
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sandro Almeida | + 4408 word(s) | 4408 | 2021-01-29 03:18:39 | | | |
| 2 | Peter Tang | Meta information modification | 4408 | 2021-02-22 14:04:05 | | |
Video Upload Options
Chromoblastomycosis (CBM) is a neglected, chronic, and progressive subcutaneous mycosis caused by different species of fungi from the Herpotrichiellaceae family. CBM disease is usually associated with agricultural activities, and its infection is characterized by verrucous, erythematous papules, and atrophic lesions on the upper and lower limbs, leading to social stigma and impacts on patients’ welfare. The economic aspect of disease treatment is another relevant issue. There is no specific treatment for CBM, and different anti-fungal drug associations are used to treat the patients.
1. Introduction
Chromoblastomycosis (CBM) is a chronic, subcutaneous, and progressive mycosis caused by fungi from the Herpotrichiellaceae family. The main etiological agents of chromoblastomycosis are Fonsecaea pedrosoi, F. monophora, F. nubica, F. pugnacious, Phialophora verrucosa, Cladophialophora carrionii, Rhinocladiella aquaspersa, Exophiala spinifera, Aureobasidium pullulans, and Chaetomium funicol [1][2]. Like almost all fungal infections (maybe except for candidiasis and aspergillosis), CBM is a neglected tropical disease, affecting mainly rural workers and low-income people. The first report of the disease dates back to 1911 when two Brazilian physicians observed ulcerative and nodular skin lesions (similar to the lesions found in cutaneous blastomycosis) in four patients from the rural area of the Minas Gerais state [3]. Skin biopsies showed a brownish fungal structure while a filamentous colony with velvety-cotton aspects was observed in the lesion culture. Although the skin lesions were similar to those observed in cutaneous blastomycosis, the biopsy and culture findings suggested a different fungal disease. Therefore, Dr. Pedroso and Dr. Gomes temporarily named the disease as “black blastomycosis” and shipped the patients’ samples to Paris, for a complete mycology analysis. However, due to a critical moment in the world’s history (World War I, 1914–1918), these findings were only published in 1920 [4]. During this 9-year gap, between the initial contact with those four patients and the publishing of the article, a German physician working in Brazil, Dr. Maximilliano Willibaldo Rudolph, published a related case in a German journal in 1914 of about six patients with lower limb lesions caused by a black fungus. At that time, Dr. Rudolph named the disease “figueira disease”, since the patients were farmworkers working strictly with fig crops [5]. In 1936, an Argentinian researcher, Dr. Pablo Negroni, classified the fungus observed in the Brazilian patients (by Dr. Pedroso and Dr. Gomes, and also by Dr. Rudolph) as belonging to the Fonsecaea genus and, in honor of Dr. Pedroso, named the species as pedrosoi [6]. Since Dr. Rudolph has published the first case report of chromoblastomycosis in 1914, he is considered the “father” of this disease. However, the scientific community currently gives credit for the discovery of the disease to Dr. Pedroso and Dr. Gomes as their reported case started in 1911, despite not being published until 1920.
Although the disease was first reported in 1911–1920, the term chromoblastomycosis was suggested only in 1922 [7]. However, this term was not a concept at that time, and different names have been used all around the world to describe this disease, e.g., figueira disease, black blastomycosis, new blastomycosis, verrucous dermatitis caused by Phialophora verrucose, Pedroso disease, Fonseca disease, and Lane and Medlar disease, among others. However, in 1992, the International Society for Human and Animal Mycology stated that the nomenclature chromoblastomycosis is the correct one to describe this disease [8].
2. Epidemiology
Although observed worldwide, CBM is a disease that is often associated with tropical and subtropical climates with the majority of cases occurring in Brazil [9][10], Madagascar [11], Mexico [12], China [13], and Australia [4]. Since it is not a notifiable disease, it is difficult to precisely know the incidence and prevalence of CBM. Surveys and case reports in the literature suggest a prevalence of 1:6800 in Madagascar and 1:196,000 in Brazil [4][14]. CBM is a disease associated with rural workers, so it has been suggested that the natural habitat of the fungus is the environment, such as the soil and in several species of plants. However, the isolation of the pathogenic species from the environment is difficult. A couple of studies have isolated the saprophytic species from their natural habitat, but the pathogenic species have only been isolated in a few cases. Recently, a Brazilian group isolated pathogenic species from insects like bees, ants, and termites [15]. A typical example of pathogenic species recovered from the environment was observed in the state of Maranhão, in Brazil. A CBM outbreak was observed in this region in the 1990s, and the disease was diagnosed in farmworkers, specifically those working with babassu (Orbignya phalerata) crops, an economically important resource for the local population in Maranhão [16][17].
CBM infection is associated with skin lesions caused by infected plants, plant debris, thorns, or with the contact of a previous open wound with contaminated soil and leaves. Lesions in the upper and lower limbs are the most frequent symptom in CBM patients, and these are the body parts that are usually in contact with soil and plant debris. However, some patients have unusual lesions on the face, cornea, brain, chest, and abdomen [10][18][19][20][21]. Recently, a case of chronic fungal meningitis caused by F. pedrosoi was reported in a patient from an endemic region for tuberculosis in India [22], demonstrating the high virulence capacity of this fungus to cause human disease. Similar to other fungal infections, CBM treatment is challenging since it is costly, of long length, has moderate efficacy, and can present several side effects.
3. Immune Response in CBM
Since the first case report, in the early twentieth century, researchers worldwide have been working to understand how our immune system works against CBM infection. After more than 100 years of the discovery, a lot has been done to understand the immune response against this fungus. In the sections below, we discuss the most important data on the CBM immune response in the literature.
3.1. Adaptive Immune Response in CBM
One of the first published works in the literature observed that murine models of CBM showed disseminated disease, with live fungi in organs like the brain, liver, lungs, heart, spleen, and kidneys. The authors suggested that the cellular response is more protective to the host than the humoral response [23]. The significance of T lymphocytes in the control of CBM disease has been observed in experiments using nude animals (T-cell deficient mice). In the absence of T-cells, a severe disease was observed in these animals. However, after an adoptive T-cell transfer, the infected animals showed complete remission from the disease [24]. The following studies demonstrated that the CD4+ T lymphocytes are the subpopulation responsible for the control of the disease. The absence of CD8+ T lymphocytes did not interfere with the severity of the disease, at least in murine CBM [25]. Since CD4+ lymphocytes (especially Th1 subset) are important to IFN-γ production, the role of CD4+ lymphocytes was also observed in humans, whereby patients with mild symptoms of CBM showed higher levels of IFN-γ and lower levels of IL-10 than patients with severe symptoms of CBM [26][27]. Recently, in vitro experiments showed that F. pedrosoi drives CD4+ Th17 cell differentiation [28][29], while an increase in the IL-17 level was observed in skin lesions of murine CBM [30]. IL17 was also showed to be important in the early stage of the disease. Animals that had IL-17 blocked by anti-IL-17 injections showed a greater fungal burden during the early stages of the disease [31]. IL-17 is an important cytokine that is related to AMP release in the skin and fungal infections [32][33][34], helping with the killing of extracellular pathogens, such as hyphae. Although related to the polarization of anti-inflammatory macrophages (M2 macrophages) [35][36], IL-17 is an important cytokine for neutrophil migration. It is related to the increases in MIP-2 and KC, important chemokines in neutrophil attraction [37][38]. Therefore, the blockage of IL-17 could interfere with AMP release and neutrophil attraction to the infection site, leading to an increase in the fungal burden; thus, neutrophils are important in F. pedrosoi conidia and hyphae killing (as discussed below). However, the roles of the Th17 cell population and IL-17 in the control of CBM disease still remain unclear.
