
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Antonella Curulli | + 4246 word(s) | 4246 | 2021-01-22 06:52:37 |
Video Upload Options
Recently, nanomaterials have received increasing attention due to their unique physical and chemical properties, which make them of considerable interest for applications in many fields, such as biotechnology, optics, electronics, and catalysis. The development of nanomaterials has proven fundamental for the development of smart electrochemical sensors to be used in different application fields such, as biomedical, environmental, and food analysis. In fact, they showed high performances in terms of sensitivity and selectivity.
1. Introduction
The introduction of novel functional nanomaterials and analytical technologies indicate the possibility for advanced electrochemical (bio)sensor platforms/devices for a wide number of applications, including biological, biotechnological, clinical and medical diagnostics, environmental and health monitoring, and food industries.
Nanoscale materials and nanomaterials are known as materials where any measurement is not as much as 100 nm. Nanomaterials reveal exciting properties that make them appeal to be exploited in electrochemistry and in the improvement of the (bio)sensors. Recent advances in nanotechnology have created a growing demand for their possible commercial application [1].
Nanotechnology involves the synthesis and characterization of nanomaterials, whereby nanomaterial can be defined as a natural or synthesized material containing particles, in an unbound state or as an aggregate or as an agglomerate, and where, for 50% or more of the particles in the number size distribution, one or more external dimensions is in the size range of 1–100 nm [2],[3].
By reducing the material dimensions at the nanometre level, the chemical and physical properties of such a material can be modified and they are totally different with respect to the same corresponding bulk material [4] [5] [[6].
Carbon nanotubes (CNTs) and gold nanoparticles (AuNPs) are among the most broadly explored nanomaterials because of their exceptional properties, which can be connected in different applications, e.g., detecting, and imaging. Yet, to date, the exploration field of advancement for the synthesis of new functionalized AuNPs and CNTs for sensing applications is a dynamic research territory. The combination of these nanomaterials has been developed, promoting improvements in controlling their size and shape [7],[8]
Electroanalytical methods and electrochemical sensors have improved the analytical approach in different application fields, ranging from the biomedical to the environmental ones [9].
Particularly the modification and/or functionalization of the electrodic surface with nanomaterials involves an amplification of the corresponding electrochemical signal and it has proven very attractive for developing sensors with high sensitivity and selectivity [9].
2. Electrochemical Techniques
Electrochemistry offers a wide range of electroanalytical techniques. A typical electrochemical experiment includes a working electrode made of a solid conductive material, such as platinum, gold, or carbon, a reference electrode, and a counter electrode, all the electrodes are generally immersed in a solution with a supporting electrolyte to guarantee the conductivity in the solution [10].
Electrochemical sensors belong to the largest family of chemical sensors. A chemical sensor can be defined as ‘‘a small device that, as the result of a chemical interaction or process between the analyte and the sensor device, transforms chemical or biochemical information of a quantitative or qualitative type into an analytically useful signal” [11]. This definition can be extended to the electrochemical ones modifying it in this way: a small device that, as the result of an electrochemical interaction or process between the analyte and the sensing device, transforms electrochemical information of a quantitative or qualitative type into an analytically useful signal [11]. The use of a nanomaterial together with the analyte kind and nature have proven crucial for the sensor sensitivity, selectivity, and stability [12]. As for all the chemical sensors, the critical parameters of electrochemical sensors are sensitivity, detection limit, dynamic range, selectivity, linearity, response time, and stability [13].
Several electrochemical methods have been employed for the detection of food additives, biological contaminants, and heavy metals [9].
In general, an electrochemical reaction can generate different measurable data, depending on the electrochemical technique adopted. In fact, a measurable current can be generated, and in this case, the corresponding electrochemical techniques are the amperometric ones. Alternatively, a potential can be measured and/or controlled, and in this case, the corresponding electrochemical techniques are the potentiometric ones. Finally, the electrochemical techniques, involving measurements of impedance at the electrode/solution interface are included in the electrochemical impedance spectroscopy (EIS) method [14].
Starting from the presentation of EIS, we propose a brief presentation and overview of the best known and used electrochemical techniques.
EIS is an electroanalytical method used for the evaluation of electron-transfer properties of the modified surfaces and in understanding of surface chemical transformations. EIS analysis provides mechanistic and kinetic information on a wide range of materials, such as batteries, fuel cells, corrosion inhibitors, etc. [14].
The overall electrochemical behaviour of an electrode can be represented by an equivalent circuit comprising resistance, inductance, and capacitance. The equivalent circuit elements, useful for analyte detection are resistance to the charge transfer Rct and the double layer capacitance Cdl. The measured capacitance usually arises from the series combination of several elements, such as analyte binding (Canal) to a sensing layer (Csens) on the electrode (Cel). The sensitivity is then determined by the relative capacitance of the analyte layer and the sensing layer. One difficulty with capacitive sensors is that their sensitivity depends on obtaining the proper thickness of the original sensing layer.
Voltammetry belongs to the class of the amperometric techniques because the current produced from an electrochemical reaction is measured whilst varying the potential window. Since there are many ways to vary the potential, we can consider many voltammetric techniques. Among others, the most common and employed are the following: cyclic voltammetry (CV), linear sweep voltammetry (LSV), differential pulse voltammetry (DPV), and square wave voltammetry (SWV) [15],[16],[17],[18].
. In Figure 1, an overview of the electrochemical methods of analysis, namely voltammetry, amperometry, electrochemical impedance spectroscopy (EIS), and potentiometry, is reported.
