020,190,110891.
- Lu,Y.;etal. Enhancing osteosarcoma killing and CT imaging using ultrahigh drug loading and NIR-responsive bismuth sulfide@mesoporous silica nanoparticles. Healthc. Mater. 2018,7,1800602.
- Si,X.-Y.;Merlin,D.;Xiao,B. Recent advances in orally administered cell-specific nano therapeutics for inflammatory bowel disease. World J. Gastroenterol. 2016,22,7718.
- Weng,Y.;etal. Nanotechnology-based strategies for treatment of ocular disease. Acta Pharm. Sin. B 2017,7,281–291.
- Khandan,F.M.;etal. Novel uranyl-curcumin-MOF photocatalysts with highly performance photocatalytic activity toward the degradation of phenol red from aqueous solution: effective synthesis route, design and a controllable systematic study. Mater. Sci. Mater. Electron. 2018,29,18600–18613.
- Zahin,N.;etal. Nanoparticles and its biomedical applications in health and diseases: Special focus on drug delivery. Sci. Pollut. Res. 2019,1–18,doi:10.1007/s11356-019-05211-0.
- Nematollahi,M.H.;etal. Changes in physical and chemical properties of niosome membrane induced by cholesterol: a promising approach for niosome bilayer intervention. Rsc Adv. 2017,7,49463–49472.
- Goudarzi,K.A.;etal. Targeted Delivery of CRISPR/Cas13asa Promising Therapeutic Approach to Treat. SARS-CoV-2. Pharmaceut. Biotechnol.2020,doi:10.2174/1389201021666201009154517.
- Yu,Z.;etal. Epidermal growth factor receptor aptamer‑conjugated polymer‑lipid hybrid nanoparticles enhance salinomycin delivery to osteosarcoma and cancer stem cells. Ther. Med. 2018,15,1247–1256.
- Lupusoru,R.V.;etal. Effect of TAT-DOX-PEG irradiated gold nanoparticles conjugates on human osteosarcoma cells . Rep. 2020,10,1–14.
- Yuan,Y.;etal. A multiple drug loaded, functionalized pH-sensitive nanocarrier as therapeutic and epigenetic modulator for osteosarcoma. Rep. 2020,10,1–11.
- Raghubir,M.;etal. Osteosarcoma growth suppression by riluzole delivery via iron oxide nanocage in nude mice. Rep. 2020,43,169–176.
- Mishra,S.;etal. Bioinspired nanocomposites: applications in disease diagnosis and treatment. Nanotechnol. 2019,7,206–219.
- Wang,S.-Q.;etal. Ifosfamide-loaded lipid-core-nanocapsules to increase the anticancer efficacy in MG63 osteosarcoma cells. Saudi J. Biol. Sci. 2018,25,1140–1145.
- Haghiralsadat,F.;etal. EphA2 targeted doxorubicin-nanoliposomes for osteosarcoma treatment. Res. 2017,34,2891–2900.
- Ma,H.;etal. PLK1shRNA and doxorubicin co-loaded thermosensitive PLGA-PEG-PLGA hydrogels for osteosarcoma treatment. Biomaterials 2014,35,8723–8734.
- Bukchin,A.;etal. Glucosylated nanomicelles target glucose-avid pediatric patient-derived sarcomas. Of Control. Release 2018,276,59–71.
- Fan,T.M.;Roberts,R.D.;Lizardo,M.M. Understanding and modeling metastasis biology to improve therapeutic strategies for combating osteosarcoma progression. Oncol. 2020,10,doi:10.3389/fonc.2020.00013.
- Wang,Q.;etal.Long-circulating iodinated albumin–gadolinium nanoparticles as enhanced magnetic resonance and computed tomography imaging probes for osteosarcoma visualization. Chem. 2015,87,4299–4304.
- Barani,M.;etal. Nanodiagnosis and nanotreatment of colorectal cancer: an overview. Nanoparticle Res. 2021,23,1–25.
- Barani,M.;etal. Nanotreatment and Nanodiagnosis of Prostate Cancer: Recent Updates. Nanomaterials 2020,10,1696.
- Bilal,M.;etal. Nanomaterials for the treatment and diagnosis of Alzheimer's disease: An overview. NanoImpact2020,100251,doi:10.1016/j.impact.2020.100251.
- Nikazar,S.;etal. Photo‐and Magnetothermally Responsive Nanomaterials for Therapy, Controlled Drug Delivery and Imaging Applications. ChemistrySelect2020,5,12590–12609.