Although the cellular response is important for CBM control, the role of the humoral response in the disease is not fully determined yet. In the 1980s, one group demonstrated the capacity of F. pedrosoi-specific IgG antibodies to decrease 50% to 60% of fungal growth in vitro [39]. Later, a specific antibody to F. pedrosoi melanin was isolated and demonstrated to inhibit fungal growth and increase human neutrophil phagocytosis, as well as reactive oxygen species (ROS) production [40]. In 2010, Machado and colleagues observed that B-cell-deficient mice (Xid animals) had more severe CBM than wild-type animals, suggesting that the humoral response is essential for the control of the disease [41]. However, the patients’ samples showed conflicting results about the importance of the humoral response in CBM disease control. Esterre and colleagues first observed that IgG levels were directly related to disease severity [42], but this finding was not observed by Gimenez and colleagues [26]. Therefore, the actual consensus is that the CD4+ Th1 lymphocyte response is associated with better control of the disease, while the CD4+ Th2 lymphocytes are related to poor control and higher disease severity [43] (Figure 1). The importance of a humoral response in CBM disease needs deeper and specific studies.
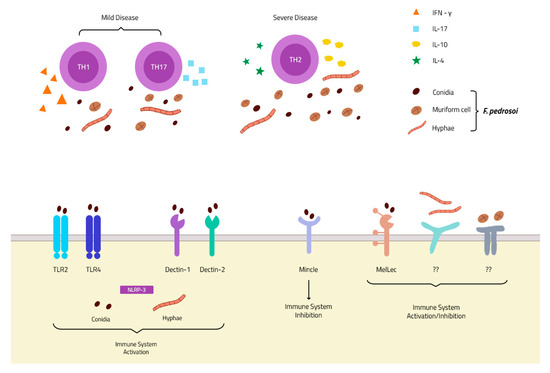
Figure 1. PRRs and immune response in chromoblastomycosis (CBM) infection: Membrane receptors of the TLR and CLR families described in F. pedrosoi conidia recognition. To date, only one receptor is described in hyphae recognition—the intracellular NLRP-3 inflammasome—and none is described in muriform cell recognition (Bottom). The Th1 and Th17 response is associated with mild disease, and an increase in IFN-γ and IL-17 secretion, while a Th2 response is related to severe disease with increases in IL-4 and IL-10 secretion and fungal spread (Top).
3.2. Innate Immune Response in CBM
Most studies of CBM’s immune response have focused on the innate immune response. In 1984, Torinuki and colleagues observed the activation of the alternative pathway of the complement system over F. pedrosoi [44]. Thereafter, it was identified that melanin, present in a high concentration in the F. pedrosoi cell wall, was triggering the activation of the alternative pathways of the complement system [45][46]. The complement system’s activation seems to be important for the opsonization (C3b) and chemotaxis process (C3a and C5a), which drives the innate immune response towards fungal clearance. However, due to the F. pedrosoi cell wall thickness, it is highly unlikely that the complement system acts to directly eliminate F. pedrosoi through formation of the membrane attack complex. Another important innate immune component is the macrophage. In vitro experiments have demonstrated that F. pedrosoi conidia ingested by peritoneal resident macrophages can survive and grow inside the macrophages, transforming into hyphae particles and leading to macrophage death through mechanical disruption of the cytoplasmic membrane [47]. Previous activation with IFN-γ will enable macrophages to block fungal growth inside it, acting as a Trojan horse and carrying live fungal particles around the host [48]. The inability of macrophages to kill ingested conidia is due to the capacity of F. pedrosoi to block nitric oxide production in macrophages, even after IFN-γ activation [49][50]. Therefore, although the levels of IFN-γ are related to a mild form of the disease, the mechanism of IFN-γ to act against CBM agents is still unclear. Unlike macrophages, neutrophils show a high in vitro microbicidal activity against F. pedrosoi [51][52]. In 2011, in an analysis of skin biopsies from mice infected with three different forms of F. pedrosoi (conidia, sclerotic bodies, and hyphae), Machado and colleagues observed dead fragments or injured fungal particles in neutrophilic areas, while intact or particles similar to the live particles were observed in regions of macrophage cells. These findings suggest that neutrophils are important for F. pedrosoi killing, not only in vitro but also in vivo. The authors also suggested that neutrophils release Neutrophil Extracellular Traps (NETs) to kill hyphae particles since they found an area of degenerative neutrophils surrounding hyphae particles in the skin biopsies [53], findings also observed by Dr. Ogawa and colleagues later on [54]. Recently, our group demonstrated that neutrophils kill conidia and hyphae in vitro in a distinct manner. Whilst conidia killing relies on phagocytosis and ROS production, neutrophils kill hyphae particles through ROS-independent Neutrophil Extracellular Trap (NET) release [51]. These findings, together with the increase of IL-17 in skin lesions of CBM patients, suggest that neutrophil attraction and activation are the most important roles of IL-17 in CBM infection, leading to F. pedrosoi killing and avoiding the spread of the disease.
Another important type of innate immune cell is the DC. These cells are important in the defense against several fungal infections and have a high migratory capacity, cytokine production, and expression of co-stimulatory molecules upon activation, which makes them the perfect bridge between the innate and adaptive immune systems (for a review of DCs and fungal infection, see [55]). Unlike macrophages and neutrophils, the role of DCs in CBM reported in the literature is sometimes contradictory. One of the studies using DCs obtained from the mouse skin (Langerhans cells) showed that the F. pedrosoi conidia block T-lymphocyte activation by inhibiting the CD40 and B7.2 expression in Langerhans cells in vitro [56]. However, our group demonstrated that monocyte-derived dendritic cells from CBM patients positively modulated HLA-DR and CD86 after conidia stimulation in vitro, leading to CD4+ Th1 lymphocyte differentiation and proliferation [57]. Since these studies focused on DCs in different parts of the body, more studies are needed to improve the understanding of the DCs’ role in CBM disease.