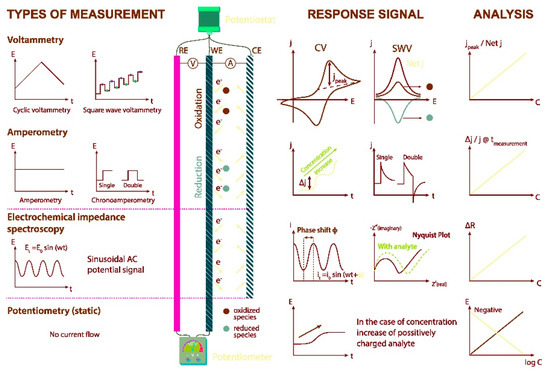
All the above-mentioned techniques have been widely employed in the development of electrochemical sensors for different application fields.
3. Nanomaterials, Nanotubes, Nanoparticles, and Nanocomposites
Recent developments in nanomaterial synthesis have allowed the development of advanced sensing systems [9],[19].Generally, we have considered modifications of the working electrode with different nanomaterials, ranging from the classical nanotubes to nanocomposites, among different nanostructures, such as graphene, metal nanoparticles, and/or nanostructured polymers. In addition, non-conventional sensing platforms, such as paper and/or screen-printed electrodes (SPE), also modified with different nanomaterials and/or nanostructures, have been considered. Hence, here, we report some example of these non-conventional sensing platforms, present in the literature and employed for different application fields [20][21].Nanomaterials play a crucial role in the development of electrochemical sensors, improving the sensor stability, sensitivity, and selectivity in the presence of the common interferences.In the following subparagraphs we introduce and describe nanomaterials and examples of the related electrochemical sensors just to show their applicability in the electrochemical sensing area.
3.1. Carbon-Based Nanomaterials
Carbon-based nanomaterials (single-walled carbon nanotubes (SWNTs), multi-walled carbon nanotubes (MWNTs), single-walled carbon nanohorns (SWCNHs), buckypaper, graphene, fullerenes (e.g., C60), etc. present very interesting properties, such as high surface-to-volume ratio, high electrical conductivity, chemical stability/durability, and strong mechanical strength, and for these reasons they have found a large applicability in the sensing area [22],[23],[24],[25],[26],[27],[28].
Carbon nanotubes (CNTs) present several properties associated to their structure, functionality, morphology, and flexibility to be employed in synthesis of hybrid or composite materials due to their hollow cylindrical structure.
Carbon nanotubes can be classified as single-walled nanotubes (SWNTs), double-walled nanotubes (DWNTs), and multi-walled nanotubes (MWNTs) depending on the number of graphite layers. Functionalized CNTs have been used in several application fields. The chemical functionalities can easily be designed and tuned through the tubular structure modification.
Some interesting examples of carbon nanomaterials based electrochemical sensors, related to different application fields not only to food analysis, are illustrated below.
Venton and co-workers have used metal microelectrodes modified with CNTs for assembling an electrochemical sensor for detecting dopamine in vitro and in vivo. [29]. It has been found that CNTs-coated niobium (CNTs-Nb) microelectrode showed a low detection limit of 11 nM for dopamine. The CNTs-Nb sensor was also employed to detect stimulated dopamine release in anesthetized rats and showed high sensitivity for in vivo measurements.
The design and synthesis of functionalized CNTs for biological and biomedical applications are highly attractive because in vivo sensing requires high selectivity, accuracy, and long-term stability. Zhang et al. have prepared an electrochemical ascorbic acid sensor for measuring ascorbic acid in brain using aligned carbon nanotube fibers (CNF) as a microsensor [30] obtaining very interesting results. The sensor measured ascorbic acid concentration of 259.0 μM in the cortex, 264.0 μM in the striatum, and 261.0 μM in the hippocampus, respectively, under normal conditions.
Graphene is one of most applied nanomaterial in the sensing area. Different graphene-based materials have been produced (e.g., electrochemically and chemically modified graphene) using many procedures [31]. Graphene shows properties such as high conductivity, accelerating electron transfer, and a large surface area, very similar indeed to the corresponding properties of CNTS, so it is considered a good candidate for assembling sensors to determine several target molecules [9],[31].
Graphene oxide (GO) is hydrophilic and can be dispersed in water solution because of hydrophilic functional groups (OH, COOH and epoxides) at the edge of the sheet and on the basal plane.
On the other hand, GO has a low conductivity in comparison to graphene, so reduced GO (rGO) is more employed as electrode modifier in electrochemical sensing/biosensing area [31]
Fluorine doped graphene oxide was used to prepare an electrochemical sensor for the detection of heavy metal ions such as Cd2+, Pb2+, Cu2+, and Hg2+. Square wave anodic stripping voltammetry was employed for the detection of the heavy metal ions. The authors have evidenced that the presence of fluorine builds a more appropriate platform for the stripping process, from the comparison between the sensor based on GO and the sensor based on F-GO [9].
Li and co-workers developed a based electrochemical sensor for the detection of metal ions, Pb2+ and Cd2+, employing a Nafion–graphene composite film. The synergistic effect of graphene nanosheets and Nafion gave rise to a better sensitivity for detecting metal ion and enhanced the electrochemical sensor selectivity [32].
Li and co-workers have reported a graphene potassium doped modified glassy carbon electrode, for the determination of sulphite in water solution. A linear response in the concentration range of 2.5 μM–10.3 mM with a detection limit of 1.0 μM for SO32− has been obtained. The graphene electronic properties resulted modified by the K doping [33].
A glassy carbon electrode was modified by means of hexadecyl trimethyl ammonium bromide (CTAB) functionalized GO/multiwalled carbon nanotubes (MWCNTs) for the detection of ascorbic acid (AA) and nitrite. The combination of GO and MWCNTs provided a sensing platform with high electrocatalytic activity, increased surface area, as well as good stability and sensitivity, allowing for the determination of nitrite and AA at the same time [34].
