
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mehdi Mirsaeidi | + 1062 word(s) | 1062 | 2021-01-26 09:26:31 | | | |
| 2 | Catherine Yang | Meta information modification | 1062 | 2021-02-02 04:16:53 | | |
Video Upload Options
The melanocortin system encompasses melanocortin peptides, five receptors, and two endogenous antagonists. Besides pigmentary effects generated by α-Melanocytic Hormone (α-MSH), new physiologic roles in sexual activity, exocrine secretion, energy homeostasis, as well as immunomodulatory actions, exerted by melanocortins, have been described recently.
1. Introduction
The melanocortin system encompasses melanocortins, five transmembrane G protein-coupled receptors, plus two endogenous antagonists: the agouti-signaling protein and agouti-related peptide. The melanocortins comprise adrenocorticotropic hormone (ACTH), α-, β-, and γ-melanocyte-stimulating hormones (α-, β-, γ-MSHs), which are generated from proopiomelanocortin (POMC) processing. Besides steroidogenesis and pigmentary effects caused by ACTH and α-MSH, respectively, new roles in energy homeostasis, reproductive system functions, exocrine glands secretion, immunomodulatory, and anti-inflammatory, have been explained [1][2].
Prior to identification and cloning of melanocortin receptor family (MCR), ACTH-induced improvement in subjects with arthritis was presumed to be due to activation of the hypothalamus-pituitary-adrenal (HPA) axis and cortisol production. Today, 53 years later, Getting et al. showed that melanocortin-3 receptor (MC3R) signaling, triggered by ACTH, regardless of steroid synthesis, was likewise responsible for ACTH effectiveness in inflammatory arthritis and proposed MC3R agonists as novel therapeutics for chronic inflammatory diseases [3].
2. α-MSH and Tissue Repair and Remodeling
Replacement of damaged tissue with new living tissue is referred to as tissue repair (healing). The basic cellular and molecular mechanisms underlying restoration of tissue architecture and function after an injury and its failure to heal are still poorly understood, and treatments are dissatisfying. Defective tissue repair after trauma, surgery, and acute or chronic disease states affect millions of people worldwide each year and arises from malregulation of tissue repair responses, including inflammation, angiogenesis, matrix deposition and degradation, and cell recruitment. Impaired repair can lead to fibrosis and organ dysfunction. Several possible anti-fibrotic properties of α-MSH are discussed below (summarized in Figure 1).
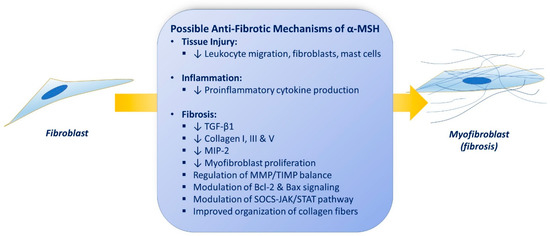
Figure 1. Possible anti-fibrotic properties of α-MSH (see text).
Hepatic fibrosis arises from escalating deposition of extracellular matrix components in the hepatic parenchyma due to recurring tissue injury. Lee et al. developed a mouse model of hepatic fibrosis with administration of carbon tetrachloride (CCl4) for 10 weeks. α-MSH expression vector was delivered via electro-permeabilization after full-blown liver fibrosis. Histologic examination and assessment of extracellular matrix contents of the livers revealed that transfected animals markedly reversed CCL4-induced fibrosis, compared to untreated animals (collagen content in intervention group was 23.7 ± 4.7 vs. 59.7 ± 5.0 μg/mg in untreated animals, p < 0.01). The over-expression of TGF-β1, collagen 1, fibronectin, TNF-α, ICAM-1, and VCAM-1 mRNA were reported in the experimental models of hepatic fibrosis. Gene therapy with α-MSH significantly attenuated this over-expression. They further showed that the intervention reversed established hepatic cirrhosis by increasing MMP activity and decrease in their tissue inhibitors (TIMP), suggesting that extracellular matrix metabolism modification might play a role in the tissue repair properties of α-MSH [4]. Wang et al. introduced a liver fibrosis model induced by chronic thioacetamide (selective hepatotoxin) administration and investigated the effects of α-MSH gene therapy on tissue remodeling. Hepatic ECM collagen content in the treated animals was 32.2 ± 6.2 μg/mg while it was 71.6 ± 10.0 μg/mg in control group (p < 0.01). Treatment significantly inhibited TGF-β1, procollagen I, TNF-α, ICAM-1, VCAM-1 and TIMP-1 mRNA over-expression in intervention group. They proposed that the collagenolytic actions of α-MSH can be due to MMP and TIMP balance modulation [5]. Lonati et al. aimed to experiment if treatment with melanocortin adjusts tissue remodeling after performing partial hepatectomy (PH) or sham procedure in rats. Immediately prior to surgery the intervention group received a single dose of NDP-MSH, while controls received only saline. RT-PCR analyses demonstrated that NDP-MSH altered the expression of a substantial proportion of transcripts, including multiple cytokines and their receptors. The critical signaling pathway IL-6/STAT/SOC was significantly enhanced by the α-MSH agonist [6]. Another older study had shown the regenerative effects of α-MSH on hepatectomized rats [7].