3.3. Pattern Recognition Receptors (PRRs)
The PRRs–PAMPs interaction is one of the most important and most-studied parts of the host’s strategy in pathogen recognition. The PRRs are located in the cell surface or in intracellular vacuoles, and several pathogen-associated molecular patterns (PAMPS) have been recognized. They are present in large amounts in APCs and innate immune cells, but it can also be found in other immune cells, such as B and T lymphocytes, and also in non-immune cells, such as keratinocytes [58][59], and melanocytes [60], present in our skin. Two of the most studied PRR classes are Toll-like receptors (TLRs) and C-type lectin receptors (CLRs).
Although a variety of studies have demonstrated the importance of these two classes of receptors in different fungal infections [61][62][63][64][65][66][67], little is known about their roles in F. pedrosoi recognition. Recently, a new receptor of DHN-melanin was discovered by Stappers and colleagues and named the Melanin sensing C-type Lectin receptor (MelLec). This discovery might be important for understanding the host immune response in CBM infection, where muriform cells have a higher concentration of melanin in the cell wall. It is the infection morphotype of the fungus, leading to the chronicity of the disease [68] (Figure 1). Another recent study demonstrated that Dectin-1 [69] and Dectin-2 [29] are important in F. pedrosoi recognition, stimulating the Th1 and Th17 responses, respectively. However, CBM agents bind to MINCLE, a new discovery receptor of the CLR family that inhibits and evades the host immune system. Fungal interaction with MINCLE leads to intracellular signaling that blocks IL-12 production (which was stimulated by Dectin-1 activation), inhibiting the Th1 differentiation of CD4+ lymphocytes [70] (Figure 1). This inhibition occurs through the IRF1 degradation after fungal recognition by the MINCLE receptor [69], leading to Th2 differentiation favoring the pathogen survival and infection spread. The MINCLE activation also inhibits Th17 differentiation, but its inhibition mechanism is not clear yet. Recently, Castro and colleagues demonstrated that F. pedrosoi conidia and hyphae activate the NLRP3 inflammasome, an important intracellular receptor belonging to the NOD-like receptor family (Figure 1). NLRP3 activation leads to IL-1β production in DCs and macrophages; however, IL-1β was not essential for the of control CBM disease, since no differences were observed in in vitro and in vivo experiments using NLRP3 KO animals [71]. Although several studies have focused on understanding the role of TLRs in fungal infections, almost no studies can be found in the literature regarding the roles of these receptors in CBM infection. A recent study showed an increase in tlr-2 and tlr-4 gene expression by macrophages infected with F. pedrosoi sclerotic bodies, but not with conidia. Later, our group demonstrated that TLR-2 and TLR-4 are essential receptors for F. pedrosoi conidia recognition and killing by murine neutrophils [51] (Figure 1). Although these receptors are important for conidia phagocytosis and ROS production, they are dispensable for hyphae killing through NET release.
Confirming the in vitro findings, TLR-2KO, TLR-4KO, and Myd88-KO animals infected with F. pedrosoi conidia showed poor prognoses compared with wild-type animals, demonstrating that the TLRs are an essential class of PRRs for the recognition and control of CBM agents [51][70]. Although fungal recognition by TLRs has proved to be important in the early stages of the disease, it seems that F. pedrosoi has the ability to hide from the host’s immune system. Sousa and colleagues (2011) observed that animals pretreated with LPS had a better immune response in CBM, leading to a mild disease followed by its cure. This work demonstrated that F. pedrosoi has the ability to hide or to trick the host immune system by being invisible to the host immune system, leading to the settlement, spread, and chronicity of the disease [70]. This is possible probably due to the cell wall complexity of this fungus, which insufficiently stimulates PRRs, leading to an inappropriate host immune response that favors the fungal survival and disease spread. The authors demonstrated that, after exogenous stimulation of the innate immune system by LPS-TLR4 or imiquimoid-TLR9 activation, the host immune response was restored, providing disease resolution. This costimulatory response also requires the CLR Mincle and signaling via the Syk/CARD9 pathway. LPS is a well-known macromolecule that is able to induce a strong immune response in mammalian cells via the TLR4 pathway, promoting the secretion of proinflammatory cytokines, including tumor necrosis factor-α (TNF-α) and interleukin-1 (IL-1) [72]. Therefore, the treatment of patients with LPS can boost the immune response against fungi and improve their clearance; however, its toxicity impairs its medicinal use [73]. Similar to LPS, imiquimod is a cytokine inducer and an innate immune booster that works through the agonistic activity of TLR7, inducing cell recruitment and activation with IFN-gamma production [74]. Imiquimod is a well-characterized compound. It is FDA-approved and the topical 5% cream is already being manufactured and commercialized [74].
Based on these findings, Souza and colleagues (2014) later reported a new treatment for CBM patients, including a topical cream with the TLR agonist, which was demonstrated to lead to significant improvement of the skin lesion (as discussed below) [75].
4. Immunotherapy Approaches
As mentioned above, CBM treatment is a therapeutic challenge. Although several methods and antimicrobials agents have been used in attempts to cure or control the disease, the high rate of relapses and the low cure rate, observed especially in severe and chronic cases, make CBM treatment a real challenge. Currently, CBM agents are not effectively controlled by a specific drug or by drug association, so the search for alternative methods, particularly those based on immune modulation, is necessary. Nevertheless, a defect in innate recognition results in the failure to mount robust inflammatory responses, causing infection susceptibility and chronicity [70]. Immunotherapy aim to stimulate or restore the ability of the immune system to fight against an infection, overcoming the host immune impairment. Currently, antifungal immunotherapies may target not only the host—such as in adoptive cell transfer, the cytokine therapies, the modulatory mAbs/AMPs, and the vaccine approaches—but also the pathogens, such as the monoclonal antibody (mAb) and the antimicrobial peptide (AMPs) treatments [76].
Studies aimed at enhancing host immune responses against CBM agents are being developed (Table 1). Some immunostimulant agents, like imiquimod and glucan, are capable of overcoming this defect, enhancing the inflammatory responses, which results in clearance of the pathogen and resolution of the infection. Imiquimod is a synthetic compound that stimulates both the innate and adaptive immune pathways through Toll-like receptor 7 (TLR7). This synthetic compound has potent antiviral, antitumor, and immunoregulatory activities. It was approved by the Food and Drug Administration (FDA) for the treatment of external genital and perianal warts (condylomata acuminata) as well as for the treatment of actinic keratosis and superficial basal cell carcinoma [77]. Recently, the role of exogenous administration of a TLR7 agonist (imiquimod) in stimulating the inflammatory response mediated by Syk/CARD9 through a Mydd88-dependent pathway was demonstrated [70]. According to this study, four patients were treated with topical imiquimod (5%) five times per week, with or without antifungal drugs. The treatment was effective and provided not only clinical improvement but also a cure [75]. Belda and colleagues also reported a successful regression of lesions in patients treated with imiquimod as a monotherapy. It is interesting that both reports observed a transitory exacerbation of the verrucous and infiltrative characteristics of the lesion that preceded its gradual evolution to healing [78]. This finding highlights the ability of imiquimod to restore the host’s immune system, inducing a beneficial inflammatory response against the CBM agent.