3.2. Gold Based Nanomaterials
Since the first examples of gold nanoparticles (AuNPs) synthesis, AuNPs have been employed for the assembly of different sensors. From an electroanalytical point of view, Au nanoparticles and in general gold nanomaterials were employed in the electrochemical sensing area because of their high conductivity, their compatibility, and a high surface to volume ratio [35],[36]. Gold nanomaterials have been used for the selective oxidation processes, or rarely, for the reduction ones.
Important improvements have been performed in the Au nanoparticles and nanomaterials synthesis for electrochemical sensing. However, researchers are dealt with several challenges/criticalities, such as size control, morphology, and suitable dispersion and/or stabilizing agents and/or media. The recent development of nanoporous Au materials seems to be fundamental in overcoming these challenges and hierarchical gold nanoporous materials have been recently reported for biomedical applications [37]. Some interesting examples of gold nanomaterials based electrochemical sensors, related to different application fields not only to food analysis, are illustrated below.
An electrochemical sensor using a gold microwire electrode for the detection of heavy metals ions, such as copper and mercury, in seawater was described by Salaün and coworkers. The sensor using gold microwires was able to detect the two metals ions at the same time by means of anodic stripping voltammetry [38].
Concerning the same analytical issue, i.e., the detection of different heavy metals ions at the same time, Soares and co-workers developed an electrochemical sensor using a vibrating gold microwire, and determined arsenic, copper, mercury, and lead ions in freshwater by means of stripping voltammetry [39].
Recently, gold nanopores were synthesized by the alloying/dealloying method for increasing the electrochemical performance of an analyte under investigation and several examples are reported below.
Ding and co-workers prepared a nanoporous gold leaf by dealloying the Au/Ag alloy in nitric acid. The resulting 3D gold nanostructure allowed the small molecules movement inside. For example, it was employed to modify a GCE for detecting nitrite. The increased surface area and the high conductivity of the 3D nanostructured gold network justify the good performance of the sensor [40].
Other examples concerning the use of nanoporous gold have involved more properly the electrochemical investigation of different analytes with this new nanomaterial as proof of concept to apply it in the sensing area.
A green synthesis of nanoporous gold materials was proposed by Jia and co-workers by means of a cyclic alloying/de-alloying procedure. The resulting nanoporous gold film modified electrodes showed very interesting electrochemical performances in terms of a high surface area and good selectivity [41].
Lin et al. modified a GCE via the electrodeposition of Au nanoparticles on polypyrrole (PPy) nanowires. AuNPs enhanced the conductivity of the polymer nanowires and consequently the electron transfer rate resulted higher than that at bare GCE and/or at GCE just modified with the polymer nanowires [42].
Finally, a nanoporous Au 3D nanostructure was synthesized as a proof of concept to be applied as a sensing platform for detecting hydrazine, sulphite, and nitrite, present in the same sample. The nanostructured sensor showed good performances in terms of selectivity and sensitivity [43].
3.3. Hybrid Nanocomposites
To improve and amplify the performances of a sensor and/or a sensing platform, nanomaterials such as carbon and/or metal nanomaterials were incorporated in different polymers both natural (e.g., chitosan) or (electro)synthesized (e.g., PEDOT, polypyrrole). Some interesting examples of hybrid nanocomposite based electrochemical sensors, related to different application fields not only to food analysis, are illustrated below [44].
As a first example, we can introduce an electrochemical sensor using a polypyrrole–chitosan–titanium dioxide (PPy–CS–TiO2) nanocomposite for glucose detection. Interactions between the TiO2 nanoparticles and PPY enhanced the sensor properties in terms of sensitivity and selectivity [45].
Bimetallic Au–Pt nanoparticles have been incorporated in rGO and the electrochemical behaviour of resulting nanocomposite was investigated and compared with the electrochemical behaviour of the bimetallic nanoparticles and of the rGO. An enhanced electrochemical activity is observed, probably due to the increase of the surface area and to the increase of the nanocomposite conductivity respect to the bimetallic nanoparticles and to the rGO [46].
Feng et al. assembled a sensor for caffeic acid detection through a nanocomposite obtained by the combination of worm-like Au–Pd nanostructures and rGO [47]. From the spectroscopic characterization, the nanotubular worm-like Au–Pd nanostructures were uniformly distributed on rGO.
A carbon paste electrode was modified with a nanocomposite obtained by combining AuNPs and MWCNTs. The oxidation of nitrite was investigated at such a modified electrode and showed better performances in terms of electrocatalytic behaviour respect to those at the bare carbon paste electrode and/or to those at CPE modified with only AuNPs or with only MWCNTs [48].
A hybrid nanocomposite was prepared by assembling Pt nanoparticles on a graphene surface. The modified electrode was used for the detection of ascorbic acid (AA), uric acid (UA), and dopamine (DA), obtaining interesting results in terms of selectivity [49].
Chen and co-workers proposed a Pt nanocomposite combining Pt nanoparticles and single-walled carbon nanotubes (SWCNTs), instead of graphene or MWCNTs.
A GCE modified with this nanocomposite was used for the electrochemical detection of α-methylglyoxal. A good linearity in the concentration range of 0.1–100.0 μM, and a detection limit of 2.80 nM were obtained. The sensor was applied to detect α-methylglyoxal in real samples of wine and beer [50].
Yegnaraman and co-workers reported an Au based nanocomposite for the detection of AA, UA, and DA to test the selectivity for detecting analytes present in the same solution. The nanocomposite film was synthesized by introducing Au nanoparticles into the PEDOT polymer matrix. The modified GCE determined AA, UA, and DA simultaneously, with improved sensitivity and selectivity [51].
