| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Claudia Raposo | + 634 word(s) | 634 | 2021-02-07 07:35:24 |
Video Upload Options
Lectins are a class of proteins responsible for several biological roles such as cell-cell interactions, signaling pathways, and several innate immune responses against pathogens.
1. Introduction
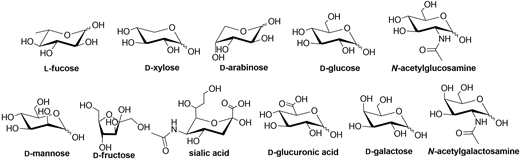
Lectins are an attractive class of proteins of non-immune origin that can either be free or linked to cell surfaces, and are involved in numerous biological processes, such as cell-cell interactions, signaling pathways, cell development, and immune responses[1]. Lectins selectively recognize carbohydrates and reversibly bind to them as long as the ligands are oriented in a specific manner. Some of the commonly occurring carbohydrates that are found in Nature are D-fructose, D-galactose, L-arabinose, D-xylose, D-mannose, D-glucose, D-glucosamine, D-galactosamine, L-fucose, various uronic acids, sialic acid, and their combinations to form other di- and oligosaccharides, or other biomolecules (Figure 1) [2].
Figure 1. Structures of some of the carbohydrate building blocks found in Nature.
Lectins in vertebrates can be classified either by their subcellular location, or by their structure. Division based on their location includes integral lectins located in membranes as structural components, or soluble lectins present in intra- and intercellular fluids, which can move freely.
Division according to lectin structure consists of several different types of lectins, such as C-type lectins (binding is Ca2+ dependent), I-type lectins (carbohydrate recognition domain is similar to immunoglobulins), galectin family (or S-type, which are thiol dependent), pentraxins (pentameric lectins) and P-type lectins (specific to glycoproteins containing mannose 6- phosphate) [3].
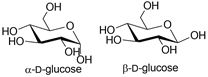
Different lectins have high similarity in the residues that bind to saccharides, most of which coordinate to metal ions, and water molecules. Nearly all animal lectins possess several pockets that recognize molecules other than carbohydrates, meaning that they are multivalent and can present 2 to 12 sites of interaction, allowing the binding of several ligands simultaneously. The specificity and affinity of the lectin-carbohydrate complex depends on the lectin, which can be very sensitive to the structure of the carbohydrate (e.g., mannose versus glucose, Figure 1), or to the orientation of the anomeric substituent (α versus β anomer, e.g., in Figure 2), or both. Lectin-carbohydrate interactions are achieved mainly through hydrogen bonds, van der Waals (steric interactions), and hydrophobic forces (example is given in Figure 3) [3][4].
Figure 2. Structures of α- and β-D-glucose.
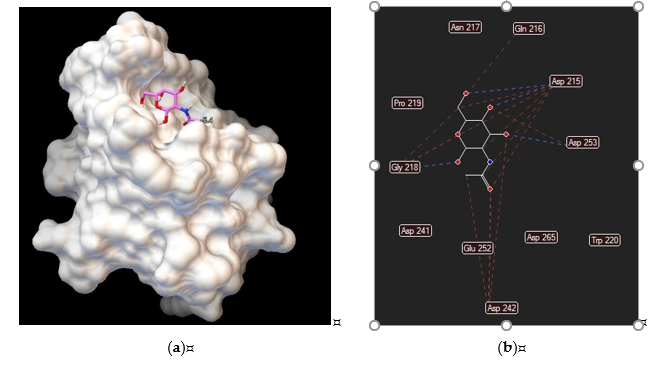
Figure 3. Asialoglycoprotein receptor (Protein Data Bank entry 1DV8, gene symbol ASGR1) binding interactions with N–acetylgalactosamine: (a) ligand conformation inside the binding site; (b) specific interactions are hydrogen bonds (blue dashed lines) and steric interactions (red dashed lines).
It has been shown that the majority of lectins are conserved through evolution, suggesting that these proteins play a crucial role in the sugar-recognition activities necessary for the living process and development [5][6].