In addition to imiquimod, treatment with Glucan is an immunotherapy that has been suggested for CBM treatment. Glucan is a carbohydrate that is a compound of the cell wall of several fungi, and there is evidence that this molecule has a positive effect on the treatment of diseases since it can stimulate innate immune responses. Clinical improvement of a CBM patient treated with glucan was reported by Azevedo and colleagues [79]. The authors reported significant lesion regression in a severe form of CBM after Glucan and itraconazole (ITZ) treatment in a patient with a 3-year history of unsuccessful treatment with only antifungal drugs. Glucan administration once a week with ITZ improved the cellular immune response of the patient and might be an alternative/adjuvant to treat this disease. Similar results were also demonstrated in Candida spp. [80] and Aspergillus spp. [81] infection.
Another important strategy to prevent and cure fungal disease is the development of vaccines. Several studies have uncovered components of the fungal cell wall that stimulate protective antibody production, making them good candidates for vaccines. Although it is not clear whether the humoral immune response is protective in CBM, it is known that CBM agents induce a highly specific humoral response in people living in endemic areas [82]. Some studies using mAb against glucosylceramide (GlcCer) and melanin have demonstrated the great potential of this therapeutic approach in CBM treatment. In vitro experiments using Mab against fungal GlcCer showed the inhibition of F. pedrosoi conidial growth and an the enhancement of the antifungal functions of murine macrophages [83]. However, this mAb is not completely effective against muriform cells because they were only able to recognize them at cell wall regions without melanization [84]. mAb treatment against melanin was also shown to be protective for the host. Melanin is a pigment present in a high concentration in chromoblastomycosis agents’ cell walls, and it is an important virulent factor that protects the fungi against destruction by immune cells [4][47]. The melanin of Aspergillus protects conidia from macrophage phagocytosis through Ca2+ sequestration, impairing cell homeostasis, and inhibiting the LC3-associated phagocytosis (LAP) pathway [85]. Due to the importance of this wall cell component in pathogen virulence, antibodies targeting F. pedrosoi melanin have been developed. The human antibody anti-melanin inhibits the in vitro growth of conidial and sclerotic cells [40]. In addition, soluble melanin improves the antifungal capacity of human neutrophils by enhancing phagocytosis and oxidative burst [40]. Although anti-melanin antibodies increase the phagocyte efficiency, some patients become close to achromic, either during the course of the disease or after the treatment [86]. This side effect suggests the occurrence of a cross-reaction with human melanocytes, leading to the development of vitiligo after immunotherapy.
Table 1. Features of the reported chromoblastomycosis immunotherapeutic approaches.
|
Immunotherapy Class |
Immunotherapy Agent |
Mechanisms |
Dose |
Study |
Protocol |
Antifungal Association |
Treatment Outcome |
|
Immunostimulants |
Imiquimod |
Toll receptor 7 agonist |
5% of imiquimod |
Human |
Five times a week (topical) for 6 to 19 months |
Monotherapy |
Lesions healed after 6 to 7 months120 |
|
Five times a week (topical) for 6 to 17 months |
Monotherapy or ITZ and/or TERB |
Improvement in clinical aspects117 |
|||||
|
β1,3 glucan |
T cell proliferation and IFN-γ production |
5 mg |
Human |
Once a week (IM) for 2 years |
ITZ |
Resolution of the majority of lesions121 |
|
|
DNA Vaccine |
DNA-hsp65 vaccine |
Reduction in NO production |
9 mg |
Mice |
Once each 15 days (IM) during 15 or 30 days |
Monotherapy or ITZ and/or AMB |
Healed injuries and eliminated F. pedrosoi from lesions127 |
|
Monoclonal antibody (Mab) therapy |
Mab anti-GlcCer |
Fungistatic and fungicidal activities |
NA |
In vitro |
NA |
NA |
Inhibition of F. pedrosoi growth in vitro and enhanced antifungal activity of murine macrophages37 |
|
Purified antibodies |
Purified antibodies anti-Melanin |
Fungicidal activities |
NA |
in vitro |
NA |
NA |
Inhibition of F. pedrosoi growth in vitro86 |
Abbreviations: AMB, amphotericin B; CBM, chromoblastomycosis; IM, intramuscular; ITZ, itraconazole; Mab, monoclonal antibodies; NA, not applied; TERB, terbinafine; GlcCer, glucosylceramides.
Immunostimulants or vaccines may improve the immune system, leading to host protection, and can be an adjuvant to antifungal drugs, which could reduce the treatment time and costs, increasing its success. Currently, there is no licensed vaccine for the prevention or treatment of human CBM or any mycosis. A conventional vaccine may be a live-attenuated or inactivated microorganism or a subunit that is able to stimulate the adaptive immune system, leading to host immune memory. A DNA-hsp65 vaccine either in association or not with intralesional administration of itraconazole and amphotericin B or not was effective for treating experimental CBM [87]. With regard to vaccine subunits, several antigens from fungal pathogens have been described, such as proteins, carbohydrates and polysaccharides, and lipids, and the peptide vaccine has emerged as a promising advance in immunotherapy. The advantages of peptide-based vaccines include easy production on a large scale with high purity; the possibility of being stored at room temperature, since it may be freeze-dried; the target specificity and induction of a proper immune response; and the reduced risk of side effects [88]. The main weakness of this vaccine, on the other hand, is the low immunogenicity; however, it can be improved through the development of better adjuvant-based delivery systems [89] or bioengineered molecules with more than two peptides [90]. Dendritic cells (DCs), as described above, play an important role in both innate and adaptive immune responses, and because of their efficiency in activating T cells and inducting memory-like responses in vivo [91], DCs may be a promising approach for vaccination. Mice protectively immunized with C. neoformans displayed a DC polarization phenotype inducing high IFN-γ and enhanced pro-inflammatory cytokine responses upon a subsequent challenge. In 2011, Magalhães and colleagues showed the therapeutic or prophylactic effects of DCs primed with peptide 10 (P10), a gp43 peptide derived from Paracoccidioides brasiliensis, in mice challenged with a virulent isolate, improving the lung fungal clearance [92]. Likewise, the administration of P10-primed DCs to an immunosuppressed mouse was also effective in combating P. brasiliensis infection. Until now, neither therapeutic DCs nor immunogenic peptides towards CBM treatment have been reported, but there are some research groups involved in this field for the development of peptide vaccines and DC pulses.
References
- Queiróz, A.J.R.; Pereira Domingos, F.; Antônio, J.R. Chromoblastomycosis: Clinical experience and review of literature. Int. J. Derm. 2018, 57, 1351–1355.
- Caligiorne, R.B.; De Resende, M.A.; Dias-Neto, E.; Oliveira, S.C.; Azevedo, V. Dematiaceous fungal pathogens: Analysis of ribosomal DNA gene polymorphism by polymerase chain reaction-restriction fragment length polymorphism. Mycoses 1999, 42, 609–614.