A glucose impedimetric biosensor [52] was assembled using a metal composite composed by a gold microtubes (AuμTs) architecture and polypyrrole overoxidized by Curulli and co-workers. A platinum (Pt) electrode was coated by gold microtubes, synthesized via electroless deposition within the pores of polycarbonate particle track-etched membranes (PTM). This platform was successfully used to deposit polypyrrole overoxidized film (OPPy) and to verify the possibility of developing a biosensor using OPPy, the characteristics of the H2O2 charge transfer reaction were studied before the enzyme immobilization. This composite material seems to be suitable in devices as biosensors based on oxidase enzymes, just because hydrogen peroxide is a side-product of the catalysis and could be directly related to the concentration of the analyte. Finally, a biosensor consisting of a Pt electrode modified with AuμTs, OPPy, and glucose oxidase was assembled to determine the glucose. The most important result of this biosensor was the wide linear range of concentration, ranging from 1.0 to 100 mM (18–1800 mg·dL−1), covering the hypo- and hyperglycaemia range, useful in diabetes diagnosis, with limit of detection of 0.1 mM (1.8 mg·dL−1) and limit of quantification of 1.0 mM (18 mg·dL−1).
A wide range of glucose biosensor prototypes have been developed during these years, but the challenge is to obtain a biosensor capable of measuring the glucose concentration in the normal range (i.e., representative of healthy patients) and in the range of hyperglycaemia values (especially for diabetes patients, for clinical diagnosis of this widespread pathology). Very few examples propose biosensors with improved analytical performances, especially in terms of an extended linearity (still 50 mM ≈ 901 mg·dL−1, useful for the hyperglycaemia pathology values), high selectivity toward the most common interferents and an improved stability [53],[54],[55].
4. Electrochemical Sensors for Food Analysis: Some Examples
4.1. Phenolic Antioxidants
Natural antioxidants are species of great interest in many areas, as food chemistry, health care and clinical applications. They have beneficial effects on human health and could play an important role in the prevention and treatment of many pathologies (such as cardiovascular disorders, cancer, etc.) and to protect from oxidative stress [56],[57],[58],[59],[60],[61]. The classification of antioxidants is commonly carried out based on the chemical structure, determining their reactivity. However, their antioxidant action is also strictly related to the redox properties and consequently their knowledge is very crucial for a better understanding of antioxidant mechanisms.
Among the natural antioxidants found in fruits and plants, hydroxycinnamic acids (HAs) are very important and present in all parts of the fruit and/or plant [62],[63],[64]. Undoubtedly, these compounds in food provide added value for their well-known health benefits, for their technological role, and marketing. The electrochemical methods have been extensively used to investigate the redox properties of various species and as analytical tool for the determination of redox target molecules. At present, as for other classes of antioxidants, the analysis of HAs and phenolic antioxidants is usually carried out using chromatographic techniques, which require sophisticated equipment and laborious analytical procedures [57]. The use of electrochemical methods for analytical purposes is receiving increasing interest [57], since they are fast, accurate, sensitive and can be used for the analysis of different and complex matrices with a low cost.
Both electrochemical sensors and biosensors are widely used for the determination of HAs and phenolic antioxidants. However, the electrochemical responses have been studied only from an analytical point of view, whereas the relationship between the antioxidant chemical structure and electrochemical behaviour has been neglected [65]. The understanding of key factors that affect the electrochemical response of analytes could promote the design of highly efficient sensors. To this aim, the role of the chemical structural features of antioxidants and nature of the electrode surface must be evaluated.
Recently, electrodes modified with nanocomposite films were successfully used for the analysis of antioxidants, such as caffeic acid, in complex matrices [66],[67]. These films consist of gold nanoparticles (AuNPs) embedded into chitosan, a biodegradable and biocompatible polymer containing many hydroxyl and amino groups that can interact with the analytes. Later, the electrochemical response of structurally related antioxidants on electrodes modified with different gold–chitosan nanocomposite films was investigated and discussed [65],[66].
To evaluate how the chemical structural features of analytes affect the electrochemical behaviour, different types of antioxidants, such as catechols, hydroxycinnamic acids, and flavonoids, structurally correlated and bearing different functional groups and steric hindrances, have been studied. This investigation [66] has demonstrated that the electrochemical response for structurally related antioxidants at AuNPs–chitosan modified electrodes depends on several parameters. The chemical structural features of the analytes affect the interaction with the electrode surface. However, their electrochemical behaviour cannot be explained only on these bases.
The nanostructure and surface functional groups of AuNPs-chitosan modified electrodes have also a key role. In particular, the formation of a collaborative network with interconnected metal nanoparticles in chitosan film significantly affects the electron transfer properties, whereas the surface functional groups can promote the interaction with the antioxidants. An overview of the behaviour of catechols, hydroxy cinnamic acids, and flavonoids derivatives at different AuNPs–chitosan modified electrodes has been illustrated by Curulli and co-workers [66].
A better response was observed for molecules with two hydroxyl groups in ortho position of the catechol ring, with a peculiar molecular symmetry (i.e., rosmarinic acid) and with a low steric hindrance, in particular for caffeic acid, chlorogenic acid, and rosmarinic acid. Moreover, the interaction with the antioxidants is also affected by the functional groups at modified electrode surface and the size and distribution of AuNPs into the polymeric matrix. The understanding of the parameters affecting the electrochemical behaviour of analytes at modified electrodes is a key issue and could significantly promote the design of highly efficient sensors.