Lee et al. evaluated the anti-fibrotic properties of an α-MSH agonist (STY39) on a cyclosporine-induced tubulointerstitial fibrosis rat model. STY39 counterbalanced the Bax and TGF-α increase and induced synthesis of anti-apoptotic Bcl2 protein, as well as inhibition of inflammation and tubulointerstitial renal fibrosis [8].
Verhaagen et al. have explained the effects of α-MSH in nerve regeneration [9]. This is also confirmed by Dekker et al., who injected 10 µg of α-MSH into rats every 48 h after a sciatic nerve crush and tested the number of myelinated axons in cross sections of sciatic nerve at several time points and observed that α-MSH increased the number and diameter of axons after nerve injury [10]. Later, the effectiveness of α-MSH on peripheral nerve regeneration was further established [11][12][13].
Bonfiglio et al. investigated the effects of KPV on corneal wound re-epithelization in rabbits and the potential role of nitric oxide. Denuded corneas of rabbits were treated four times a day with KPV 1, 5, or 10 mg/mL (30 mL) or sodium nitroprusside (NO donor) instantaneously after corneal abrasion while control group only received normal saline. Then, 60 hours later, 100% of the corneas treated with KPV and SP were fully re-epithelized while none from untreated rabbits were re-epithelized. They concluded that the availability of nitric oxide might be of specific importance in therapeutic efficacy of topical KPV in experimental corneal abrasion model [14]. Pavan et al. evaluated the influence of topical α-MSH on the healing of corneal wound healing in rats. Topical α-MSH eye-drop in a concentration of 1 × 10−4 mg/mL improved corneal wound healing significantly, while non from control group were healed [15]. Zhang and co-workers tested the anti-fibrotic effect of α-MSH on TGF-β1-stimulated human Tenon’s capsule fibroblasts (HTFs) since these fibroblasts play a central role in the initiation and handling of wound healing and tissue remodeling after trabeculectomy. α-MSH inhibited the proliferation of TGF-β1-induced HTFs in a concentration-dependent fashion and demonstrated inhibitory effect on the mRNA expression of type I collagen, TNF-α, ICAM-1, and VCAM-1, which were upregulated by TGF-β1. They proposed an opposite effect of α-MSH on the disparity between MMPs and TIMPs compared with TGF-β1 [16].
3. Future Perspectives
Tissue injury and inflammation are crucial triggers for either regeneration or fibrosis. Melanocortin agonists might have favorable effects on the processes leading from injury to tissue inflammation, from inflammation to tissue fibrosis, and from fibrosis to organ dysfunction. α-MSH may have significant potentials in inflammation control and repairment process in numerous inflammatory lung diseases including sarcoidosis, interstitial lung disease, and COVID-19 related pulmonary fibrosis, with fewer safety concerns than other immunomodulatory medications. Validation via further investigation is recommended to prove the therapeutic properties of MSH agonists in lung diseases.
References
- Tao, Y.X. Melanocortin receptors. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 2411–2413.
- Gallo-Payet, N. 60 YEARS OF POMC: Adrenal and extra-adrenal functions of ACTH. J. Mol. Endocrinol. 2016, 56, T135–T156.