- Pedroso, A. Sobre quatro casos de dermatite verrucosa produzida pela Phialophora verrucosa. Gomes J. Ed. Paul Med. Cir. 1920, 11, 53–61.
- Queiroz-Telles, F.; de Hoog, S.; Santos, D.W.; Salgado, C.G.; Vicente, V.A.; Bonifaz, A.; Roilides, E.; Xi, L.; Azevedo, C.M.; da Silva, M.B.; et al. Chromoblastomycosis. Clin. Microbiol. Rev. 2017, 30, 233–276.
- Rudolph, M. Über die brasilianische “Figueira” (Vorläufige Mitteilung). Arch. Schiffs Trop. Hyg. 1914, 18, 498–499.
- Negroni, P. Estudio del primer caso Argentino de cromomicosis, Fonsecaea (Negroni) pedrosoi (Brumpt 1921). Rev. Inst. Bacteriol. 1936, 7, 419–426.
- Terra, F.; Torres, M.; da Fonseca, O.; Area Leao, A.D. Novo typo de dermatite verrucosa mycose por Acrotheca com associacao de leishmaniosa. Bras. Med. 1922, 36, 363–368.
- Odds, F.C.; Arai, T.; Disalvo, A.F.; Evans, E.G.; Hay, R.J.; Randhawa, H.S.; Rinaldi, M.G.; Walsh, T.J. Nomenclature of fungal diseases: A report and recommendations from a Sub-Committee of the International Society for Human and Animal Mycology (ISHAM). J. Med. Vet. Mycol. 1992, 30, 1–10.
- Silva, J.P.; de Souza, W.; Rozental, S. Chromoblastomycosis: A retrospective study of 325 cases on Amazonic Region (Brazil). Mycopathologia 1998, 143, 171–175.
- Minotto, R.; Bernardi, C.D.; Mallmann, L.F.; Edelweiss, M.I.; Scroferneker, M.L. Chromoblastomycosis: A review of 100 cases in the state of Rio Grande do Sul, Brazil. J. Am. Acad Derm. 2001, 44, 585–592.
- Esterre, P.; Andriantsimahavandy, A.; Ramarcel, E.R.; Pecarrere, J.L. Forty years of chromoblastomycosis in Madagascar: A review. Am. J. Trop. Med. Hyg. 1996, 55, 45–47.
- Bonifaz, A.; Carrasco-Gerard, E.; Saúl, A. Chromoblastomycosis: Clinical and mycologic experience of 51 cases. Mycoses 2001, 44, 1–7.
- Lu, S.; Lu, C.; Zhang, J.; Hu, Y.; Li, X.; Xi, L. Chromoblastomycosis in Mainland China: A systematic review on clinical characteristics. Mycopathologia 2013, 175, 489–495.
- Gomes, R.R.; Vicente, V.A.; Azevedo, C.M.; Salgado, C.G.; da Silva, M.B.; Queiroz-Telles, F.; Marques, S.G.; Santos, D.W.; de Andrade, T.S.; Takagi, E.H.; et al. Molecular Epidemiology of Agents of Human Chromoblastomycosis in Brazil with the Description of Two Novel Species. PLoS Negl. Trop. Dis. 2016, 10, e0005102.
- Lima, B.J.F.S.; Voidaleski, M.F.; Gomes, R.R.; Fornari, G.; Soares, J.M.B.; Bombassaro, A.; Schneider, G.X.; Soley, B.D.S.; de Azevedo, C.M.P.E.; Menezes, C.; et al. Selective isolation of agents of chromoblastomycosis from insect-associated environmental sources. Fungal Biol. 2020, 124, 194–204.
- Silva, C.M.; da Rocha, R.M.; Moreno, J.S.; Branco, M.R.; Silva, R.R.; Marques, S.G.; Costa, J.M. The coconut babaçu (Orbignya phalerata martins) as a probable risk of human infection by the agent of chromoblastomycosis in the State of Maranhão, Brazil. Rev. Soc. Bras. Med. Trop. 1995, 28, 49–52.
- Mello e Silva, A.C.; Serra Neto, A.; Galvão, C.E.; Marques, S.G.; Saldanha, A.C.; Pedroso e Silva, C.M.; Fischman, O.; da Silva, R.R.; Costa, M.o.R.; Costa, J.M. Fonsecaea pedrosoi-caused chromoblastomycosis in the state of Maranhão. I. The clinical, epidemiological and evolutionary aspects. Rev. Soc. Bras. Med. Trop. 1992, 25, 37–44.
- Rippon, J.W. Chromoblastomycosis; Harbour Brace Jovanovich Inc.: Phildelphia, PA, USA, 1988.
- Bittencourt, A.L.; Londero, A.T.; Andrade, J.A. Auricular chromoblastomycosis. A case report. Rev. Inst. Med. Trop. Sao Paulo 1994, 36, 381–383.
- Höfling-Lima, A.L.; Guarro, J.; Freitas, D.; Godoy, P.; Gené, J.; Souza, L.B.; Zaror, L.; Romano, A.C. Clinical treatment of corneal infection due to Fonsecaea pedrosoi—Case report. Arq Bras. Oftalmol. 2005, 68, 270–272.
- Salgado, C.G.; da Silva, J.P.; da Silva, M.B.; da Costa, P.F.; Salgado, U.I. Cutaneous diffuse chromoblastomycosis. Lancet Infect. Dis. 2005, 5, 528.
- Hesarur, N.; Seshagiri, D.V.; Nagappa, M.; Rao, S.; Santosh, V.; Chandrashekar, N.; Reddy, N.; Sharma, P.P.; Kumari, P.; Pruthi, N.; et al. Case Report: Chronic fungal Meningitis Masquerading as Tubercular Meningitis. Am. J. Trop. Med. Hyg. 2020, 103, 1473–1479.
- Kurita, N. Cell-mediated immune responses in mice infected with Fonsecaea pedrosoi. Mycopathologia 1979, 68, 9–15.
- Ahrens, J.; Graybill, J.R.; Abishawl, A.; Tio, F.O.; Rinaldi, M.G. Experimental murine chromomycosis mimicking chronic progressive human disease. Am. J. Trop. Med. Hyg. 1989, 40, 651–658.
- Teixeira de Sousa, M.a.G.; Ghosn, E.E.; Almeida, S.R. Absence of CD4+ T cells impairs host defence of mice infected with Fonsecaea pedrosoi. Scand. J. Immunol. 2006, 64, 595–600.
- Mazo Fávero Gimenes, V.; Da Glória de Souza, M.; Ferreira, K.S.; Marques, S.G.; Gonçalves, A.G.; Vagner de Castro Lima Santos, D.; Pedroso e Silva, C.E.M.; Almeida, S.R. Cytokines and lymphocyte proliferation in patients with different clinical forms of chromoblastomycosis. Microbes Infect. 2005, 7, 708–713.