4.2. Caffeine
Caffeine (CAF, chemical structure in Figure 2) a natural alkaloid, distributed in seeds, nuts, or leaves of a number of plants, mainly coffee, cocoa, tea, with the natural function as insecticide, is the most widely consumed psychoactive substance in human dietary. Many physiological effects of CAF are well known, from stimulation of the central nervous system, diuresis, and gastric acid secretion to nausea, seizures, trembling, and nervousness. Mutation effects on DNA have been also reported. Moreover, it is considered a risk molecule for cardiovascular diseases. Recently, also an antioxidant activity has been suggested for CAF, showing protective effects against oxidative stress. The presence of CAF in many beverages and drug formulations of worldwide economic importance [68] makes it an analyte of great interest and although many different analytical methods are currently applied, novel analytical methods for fast, sensitive and reliable determination of CAF are always necessary, especially for particular purposes, as the determination in specific matrix in the presence of interfering agents, or in a specific concentration range, besides under beneficial conditions in terms of time consumption, material cost, and procedure ease [68].

Figure 2. Scheme of the approach and method used for the caffeine detection reprinted with the permission from [68]. Copyright 2017 Elsevier.
4.3. Ascorbic Acid
L-Ascorbic acid (AA) or Vitamin C is a water-soluble vitamin and antioxidant, present in many biological systems and food. The biochemically and physiologically active form is the L-enantiomer, with a γ-lactone structure [69]. AA is an ideal scavenger free-radical and singlet oxygen or act as a chelating agent. As a strong antioxidant, it acts as a two-electron donor involving hydrogen atom transfer, giving rise to the ascorbate radical ion first, and finally to dehydroascorbic acid. The AA action prevents the oxidation of several compounds present in food and/or beverages. The deficiency of AA can cause several diseases, such as rheumatoid arthritis, Parkinson’s and Alzheimer’s diseases, and even cancer [69]. The excess of AA can result to several other diseases such as gastric irritation. Moreover, in the presence of heavy metal cations, the excess of AA has other drawbacks, because it can act as a pro-oxidant, in other words it limits its own antioxidant action tills to produce the reactive oxygen species, causing oxidative stress. Therefore, the determination of AA in biological fluids is very important for the diagnosis of such diseases. The quantitative determination of AA is also necessary for different application fields, including among others, cosmetics, drugs, and food [70].
Conventional bare electrodes, like Pt, Au, and glassy carbon, were used for the ascorbic acid detection but because of the vitamin C overpotential, fouling of the electrode surface was observed, involving low sensitivity and selectivity. Nanomaterials were used for the modification of conventional electrode to reduce the overpotential and to enhance the electrode sensitivity, selectivity, and reproducibility.
4.4. Nitrite
Nitrite is an additive used to extend the shelf-life of beverages and foods such as ham, salami, and other cured meats [9]. On the other hand, it proves harmful for the human body if it is added to food and beverages at levels higher than those indicated by the safety standards. The World Health Organization (WHO) has established a concentration limit of 3.0 mg·L−1 (65.22 μM) for nitrite in drinking water [71]. Therefore, the detection and quantification of nitrite is one of the critical issues of food analysis. Novel nanomaterials have been synthesized and used for the design of advanced sensors for the nitrite detection. It has been shown that nanomaterial-based electrocatalysts significantly improve analytical performances for the nitrite determination. We must evidence that the majority of them are concerned with the detection in water samples, but very significant examples are concerned with cured meats and milk.
References
- Logothetidis, S.. Nanostructured Materials and Their Applications;; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1-22.
- Roco, M.C.; Mirkin, C.A.; Dincer Hersam, M.C.. Nanotechnology Research Directions for Societal Needs in 2020: Retrospective and Outlook; Springer Science Business Media: Berlin/Heidelberg, Germany, 2011; pp. all.
- Sattler, K.D.. Handbook of Nanophysics: Principles and Methods; CRC Press: Boca Raton, FL, USA, 2010; pp. all.
- Aicheng Chen; Sanghamitra Chatterjee; Nanomaterials based electrochemical sensors for biomedical applications. Chemical Society Reviews 2013, 42, 5425-5438, 10.1039/c3cs35518g.
- Yogeswaran Umasankar; Bal-Ram Adhikari; Aicheng Chen; Effective immobilization of alcohol dehydrogenase on carbon nanoscaffolds for ethanol biofuel cell. Bioelectrochemistry 2017, 118, 83-90, 10.1016/j.bioelechem.2017.07.008.
- Emiliano Martínez-Periñán; Christopher William Foster; Michael P. Down; Yan Zhang; Xiaobo Ji; Encarnación Lorenzo; Dmitrijs Kononovs; Anatoly I. Saprykin; Vladimir N. Yakovlev; Georgiy Pozdnyakov; et al.Craig E. Banks Graphene Encapsulated Silicon Carbide Nanocomposites for High and Low Power Energy Storage Applications. J. Carbon Res. 2017, 3, 20, 10.3390/c3020020.
- Nelson Durán; Priscyla D. Marcato; Nanobiotechnology perspectives. Role of nanotechnology in the food industry: a review. International Journal of Food Science & Technology 2012, 48, 1127-1134, 10.1111/ijfs.12027.
- Srilatha, B.; Nanotechnology in Agriculture.. J. Nanomed. Nanotechnol. 2011, 2, 5-20.
- Venkatesh S. Manikandan; Bal Ram Adhikari; Aicheng Chen; Nanomaterial based electrochemical sensors for the safety and quality control of food and beverages. The Analyst 2018, 143, 4537-4554, 10.1039/c8an00497h.
- Bartlett, P.N.. Bioelectrochemistry 45: Fundamentals, Experimental Techniques, and Applications; JohnWiley & Sons: Hoboken, NJ, USA, 2008; pp. all.
- R. A. Durst; Chemically modified electrodes: Recommended terminology and definitions (IUPAC Recommendations 1997). Pure and Applied Chemistry 1997, 69, 1317-1324, 10.1351/pac199769061317.