Although lectins are present in animals, plants, lichens, bacteria, and higher fungi [3], this review focuses only on human lectins for targeted drug delivery[7] purposes, their specificity towards carbohydrates and the organs where they are expressed. When referring to gene expression (or RNA expression), one means that those specific organs or cells have that specific gene coded. If active, it produces the respective protein, and one says that the protein is expressed in that organ or cell. In this review, we focus only on protein expression, since that information is the only relevant one for the development of targeted drug delivery systems. More information about carbohydrate-based nanocarriers for targeted drug delivery systems can be found elsewhere[8][9][10]. Since lectins are able to recognize and transport carbohydrates and their derivatives, lectin targeting can be relevant in the research and development of new medicines [7][11][12]. The metabolism of cancer cells, for example, is different from normal cells due to intense glycolytic activity (Warburg effect) [13]. Cancer cells require glutamine and/or glucose for cell growth, and glucose transporter isoforms 1 and 2 (gene symbols GLUT1 and GLUT2, respectively) showed an increase in activity in several tumors (gastrointestinal carcinoma, squamous cell carcinoma of the head and neck, breast carcinoma, renal cell carcinoma, gastric and ovarian cancer)[14][15].
References
- Lepenies, Bernd; Lang, Ronald. Lectins and Their Ligands in Shaping Immune Responses; Lepenies, Bernd; Lang, Ronald, Eds.; Lausanne: Frontiers Media S: Lausanne Switzerland, 2019; pp. 1-239.
- Stick, Robert. Carbohydrates: The Sweet Molecules of Life; Academic Press: New York, NY, USA, 2001; pp. 1-256.
- Santos, A. F. S.; Silva, M. D. C.; Napoleão, T. H.; Paiva, P. M. G.; Correia, M. T. S.; Coelho, L. C. B. B.; Lectins: Function, structure, biological properties and potential applications. Current Topics in Peptide & Protein Research 2014, 15, 41-62.
- Wang, B.; Boons, G.-J.. Carbohydrate Recognition: Biological Problems, Methods and Applications; John Wiley & Sons, Inc.: MA, USA, 2011; pp. 1-448.
- Hirabayashi, J.; Kasai, K.I.. Molecular Evolution: Evidence for Monophyly of Metazoa; Springer: Berlin, Germany, 1998; pp. 1-200.
- Drickamer, Kurt; Evolution of Ca2+ dependent Animal Lectins. Prog. Nucleic Acid Res. Mol. Biol. 1993, 45, 207-232, 10.1016/s0079-6603(08)60870-3.
- Himri, I.; Guaadaoui, A.. Nanostructures for the Engineering of Cells, Tissues and Organs; Grumezescu, A., Eds.; Elsevier Inc.: Norwich, UK, 2018; pp. 1-66.
- Liu, Kegang; Jiang, Xiaohua; Hunziker, Patrick; Carbohydrate-based amphiphilic nano delivery systems for cancer therapy. Nanoscale 2016, 8, 16091-16156, 10.1039/c6nr04489a.
- Zhang, Xueqin; Huang, Gangliang; Huang, Hualiang; The glyconanoparticle as carrier for drug delivery. Drug Delivery 2018, 25, 1840-1845, 10.1080/10717544.2018.1519001.
- Mosaiab, Tamim; Farr, Dylan C.; Kiefel, Milton J.; Houston, Todd A.; Carbohydrate-based nanocarriers and their application to target macrophages and deliver antimicrobial agents. Advanced Drug Delivery Reviews 2019, 151-152, 94-129, 10.1016/j.addr.2019.09.002.
- Hossain, Farzana; Andreana, Peter R.; Developments in Carbohydrate-Based Cancer Therapeutics. Pharmaceuticals 2019, 12, 1-84, 10.3390/ph12020084.
- Keshavarz-Fathi, M.; Rezaei, N.. Vaccines for Cancer Immunotherapy; Keshavarz-Fathi, M.; Rezaei, N., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 45–59.
- Warburg, Otto; On the Origin of Cancer Cells. Science 1956, 123, 309-314, 10.1126/science.123.3191.309.
- Chiaradonna, F.; Moresco, R. M.; Airoldi, C.; Gaglio, D.; Palorini, R.; Nicotra, F.; Messa, C.; Alberghina, L.; From cancer metabolism to new biomarkers and drug targets. Biotechnology Advances 2012, 30, 30-51, 10.1016/j.biotechadv.2011.07.006.
- Wesener, D. A.; Wangkanont, K.; McBride, R.; Song, X.; Kraft, M. B.; Hodges, H. L.; Zarling, L. C.; Splain, R. A.; Smith, D. F.; Cummings, R. D.; et al.Paulson, J. C.Forest, K. T.Kiessling, L. L. Recognition of microbial glycans by human intelectin-1. Nature Structural & Molecular Biology 2015, 22, 603-610, 10.1038/nsmb.3053.