- Sousa, M.G.; de Maria Pedrozo e Silva Azevedo, C.; Nascimento, R.C.; Ghosn, E.E.; Santiago, K.L.; Noal, V.; Bomfim, G.F.; Marques, S.G.; Gonçalves, A.G.; Wagner de Castro Lima Santos, D.; et al. Fonsecaea pedrosoi infection induces differential modulation of costimulatory molecules and cytokines in monocytes from patients with severe and mild forms of chromoblastomycosis. J. Leukoc. Biol. 2008, 84, 864–870.
- Dong, B.; Li, D.; Li, R.; Chen, S.C.; Liu, W.; Chen, L.; Chen, Y.; Zhang, X.; Tong, Z.; Xia, Y.; et al. A chitin-like component on sclerotic cells of Fonsecaea pedrosoi inhibits Dectin-1-mediated murine Th17 development by masking β-glucans. PLoS ONE 2014, 9, e114113.
- Wüthrich, M.; Wang, H.; Li, M.; Lerksuthirat, T.; Hardison, S.E.; Brown, G.D.; Klein, B. Fonsecaea pedrosoi-induced Th17-cell differentiation in mice is fostered by Dectin-2 and suppressed by Mincle recognition. Eur. J. Immunol. 2015, 45, 2542–2552.
- Silva, A.A.; Criado, P.R.; Nunes, R.S.; da Silva, W.L.; Kanashiro-Galo, L.; Duarte, M.I.; Sotto, M.N.; Pagliari, C. In situ immune response in human chromoblastomycosis--a possible role for regulatory and Th17 T cells. PLoS Negl. Trop. Dis. 2014, 8, e3162.
- Siqueira, I.M.; Wüthrich, M.; Li, M.; Wang, H.; Las-Casas, L.O.; de Castro, R.J.A.; Klein, B.; Bocca, A.L. Early immune response against Fonsecaea pedrosoi requires Dectin-2-mediated Th17 activity, whereas Th1 response, aided by Treg cells, is crucial for fungal clearance in later stage of experimental chromoblastomycosis. PLoS Negl. Trop. Dis. 2020, 14, e0008386.
- Conti, H.R.; Gaffen, S.L. IL-17-Mediated Immunity to the Opportunistic fungal Pathogen Candida albicans. J. Immunol. 2015, 195, 780–788.
- Kuwabara, T.; Ishikawa, F.; Kondo, M.; Kakiuchi, T. The Role of IL-17 and Related Cytokines in Inflammatory Autoimmune Diseases. Mediat. Inflamm. 2017, 2017, 3908061.
- Kao, C.Y.; Chen, Y.; Thai, P.; Wachi, S.; Huang, F.; Kim, C.; Harper, R.W.; Wu, R. IL-17 markedly up-regulates beta-defensin-2 expression in human airway epithelium via JAK and NF-kappaB signaling pathways. J. Immunol. 2004, 173, 3482–3491.
- Shen, J.; Sun, X.; Pan, B.; Cao, S.; Cao, J.; Che, D.; Liu, F.; Zhang, S.; Yu, Y. IL-17 induces macrophages to M2-like phenotype via NF-κB. Cancer Manag. Res. 2018, 10, 4217–4228.
- Hardison, S.E.; Wozniak, K.L.; Kolls, J.K.; Wormley, F.L. Interleukin-17 is not required for classical macrophage activation in a pulmonary mouse model of Cryptococcus neoformans infection. Infect. Immun. 2010, 78, 5341–5351.
- Laan, M.; Cui, Z.H.; Hoshino, H.; Lötvall, J.; Sjöstrand, M.; Gruenert, D.C.; Skoogh, B.E.; Lindén, A. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J. Immunol. 1999, 162, 2347–2352.
- Yu, J.J.; Ruddy, M.J.; Wong, G.C.; Sfintescu, C.; Baker, P.J.; Smith, J.B.; Evans, R.T.; Gaffen, S.L. An essential role for IL-17 in preventing pathogen-initiated bone destruction: Recruitment of neutrophils to inflamed bone requires IL-17 receptor-dependent signals. Blood 2007, 109, 3794–3802.
- Ibrahim-Granet, O.; de Bièvre, C.; Jendoubi, M. Immunochemical characterisation of antigens and growth inhibition of Fonsecaea pedrosoi by species-specific IgG. J. Med. Microbiol. 1988, 26, 217–222.
- Alviano, D.S.; Franzen, A.J.; Travassos, L.R.; Holandino, C.; Rozental, S.; Ejzemberg, R.; Alviano, C.S.; Rodrigues, M.L. Melanin from Fonsecaea pedrosoi induces production of human antifungal antibodies and enhances the antimicrobial efficacy of phagocytes. Infect. Immun. 2004, 72, 229–237.
- Machado, A.P.; Silva, M.R.; Fischman, O. Prolonged infection by Fonsecaea pedrosoi after antigenic co-stimulation at different sites in experimental murine chromoblastomycosis. Virulence 2010, 1, 29–36.
- Esterre, P.; Jahevitra, M.; Andriantsimahavandy, A. Humoral immune response in chromoblastomycosis during and after therapy. Clin. Diagn Lab. Immunol. 2000, 7, 497–500.
- D’Avila, S.C.; Pagliari, C.; Duarte, M.I. The cell-mediated immune reaction in the cutaneous lesion of chromoblastomycosis and their correlation with different clinical forms of the disease. Mycopathologia 2003, 156, 51–60.
- Torinuki, W.; Okohchi, K.; Takematsu, H.; Tagami, H. Activation of the alternative complement pathway by Fonsecaea pedrosoi. J. Investig. Dermatol. 1984, 83, 308–310.
- Pinto, L.; Granja, L.F.; Alviano, D.S.; da Silva, M.H.; Alviano, C.S.; Ejzemberg, R. Activation of the human complement system by pigmented and hypopigmented mycelia of the fungus Fonsecaea pedrosoi. Mycoses 2011, 54, e474–e480.
- Pinto, L.; Granja, L.F.Z.; Almeida, M.A.; Alviano, D.S.; Silva, M.H.D.; Ejzemberg, R.; Rozental, S.; Alviano, C.S. Melanin particles isolated from the fungus Fonsecaea pedrosoi activates the human complement system. Mem. Inst. Oswaldo Cruz 2018, 113, e180120.
- Farbiarz, S.R.; De Carvalho, T.U.; Alviano, C.; De Souza, W. Fine structure and cytochemistry of the interaction between Fonsecaea pedrosoi and mouse resident macrophages. J. Med. Vet. Mycol. 1990, 28, 373–383.
- Rozental, S.; Alviano, C.S.; de Souza, W. The in vitro susceptibility of Fonsecaea pedrosoi to activated macrophages. Mycopathologia 1994, 126, 85–91.
- Hayakawa, M.; Ghosn, E.E.; da Gloria Teixeria de Sousa, M.; Ferreira, K.S.; Almeida, S.R. Phagocytosis, production of nitric oxide and pro-inflammatory cytokines by macrophages in the presence of dematiaceous [correction of dematiaceus] fungi that cause chromoblastomycosis. Scand. J. Immunol. 2006, 64, 382–387.