- Andreas Hierlemann; Ricardo Gutierrez-Osuna; Higher-Order Chemical Sensing. Chemical Reviews 2008, 108, 563-613, 10.1021/cr068116m.
- Trojanowicz, M.. Combinatorial Methods for Chemical and Biological Sensors; Potyrailo, Radislav A., Mirsky, Vladimir M., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 25-60.
- Ian Ivar Suni; Impedance methods for electrochemical sensors using nanomaterials. TrAC Trends in Analytical Chemistry 2008, 27, 604-611, 10.1016/j.trac.2008.03.012.
- Eugenii Katz; Itamar Willner; Probing Biomolecular Interactions at Conductive and Semiconductive Surfaces by Impedance Spectroscopy: Routes to Impedimetric Immunosensors, DNA-Sensors, and Enzyme Biosensors. Electroanalysis 2003, 15, 913-947, 10.1002/elan.200390114.
- Wang, J.. Analytical Electrochemistry, 2nd ed.; Wiley/VCH: New York, NY, USA, 2000; pp. all.
- Bockris, J.O.M.; Reddy, A.K.N.; Gamboa-Aldeco, M.. Modern Electrochemistry 2A: Fundamentals of Electrodics, 2nd ed. Volume 2; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2000; pp. all.
- Bard, A.J.; Faulkner, L.R.. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2001; pp. all.
- Can Dincer; Richard Bruch; Estefanía Costa‐Rama; Maria Teresa Fernández‐Abedul; Arben Merkoçi; Andreas Manz; Gerald Anton Urban; Firat Güder; Disposable Sensors in Diagnostics, Food, and Environmental Monitoring. Advanced Materials 2019, 31, e1806739, 10.1002/adma.201806739.
- Shikha Sharma; Nidhi Singh; Vartika Tomar; Ramesh Chandra; A review on electrochemical detection of serotonin based on surface modified electrodes. Biosensors and Bioelectronics 2018, 107, 76-93, 10.1016/j.bios.2018.02.013.
- Brownson, D.A.; Banks, C.E.; Graphene electrochemistry: An overview of potential applications. Analyst 2010, 135, 2768-2778, 10.1039/C0AN00590H.
- Nagappa L. Teradal; Raz Jelinek; Carbon Nanomaterials in Biological Studies and Biomedicine. Advanced Healthcare Materials 2017, 6, 1700574., 10.1002/adhm.201700574.
- Laís S. Porto; Daniela N. Silva; Ana Elisa F. De Oliveira; Arnaldo César Pereira; Keyller Bastos Borges; Carbon nanomaterials: synthesis and applications to development of electrochemical sensors in determination of drugs and compounds of clinical interest. Reviews in Analytical Chemistry 2020, 38, 20190017, 10.1515/revac-2019-0017.
- Ivan Bobrinetskiy; Nikola Ž. Knežević; Graphene-based biosensors for on-site detection of contaminants in food. Analytical Methods 2018, 10, 5061-5070, 10.1039/c8ay01913d.
- Alexandra Virginia Bounegru; Constantin Apetrei; Carbonaceous Nanomaterials Employed in the Development of Electrochemical Sensors Based on Screen-Printing Technique—A Review. Catalysts 2020, 10, 680, 10.3390/catal10060680.
- Eva-Maria Kirchner; Thomas Hirsch; Recent developments in carbon-based two-dimensional materials: synthesis and modification aspects for electrochemical sensors. Microchimica Acta 2020, 187, 1-21, 10.1007/s00604-020-04415-3.
- Ravinder Kour; Sandeep Arya; Sheng-Joue Young; Vinay Gupta; Pankaj Bandhoria; Ajit Khosla; Review—Recent Advances in Carbon Nanomaterials as Electrochemical Biosensors. Journal of the Electrochemical Society 2020, 167, 037555, 10.1149/1945-7111/ab6bc4.
- Harshita Pandey; Prateek Khare; Shiv Singh; Sheelendra Pratap Singh; Carbon nanomaterials integrated molecularly imprinted polymers for biological sample analysis: A critical review. Materials Chemistry and Physics 2020, 239, 121966., 10.1016/j.matchemphys.2019.121966.
- Cheng Yang; Christopher B. Jacobs; Michael D. Nguyen; Mallikarjunarao Ganesana; Alexander G. Zestos; Ilia N. Ivanov; Alexander A. Puretzky; Christopher M. Rouleau; David B. Geohegan; B. Jill Venton; et al. Carbon Nanotubes Grown on Metal Microelectrodes for the Detection of Dopamine. Analytical Chemistry 2015, 88, 645-652, 10.1021/acs.analchem.5b01257.
- Limin Zhang; Fangling Liu; Xuemei Sun; Guangfeng Wei; Yang Tian; Zhipan Liu; Rong Huang; Yanyan Yu; Huisheng Peng; Engineering Carbon Nanotube Fiber for Real-Time Quantification of Ascorbic Acid Levels in a Live Rat Model of Alzheimer’s Disease. Analytical Chemistry 2017, 89, 1831-1837, 10.1021/acs.analchem.6b04168.
- Martin Pumera; Graphene-based nanomaterials and their electrochemistry. Chemical Society Reviews 2010, 39, 4146-4157, 10.1039/c002690p.
- Jing Li; Shaojum Guo; Yueming Zhai; Erkang Wang; Nafion–graphene nanocomposite film as enhanced sensing platform for ultrasensitive determination of cadmium. Electrochemistry Communications 2009, 11, 1085-1088, 10.1016/j.elecom.2009.03.025.