- Rahdar,A.;etal. The synthesis of methotrexate-loaded F127 microemulsions and their in vivo toxicity in a rat model. Mol. Liq. 2020,313,113449.
- Roberts,R.D.;etal. Provocative questions in osteosarcoma basic and translational biology: A report from the Children's Oncology Group. Cancer2019,125,3514–3525.
- Sheen,H.;etal. Metastasis risk prediction model in osteosarcoma using metabolic imaging phenotypes: A multivariable radiomics model. PLoS ONE 2019,14,e0225242.
- Li,J.;etal. Label-free Raman imaging of live osteosarcoma cells with multivariate analysis. Microbiol. Biotechnol. 2019,103,6759–6769.
- Yarmish,G.;etal. Imaging characteristics of primary osteosarcoma: nonconventional subtypes Radiographics 2010,30,1653–1672.
- Murphey,M.D.;etal. Telangiectatic osteosarcoma: radiologic-pathologic comparison. Radiology2003,229,545–553.
- McAuley,G.;etal. Extraskeletal osteosarcoma: spectrum of imaging findings.. J. Roentgenol. 2012,198,W31–W37.
- Kundu,Z.S. Classification, imaging, biopsy and staging of osteosarcoma. Indian J. Orthop. 2014,48,238–246.
- O'flanagan,S.;etal. Imaging of intramedullary tumour spread in osteosarcoma. A comparison of techniques. Bone Jt. Surg. Br. Vol. 1991,73,998–1001.
- Uhl,M.;etal. Osteosarcoma: preliminary results of in vivo assessment of tumor necrosis after chemotherapy with diffusion-and perfusion-weighted magnetic resonance imaging. Radiol. 2006,41,618–623.
- Wittig,J.C.;etal. Osteosarcoma: a multidisciplinary approach to diagnosis and treatment.. Fam. Physician 2002,65,1123.
- Thoeni,R.F.;etal. Detection of small, functional islet cell tumors in the pancreas: selection of MR imaging sequences for optimal sensitivity. Radiology2000,214,483–490.
- Hallscheidt,P.J.;etal. Preoperative staging of renal cell carcinoma with inferior vena cava thrombus using multidetector CT and MRI: prospective study with histopathological correlation. Comput. Assist. Tomogr. 2005,29,64–68.
- Pichler,B.J.;Judenhofer,M.S.;Pfannenberg,C. Multimodal Imaging Approaches: Pet./Ct and Pet/Mri, in Molecular Imaging I;Springer:Berlin/Heidelberg,Germany,2008;pp.109–132.
- Barani,M.;etal. Comprehensive evaluation of gene expression in negative and positive trigger-based targeting niosomes in HEK-293 cell line. J. Pharm. Res. 2020,19,166.
- Torkzadeh-Mahani,M.;etal. A combined theoretical and experimental study to improve the thermal stability of recombinant D‐lactate dehydrogenase immobilized on a novel superparamagnetic Fe3O4NPs@ metal–organic framework. Organomet. Chem. 2020,34,e5581.
- Sonvico,F.;etal. Metallic colloid nanotechnology, applications in diagnosis and therapeutics. Pharm. Des. 2005,11,2091–2105.
- Jamali,Z.;etal. Autophagy regulation by microRNAs: Novel insights into osteosarcoma therapy. IUBMB Life 2020,72,1306–1321.
- Pereira-Silva,M.;etal. Nanomedicine in osteosarcoma therapy: Micelleplexes for delivery of nucleic acids and drugs toward osteosarcoma-targeted therapies. J. Pharm. Biopharm. 2020,148, 88–106.
- Hu,B.;etal. SPECT/CT imaging of retroperitoneal extraskeletal osteosarcoma. Nucl. Med. 2014,39,200–202.
- Gu,T.;etal. Primary pulmonary osteosarcoma: PET/CT and SPECT/CT findings. Nucl. Med. 2011,36,e209–e212.
- Chen,Z.;etal. Bone-Seeking Albumin-Nanomedicine for In Vivo Imaging and Therapeutic Monitoring. ACS Biomater. Sci. Eng. 2019,6,647–653.
- Bhushan,B.;etal. Impact of albumin based approaches in nanomedicine: Imaging, targeting and drug delivery. Colloid Interface Sci. 2017,246,13–39.