- Bocca, A.L.; Brito, P.P.; Figueiredo, F.; Tosta, C.E. Inhibition of nitric oxide production by macrophages in chromoblastomycosis: A role for Fonsecaea pedrosoi melanin. Mycopathologia 2006, 161, 195–203.
- Breda, L.C.D.; Breda, C.N.S.; de Almeida, J.R.F.; Paulo, L.N.M.; Jannuzzi, G.P.; Menezes, I.G.; Albuquerque, R.C.; Câmara, N.O.S.; Ferreira, K.S.; de Almeida, S.R. Conidia and Hyphae Activate Neutrophils Distinctly: Requirement of TLR-2 and TLR-4 in Neutrophil Effector Functions. Front. Immunol. 2020, 11, 540064.
- Rozental, S.; Alviano, C.S.; de Souza, W. Fine structure and cytochemical study of the interaction between Fonsecaea pedrosoi and rat polymorphonuclear leukocyte. J. Med. Vet. Mycol. 1996, 34, 323–330.
- Machado, A.P.; Silva, M.R.; Fischman, O. Local phagocytic responses after murine infection with different forms of Fonsecaea pedrosoi and sclerotic bodies originating from an inoculum of conidiogenous cells. Mycoses 2011, 54, 202–211.
- Ogawa, M.M.; Mariano, M.; Silva, M.R.R.; Enokihara, M.M.S.E.; Michalany, N.S.; Nishikaku, A.S.; Silvestre, A.M.; Tomimori, J. Study of tissue inflammatory response in different mice strains infected by dematiaceous fungi Fonsecaea pedrosoi. An. Bras. Dermatol. 2019, 94, 29–36.
- Roy, R.M.; Klein, B.S. Dendritic cells in antifungal immunity and vaccine design. Cell Host Microbe 2012, 11, 436–446.
- da Silva, J.P.; da Silva, M.B.; Salgado, U.I.; Diniz, J.A.; Rozental, S.; Salgado, C.G. Phagocytosis of Fonsecaea pedrosoi conidia, but not sclerotic cells caused by Langerhans cells, inhibits CD40 and B7-2 expression. FEMS Immunol. Med. Microbiol. 2007, 50, 104–111.
- Sousa, M.G.; Ghosn, E.E.; Nascimento, R.C.; Bomfim, G.F.; Noal, V.; Santiago, K.; de Maria Pedrozo, E.; Silva Azevedo, C.; Marques, S.G.; Gonçalves, A.G.; et al. Monocyte-derived dendritic cells from patients with severe forms of chromoblastomycosis induce CD4+ T cell activation in vitro. Clin. Exp. Immunol. 2009, 156, 117–125.
- Miller, L.S. Toll-like receptors in skin. Adv. Dermatol. 2008, 24, 71–87.
- Kawai, K.; Shimura, H.; Minagawa, M.; Ito, A.; Tomiyama, K.; Ito, M. Expression of functional Toll-like receptor 2 on human epidermal keratinocytes. J. Dermatol. Sci. 2002, 30, 185–194.
- Jin, S.H.; Kang, H.Y. Activation of Toll-like Receptors 1, 2, 4, 5, and 7 on Human Melanocytes Modulate Pigmentation. Ann. Dermatol. 2010, 22, 486–489.
- Carrion, S.e.J.; Leal, S.M.; Ghannoum, M.A.; Aimanianda, V.; Latgé, J.P.; Pearlman, E. The RodA hydrophobin on Aspergillus fumigatus spores masks dectin-1- and dectin-2-dependent responses and enhances fungal survival in vivo. J. Immunol. 2013, 191, 2581–2588.
- Drummond, R.A.; Dambuza, I.M.; Vautier, S.; Taylor, J.A.; Reid, D.M.; Bain, C.C.; Underhill, D.M.; Masopust, D.; Kaplan, D.H.; Brown, G.D. CD4(+) T-cell survival in the GI tract requires dectin-1 during fungal infection. Mucosal Immunol. 2016, 9, 492–502.
- Griffiths, J.S.; Thompson, A.; Stott, M.; Benny, A.; Lewis, N.A.; Taylor, P.R.; Forton, J.; Herrick, S.; Orr, S.J.; McGreal, E.P. Differential susceptibility of Dectin-1 isoforms to functional inactivation by neutrophil and fungal proteases. FASEB J. 2018, 32, 3385–3397.
- McDonald, J.U.; Rosas, M.; Brown, G.D.; Jones, S.A.; Taylor, P.R. Differential dependencies of monocytes and neutrophils on dectin-1, dectin-2 and complement for the recognition of fungal particles in inflammation. PLoS ONE 2012, 7, e45781.
- Wells, C.A.; Salvage-Jones, J.A.; Li, X.; Hitchens, K.; Butcher, S.; Murray, R.Z.; Beckhouse, A.G.; Lo, Y.L.; Manzanero, S.; Cobbold, C.; et al. The macrophage-inducible C-type lectin, mincle, is an essential component of the innate immune response to Candida albicans. J. Immunol. 2008, 180, 7404–7413.
- Goyal, S.; Castrillón-Betancur, J.C.; Klaile, E.; Slevogt, H. The Interaction of Human Pathogenic Fungi With C-Type Lectin Receptors. Front. Immunol. 2018, 9, 1261.
- Drummond, R.A.; Gaffen, S.L.; Hise, A.G.; Brown, G.D. Innate Defense against fungal Pathogens. Cold Spring Harb. Perspect. Med. 2014, 5, a019620.
- Stappers, M.H.T.; Clark, A.E.; Aimanianda, V.; Bidula, S.; Reid, D.M.; Asamaphan, P.; Hardison, S.E.; Dambuza, I.M.; Valsecchi, I.; Kerscher, B.; et al. Recognition of DHN-melanin by a C-type lectin receptor is required for immunity to Aspergillus. Nature 2018, 555, 382–386.
- Wevers, B.A.; Kaptein, T.M.; Zijlstra-Willems, E.M.; Theelen, B.; Boekhout, T.; Geijtenbeek, T.B.; Gringhuis, S.I. fungal engagement of the C-type lectin mincle suppresses dectin-1-induced antifungal immunity. Cell Host Microbe 2014, 15, 494–505.
- Sousa, M.a.G.; Reid, D.M.; Schweighoffer, E.; Tybulewicz, V.; Ruland, J.; Langhorne, J.; Yamasaki, S.; Taylor, P.R.; Almeida, S.R.; Brown, G.D. Restoration of pattern recognition receptor costimulation to treat chromoblastomycosis, a chronic fungal infection of the skin. Cell Host Microbe 2011, 9, 436–443.
- De Castro, R.J.A.; Siqueira, I.M.; Jerônimo, M.S.; Basso, A.M.M.; Veloso Junior, P.H.H.; Magalhães, K.G.; Leonhardt, L.C.; de Oliveira, S.A.M.; Bürgel, P.H.; Tavares, A.H.; et al. The Major Chromoblastomycosis Etiologic Agent. Front. Immunol. 2017, 8, 1572.