- Xiao-Rong Li; Jing Liu; Fen-Ying Kong; Xin-Chun Liu; Jing-Juan Xu; Huayong Chen; Potassium-doped graphene for simultaneous determination of nitrite and sulfite in polluted water. Electrochemistry Communications 2012, 20, 109-112, 10.1016/j.elecom.2012.04.014.
- Yu Jun Yang; Weikun Li; CTAB functionalized graphene oxide/multiwalled carbon nanotube composite modified electrode for the simultaneous determination of ascorbic acid, dopamine, uric acid and nitrite. Biosensors and Bioelectronics 2014, 56, 300-306, 10.1016/j.bios.2014.01.037.
- Fatima Mustafa; Silvana Andreescu; Nanotechnology-based approaches for food sensing and packaging applications. RSC Advances 2020, 10, 19309-19336, 10.1039/d0ra01084g.
- Ting Xiao; Jianshe Huang; Dewen Wang; Tian Meng; Xiurong Yang; Au and Au-Based nanomaterials: Synthesis and recent progress in electrochemical sensor applications. Talanta 2020, 206, 120210, 10.1016/j.talanta.2019.120210.
- Zhonggang Liu; Ashley Nemec-Bakk; Neelam Khaper; Aicheng Chen; Sensitive Electrochemical Detection of Nitric Oxide Release from Cardiac and Cancer Cells via a Hierarchical Nanoporous Gold Microelectrode. Analytical Chemistry 2017, 89, 8036-8043, 10.1021/acs.analchem.7b01430.
- Pascal Salaun; Constant M. G. Van Den Berg; Voltammetric Detection of Mercury and Copper in Seawater Using a Gold Microwire Electrode. Analytical Chemistry 2006, 78, 5052-5060, 10.1021/ac060231+.
- Georgina M.S. Alves; Júlia M.C.S. Magalhães; Pascal Salaün; Constant M.G. Van Den Berg; Eduardo V. Soares; Simultaneous electrochemical determination of arsenic, copper, lead and mercury in unpolluted fresh waters using a vibrating gold microwire electrode. Analytica Chimica Acta 2011, 703, 1-7, 10.1016/j.aca.2011.07.022.
- Xingbo Ge; Liqin Wang; Zhaona Liu; Yi Ding; Nanoporous Gold Leaf for Amperometric Determination of Nitrite. Electroanalysis 2010, 23, 381-386, 10.1002/elan.201000320.
- Falong Jia; Chuanfang Yu; Zhihui Ai; Lizhi Zhang; Fabrication of Nanoporous Gold Film Electrodes with Ultrahigh Surface Area and Electrochemical Activity. Chemistry of Materials 2007, 19, 3648-3653, 10.1021/cm070425l.
- Jing Li; Xiangqin Lin; Electrocatalytic oxidation of hydrazine and hydroxylamine at gold nanoparticle—polypyrrole nanowire modified glassy carbon electrode. Sensors and Actuators B: Chemical 2007, 126, 527-535, 10.1016/j.snb.2007.03.044.
- Venkatesh S. Manikandan; Zhonggang Liu; Aicheng Chen; Simultaneous detection of hydrazine, sulfite, and nitrite based on a nanoporous gold microelectrode. Journal of Electroanalytical Chemistry 2018, 819, 524-532, 10.1016/j.jelechem.2018.02.004.
- Hyeonseok Yoon; Current Trends in Sensors Based on Conducting Polymer Nanomaterials. Nanomaterials 2013, 3, 524-549, 10.3390/nano3030524.
- Ali M. A. Abdul Amir Al-Mokaram; Rosiyah Yahya; Mahnaz M. Abdi; Habibun Nabi Muhammad Ekramul Mahmud; The Development of Non-Enzymatic Glucose Biosensors Based on Electrochemically Prepared Polypyrrole–Chitosan–Titanium Dioxide Nanocomposite Films. Nanomaterials 2017, 7, 129, 10.3390/nano7060129.
- Maduraiveeran Govindhan; Mona Amiri; A. Chen; Au nanoparticle/graphene nanocomposite as a platform for the sensitive detection of NADH in human urine. Biosensors and Bioelectronics 2015, 66, 474-480, 10.1016/j.bios.2014.12.012.
- Shan-Shan Li; Yuan-Yuan Hu; Ai-Jun Wang; Xuexiang Weng; Jian-Rong Chen; Jiu- Ju Fenga; Simple synthesis of worm-like Au–Pd nanostructures supported on reduced graphene oxide for highly sensitive detection of nitrite. Sensors and Actuators B: Chemical 2015, 208, 468-474, 10.1016/j.snb.2014.11.056.
- Abbas Afkhami; Farzaneh Soltani-Felehgari; Tayyebeh Madrakian; Hamed Ghaedi; Surface decoration of multi-walled carbon nanotubes modified carbon paste electrode with gold nanoparticles for electro-oxidation and sensitive determination of nitrite. Biosensors and Bioelectronics 2014, 51, 379-385, 10.1016/j.bios.2013.07.056.
- Chia-Liang Sun; Hsin-Hsien Lee; Jen-Ming Yang; Ching-Chou Wu; The simultaneous electrochemical detection of ascorbic acid, dopamine, and uric acid using graphene/size-selected Pt nanocomposites. Biosensors and Bioelectronics 2011, 26, 3450-3455, 10.1016/j.bios.2011.01.023.
- Sanghamitra Chatterjee; Aicheng Chen; Voltammetric detection of the α-dicarbonyl compound: Methylglyoxal as a flavoring agent in wine and beer. Analytica Chimica Acta 2012, 751, 66-70, 10.1016/j.aca.2012.09.011.
- J. Mathiyarasu; S. Senthilkumar; K.L.N. Phani; V. Yegnaraman; PEDOT-Au nanocomposite film for electrochemical sensing. Materials Letters 2008, 62, 571-573, 10.1016/j.matlet.2007.06.004.