- Wang, W.; Guo, D.Y.; Lin, Y.J.; Tao, Y.X. Melanocortin Regulation of Inflammation. Front. Endocrinol. 2019, 10, 683.
- Lee, T.H.; Jawan, B.; Chou, W.Y.; Lu, C.N.; Wu, C.L.; Kuo, H.M.; Concejero, A.M.; Wang, C.H. Alpha-melanocyte-stimulating hormone gene therapy reverses carbon tetrachloride induced liver fibrosis in mice. J. Gene Med. 2006, 8, 764–772.
- Wang, C.H.; Lee, T.H.; Lu, C.N.; Chou, W.Y.; Hung, K.S.; Concejero, A.M.; Jawan, B. Electroporative alpha-MSH gene transfer attenuates thioacetamide-induced murine hepatic fibrosis by MMP and TIMP modulation. Gene Ther. 2006, 13, 1000–1009.
- Lonati, C.; Carlin, A.; Leonardi, P.; Valenza, F.; Bosari, S.; Catania, A.; Gatti, S. Modulatory effects of NDP-MSH in the regenerating liver after partial hepatectomy in rats. Peptides 2013, 50, 145–152.
- Vándor, E.; Simon, G.; Anda, E.; Budavári, I. The effect of alpha-melanophor-stimulating hormone on liver regeneration and incorporation of amino acid in rats’ liver protein. Endokrinologie 1975, 66, 81–87.
- Lee, S.Y.; Jo, S.K.; Cho, W.Y.; Kim, H.K.; Won, N.H. The effect of alpha-melanocyte-stimulating hormone on renal tubular cell apoptosis and tubulointerstitial fibrosis in cyclosporine A nephrotoxicity. Transplantation 2004, 78, 1756–1764.
- Verhaagen, J.; Edwards, P.M.; Jennekens, F.G.; Schotman, P.; Gispen, W.H. Alpha-melanocyte-stimulating hormone stimulates the outgrowth of myelinated nerve fibers after peripheral nerve crush. Exp. Neurol. 1986, 92, 451–454.
- Dekker, A.J. Effect of alpha-melanocyte-stimulating hormone on peripheral nerve regeneration in the rat: Histological aspects and comparison with the effect of gangliosides. Exp. Neurol. 1988, 99, 490–497.
- Laquerriere, A.; Peulve, P.; Jin, O.; Tiollier, J.; Tardy, M.; Vaudry, H.; Hemet, J.; Tadie, M. Effect of basic fibroblast growth factor and alpha-melanocytic stimulating hormone on nerve regeneration through a collagen channel. Microsurgery 1994, 15, 203–210.
- Plantinga, L.C.; Verhaagen, J.; Edwards, P.M.; Hali, M.; Brakkee, J.H.; Gispen, W.H. Pharmacological evidence for the involvement of endogenous alpha-MSH-like peptides in peripheral nerve regeneration. Peptides 1995, 16, 319–324.
- Ter Laak, M.P.; Brakkee, J.H.; Adan, R.A.; Hamers, F.P.; Gispen, W.H. The potent melanocortin receptor agonist melanotan-II promotes peripheral nerve regeneration and has neuroprotective properties in the rat. Eur. J. Pharmacol. 2003, 462, 179–183.
- Bonfiglio, V.; Camillieri, G.; Avitabile, T.; Leggio, G.M.; Drago, F. Effects of the COOH-terminal tripeptide alpha-MSH(11-13) on corneal epithelial wound healing: Role of nitric oxide. Exp. Eye Res. 2006, 83, 1366–1372.
- Pavan, J.; Lukenda, A.; Stambuk, N.; Konjevoda, P.; Kastelan, S.; Curković, M. Effects of alpha-MSH on corneal epithelial lesions in rats. Coll. Antropol. 2012, 36, 1407–1411.
- Zhang, Z.; Ma, J.; Yao, K.; Yin, J. Alpha-melanocyte stimulating hormone suppresses the proliferation of human tenon’s capsule fibroblast proliferation induced by transforming growth factor beta 1. Mol. Biol. 2012, 46, 628–633.