- Cochet, F.; Peri, F. The Role of Carbohydrates in the Lipopolysaccharide (LPS)/Toll-Like Receptor 4 (TLR4) Signalling. Int. J. Mol. Sci. 2017, 18, 2318.
- Johnson, D.A. Synthetic TLR4-active glycolipids as vaccine adjuvants and stand-alone immunotherapeutics. Curr. Top. Med. Chem. 2008, 8, 64–79.
- Vidal, D.; Alomar, A. Mode of activation and Clinical use of Imiquimod. Expert Rev. Dermatol. 2014, 2, 151–159.
- De Sousa, M.A.G.; Belda, W.; Spina, R.; Lota, P.R.; Valente, N.S.; Brown, G.D.; Criado, P.R.; Benard, G. Topical application of imiquimod as a treatment for chromoblastomycosis. Clin. Infect. Dis. 2014, 58, 1734–1737.
- Datta, K.; Hamad, M. Immunotherapy of fungal Infections. Immunol. Invest. 2015, 44, 738–776.
- Patinote, C.; Karroum, N.B.; Moarbess, G.; Cirnat, N.; Kassab, I.; Bonnet, P.A.; Deleuze-Masquéfa, C. Agonist and antagonist ligands of toll-like receptors 7 and 8: Ingenious tools for therapeutic purposes. Eur. J. Med. Chem. 2020, 193, 112238.
- Belda, W.; Criado, P.R.; Passero, L.F.D. Successful treatment of chromoblastomycosis caused by Fonsecaea pedrosoi using imiquimod. J. Dermatol. 2020, 47, 409–412.
- Azevedo, C.E.M.; Marques, S.G.; Resende, M.A.; Gonçalves, A.G.; Santos, D.V.; da Silva, R.R.; de Sousa, M.A.G.; de Almeida, S.R. The use of glucan as immunostimulant in the treatment of a severe case of chromoblastomycosis. Mycoses 2008, 51, 341–344.
- Pietrella, D.; Rachini, A.; Torosantucci, A.; Chiani, P.; Brown, A.J.; Bistoni, F.; Costantino, P.; Mosci, P.; d’Enfert, C.; Rappuoli, R.; et al. A beta-glucan-conjugate vaccine and anti-beta-glucan antibodies are effective against murine vaginal candidiasis as assessed by a novel in vivo imaging technique. Vaccine 2010, 28, 1717–1725.
- Clemons, K.V.; Danielson, M.E.; Michel, K.S.; Liu, M.; Ottoson, N.C.; Leonardo, S.M.; Martinez, M.; Chen, V.; Antonysamy, M.A.; Stevens, D.A. Whole glucan particles as a vaccine against murine aspergillosis. J. Med. Microbiol. 2014, 63, 1750–1759.
- Esterre, P.; Andriantsimahavandy, A.; Raharisolo, C. Natural history of chromoblastomycosis in Madagascar and the Indian Ocean. Natural history of chromoblastomycosis in Madagascar and the Natural history of chromoblastomycosis in Madagascar and the Indian Ocean. Bull. Soc. Pathol. Exot. 1997, 90, 312–317.
- Nimrichter, L.; Barreto-Bergter, E.; Mendonça-Filho, R.R.; Kneipp, L.F.; Mazzi, M.T.; Salve, P.; Farias, S.E.; Wait, R.; Alviano, C.S.; Rodrigues, M.L. A monoclonal antibody to glucosylceramide inhibits the growth of Fonsecaea pedrosoi and enhances the antifungal action of mouse macrophages. Microbes Infect. 2004, 6, 657–665.
- Nimrichter, L.; Cerqueira, M.D.; Leitão, E.A.; Miranda, K.; Nakayasu, E.S.; Almeida, S.R.; Almeida, I.C.; Alviano, C.S.; Barreto-Bergter, E.; Rodrigues, M.L. Structure, cellular distribution, antigenicity, and biological functions of Fonsecaea pedrosoi ceramide monohexosides. Infect. Immun. 2005, 73, 7860–7868.
- Kyrmizi, I.; Ferreira, H.; Carvalho, A.; Figueroa, J.A.L.; Zarmpas, P.; Cunha, C.; Akoumianaki, T.; Stylianou, K.; Deepe, G.S.; Samonis, G.; et al. Calcium sequestration by fungal melanin inhibits calcium-calmodulin signalling to prevent LC3-associated phagocytosis. Nat. Microbiol. 2018, 3, 791–803.
- Vidal, M.S.; Castro, L.G.; Cavalcante, S.C.; Lacaz, C.S. Highly specific and sensitive, immunoblot-detected 54 kDa antigen from Fonsecaea pedrosoi. Med. Mycol. 2004, 42, 511–515.
- Siqueira, I.M.; Ribeiro, A.M.; Nóbrega, Y.K.; Simon, K.S.; Souza, A.C.; Jerônimo, M.S.; Cavalcante Neto, F.F.; Silva, C.L.; Felipe, M.S.; Bocca, A.L. DNA-hsp65 vaccine as therapeutic strategy to treat experimental chromoblastomycosis caused by Fonsecaea pedrosoi. Mycopathologia 2013, 175, 463–475.
- Draper, S.J.; Angov, E.; Horii, T.; Miller, L.H.; Srinivasan, P.; Theisen, M.; Biswas, S. Recent advances in recombinant protein-based malaria vaccines. Vaccine 2015, 33, 7433–7443.
- Mehta, N.K.; Pradhan, R.V.; Soleimany, A.P.; Moynihan, K.D.; Rothschilds, A.M.; Momin, N.; Rakhra, K.; Mata-Fink, J.; Bhatia, S.N.; Wittrup, K.D.; et al. Pharmacokinetic tuning of protein-antigen fusions enhances the immunogenicity of T-cell vaccines. Nat. BioMed. Eng. 2020, 4, 636–648.
- Shi, H.; Dong, S.; Zhang, X.; Chen, X.; Gao, X.; Wang, L. Phage vaccines displaying Ygkdvkdlfdyaqe epitope induce protection against systemic candidiasis in mouse model. Vaccine 2018, 36, 5717–5724.
- Hole, C.R.; Wager, C.M.L.; Castro-Lopez, N.; Campuzano, A.; Cai, H.; Wozniak, K.L.; Wang, Y.; Wormley, F.L. Induction of memory-like dendritic cell responses in vivo. Nat. Commun. 2019, 10, 2955.
- Magalhães, A.; Ferreira, K.S.; Almeida, S.R.; Nosanchuk, J.D.; Travassos, L.R.; Taborda, C.P. Prophylactic and therapeutic vaccination using dendritic cells primed with peptide 10 derived from the 43-kilodalton glycoprotein of Paracoccidioides brasiliensis. Clin. Vaccine Immunol. 2012, 19, 23–29.