- C. Bianchini; D. Zane; Antonella Curulli; Gold microtubes assembling architecture for an impedimetric glucose biosensing system. Sensors and Actuators B: Chemical 2015, 220, 734-742, 10.1016/j.snb.2015.05.063.
- Joseph Wang; Mustafa Musameh; Carbon-nanotubes doped polypyrrole glucose biosensor. Analytica Chimica Acta 2005, 539, 209-213, 10.1016/j.aca.2005.02.059.
- Federica Valentini; L. Galache Fernàndez; Emanuela Tamburri; Giuseppe Palleschi; Single Walled Carbon Nanotubes/polypyrrole–GOx composite films to modify gold microelectrodes for glucose biosensors: Study of the extended linearity. Biosensors and Bioelectronics 2013, 43, 75-78, 10.1016/j.bios.2012.11.019.
- Teagan Leigh Adamson; Francis Ang Eusebio; Curtiss B. Cook; Jeffrey T. Labelle; The promise of electrochemical impedance spectroscopy as novel technology for the management of patients with diabetes mellitus. The Analyst 2012, 137, 4179-4187, 10.1039/c2an35645g.
- Denisov, E.T.; Afanas’ev, I.B. Oxidation and Antioxidants in Organic Chemistry and Biochemistry; CRC Press: Andover, MA, USA, 2005; pp. all.
- Stéphane Quideau; Denis Deffieux; Céline Douat-Casassus; Laurent Pouységu; Plant Polyphenols: Chemical Properties, Biological Activities, and Synthesis. Angewandte Chemie International Edition 2011, 50, 586-621, 10.1002/anie.201000044.
- Michael H. Gordon; Significance of Dietary Antioxidants for Health. International Journal of Molecular Sciences 2011, 13, 173-179, 10.3390/ijms13010173.
- Catherine A. Rice-Evans; Nicholas J. Miller; George Paganga; Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radical Biology and Medicine 1996, 20, 933-956, 10.1016/0891-5849(95)02227-9.
- Bors, W.; Heller, W.; Michel, C.;Stettmaier, K.. Handbook of Antioxidants; Cadenas, E., Packer, L., Eds.; Marcel Dekker: New York, NY, USA, 1996; pp. 409–466.
- Qingqing Guo; Qiaoli Yue; Jingjing Zhao; Lei Wang; Huaisheng Wang; Xilian Wei; Jifeng Liu; Jianbo Jia; How far can hydroxyl radicals travel? An electrochemical study based on a DNA mediated electron transfer process. Chemical Communications 2011, 47, 11906-11908, 10.1039/c1cc14699h.
- Kanti Bhooshan Pandey; Syed Ibrahim Rizvi; Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxidative Medicine and Cellular Longevity 2009, 2, 270-278, 10.4161/oxim.2.5.9498.
- Reşat Apak; Sema Demirci Çekiç; Ayşem Üzer; Saliha Esin Çelik; Mustafa Bener; Burcu Bekdeşer; Ziya Can; Şener Sağlam; Ayşe Nur Önem; Erol Erçağ; et al. Novel Spectroscopic and Electrochemical Sensors and Nanoprobes for the Characterization of Food and Biological Antioxidants. Sensors 2018, 18, 186, 10.3390/s18010186.
- Alexandra Virginia Bounegru; Constantin Apetrei; Voltammetric Sensors Based on Nanomaterials for Detection of Caffeic Acid in Food Supplements. Chemosensors 2020, 8, 41, 10.3390/chemosensors8020041.
- Gabriella Di Carlo; Antonella Curulli; Alessandro Trani; Daniela Zane; Gabriel M. Ingo; Enhanced electrochemical response of structurally related antioxidant at nanostructured hybrid films. Sensors and Actuators B: Chemical 2014, 191, 703-710, 10.1016/j.snb.2013.10.063.
- Gabriella Di Carlo; Antonella Curulli; Roberta G. Toro; Chiara Bianchini; Tilde De Caro; Giuseppina Padeletti; Daniela Zane; G.M Ingo; Green Synthesis of Gold–Chitosan Nanocomposites for Caffeic Acid Sensing. Langmuir 2012, 28, 5471-5479, 10.1021/la204924d.
- Antonella Curulli; Gabriella Di Carlo; G.M Ingo; Cristina Riccucci; Daniela Zane; Chiara Bianchini; Chitosan Stabilized Gold Nanoparticle-Modified Au Electrodes for the Determination of Polyphenol Index in Wines: a Preliminary Study. Electroanalysis 2012, 24, 897-904, 10.1002/elan.201100583.
- Alessandro Trani; Rita Petrucci; Giancarlo Marrosu; Daniela Zane; Antonella Curulli; Selective electrochemical determination of caffeine at a gold-chitosan nanocomposite sensor: May little change on nanocomposites synthesis affect selectivity?. Journal of Electroanalytical Chemistry 2017, 788, 99-106, 10.1016/j.jelechem.2017.01.049.
- Keerthy Dhara; Roy Mahapatra Debiprosad; Review on nanomaterials-enabled electrochemical sensors for ascorbic acid detection. Analytical Biochemistry 2019, 586, 113415, 10.1016/j.ab.2019.113415.
- Saifeldin M. Siddeeg; The Application of Nanomaterials as Electrode Modifiers for the Electrochemical Detection of Ascorbic Acid: Review. International Journal of Electrochemical Science 2020, 15, 3327-3346, 10.20964/2020.04.13.
- WHO. Guidelines for Drinking-Water Quality, 3rd ed; World Health Organization:: Geneva, Switzerland, 2004; pp. 417.