- Chen,J.;etal. Light‐triggered retention and cascaded therapy of albumin‐based theranostic nanomedicines to alleviate tumor adaptive treatment tolerance. Funct. Mater. 2018,28,1707291.
- Gao,G.;etal. Molecular Targeting‐Mediated Mild‐Temperature Photothermal Therapy with a Smart Albumin‐Based Nanodrug. Small2019,15,1900501.
- Carpentier,A.;etal. Laser thermal therapy: Real‐time MRI‐guided and computer‐controlled procedures for metastatic brain tumors. Lasers Surg. Med. 2011,43,943–950.
- Zhou,X.;etal. Bone-targeting polymer vesicles for simultaneous imaging and effective malignant bone tumor treatment. Biomaterials2020,269,120345.
- Meng,X.;etal. Negative CT Contrast Agents for the Diagnosis of Malignant Osteosarcoma. Sci. 2019,6,1901214.
- Wang,Y.;etal. Targeted imaging of damaged bone in vivo with gemstone spectral computed tomography. ACS Nano 2016,10,4164–4172.
- Yin,X.-R.;etal. The Initial Exploration of Adamkiewicz Artery Computed Tomographic Angiography With Monochromatic Reconstruction of Gemstone Spectral Imaging. Comput. Assist. Tomogr. 2016,40,820–826.
- Heilemann,M.;etal. Subdiffraction‐resolution fluorescence imaging with conventional fluorescent probes. Chem. Int. Ed. 2008,47,6172–6176.
- Rao,J.;Dragulescu-Andrasi,A.;Yao,H. Fluorescence imaging in vivo: recent advances. Opin. Biotechnol. 2007,18,17–25.
- Schäferling,M. The art of fluorescence imaging with chemical sensors. Chem. Int. Ed. 2012,51,3532–3554.
- Kawada;K;Taketo,M.M. Significance and mechanism of lymph node metastasis in cancer progression. Cancer Res. 2011,71,1214–1218.
- Yin,L.;etal. Rational design and synthesis of a metalloproteinase-activatable probe for dual-modality imaging of metastatic lymph nodes in vivo. Org. Chem. 2019,84,6126–6133.
- Zhou,H.;etal. Specific Small-Molecule NIR-II Fluorescence Imaging of Osteosarcoma and Lung. Metastasis Healthcare Mater.2020,9,1901224.
- Schenck,J.F. Health and physiological effects of human exposure to whole‐body four‐tesla magnetic fields during MRI. NY Acad. Sci. 1992,649,285–301.
- Jin,C.;etal. MRI-based three-dimensional thermal physiological characterization of thyroid gland of human body. Med Eng. Phys. 2014,36,16–25.
- Taylor,J.C.;Wiggett,A.J.;Downing,P.E. Functional MRI analysis of body and body part representations in the extrastriate and fusiform body areas. Neurophysiol. 2007,98,1626–1633.
- Mohanty,S.;etal. Nanoparticle enhanced MRI can monitor macrophage response to CD47 mAb immunotherapy in osteosarcoma. Cell Death Dis. 2019,10,1–14.
- Pourtau,L.;etal. Antibody‐functionalized magnetic polymersomes: in vivo targeting and imaging of bone metastases using high resolution MRI. Healthc. Mater. 2013,2,1420–1424.
- Ma,Z.;etal. Phage display-derived oligopeptide-functionalized probes for in vivo specific photoacoustic imaging of osteosarcoma. Nanotechnol. Biol. Med. 2017,13,111–121.
- Lee,D.-E.;etal. Multifunctional nanoparticles for multimodal imaging and theragnosis. Soc. Rev.2012,41,2656–2672.
- Kim,J.;Piao,Y.;Hyeon,T. Multifunctional nanostructured materials for multimodal imaging, and simultaneous imaging and therapy. Soc. Rev. 2009,38,372–390.
- Núñez,N.G.;etal. Tumor invasion in draining lymph nodes is associated with Treg accumulation in breast cancer patients. Commun. 2020,11,1–15.
- Faghih,Z.;etal. IL-17 and IL-4 producing CD8+ T cells in tumor draining lymph nodes of breast cancer patients: positive association with tumor progression. J. Immunol. 2013,10,193–204.
- Xu,Z.;etal. Noninvasive multimodal imaging of osteosarcoma and lymph nodes using a 99mTc-labeled biomineralization nanoprobe. Chem. 2018,90,4529–4534.