
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jill Kolesar | + 993 word(s) | 993 | 2021-01-26 09:29:14 | | | |
| 2 | Vicky Zhou | Meta information modification | 993 | 2021-02-02 03:44:27 | | |
Video Upload Options
Mithramycin is an antineoplastic antibiotic. The use of mithramycin has been previously limited by its narrow therapeutic window. Recent advances in semisynthetic methods have led to mithramycin analogs with improved pharmacological profiles. Mithramycin inhibits the activity of the transcription factor Sp1, which is closely linked with ovarian tumorigenesis and platinum-resistance.
1. Introduction
Mithramycin, also referred to as plicamycin, is an antineoplastic antibiotic that is naturally produced by three bacteria of the Streptomyces genus (S. argillaceus, S. tanashiensis, and S. plicatus) (Figure 1) [1]. Historically, some leukemias and testicular cancer were treated with mithramycin, in addition to Paget’s disease and the associated hypercalcemia. Despite its anticancer activity, the use of mithramycin was limited due to its very narrow therapeutic window and severe toxicity, resulting in discontinued commercial production in 2000 [2]. The discovery of mithramycin’s activity against Ewing sarcoma, bone and soft tissue pediatric cancer in vitro and in mouse tumor xenografts have led to a phase I/II trial of mithramycin as a monotherapy against Ewing sarcoma [3][4]. This trial, too, was terminated due to the inability to reach pharmacologically relevant mithramycin levels in plasma before the onset of adverse effects.
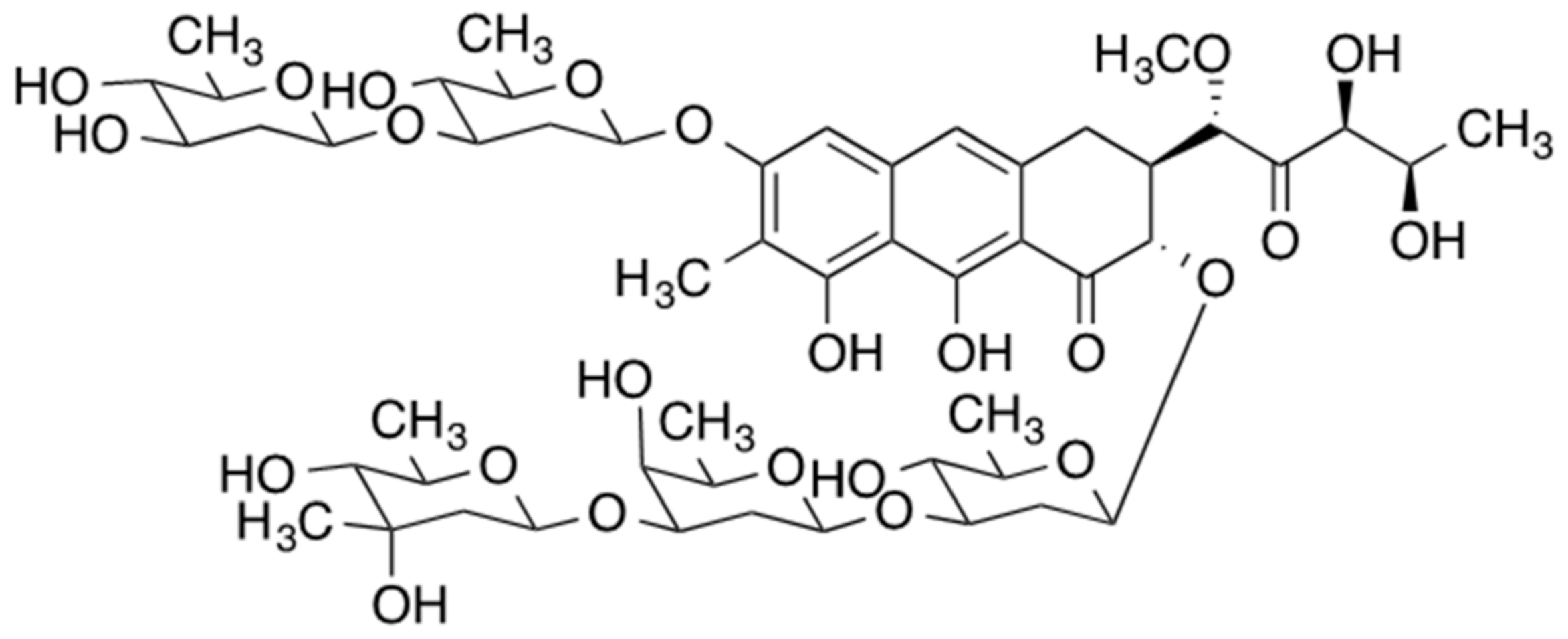
The clinical use of mithramycin for ovarian cancers has largely been limited to the 1960s. In a case series studying the use of mithramycin in “embryonal cancers”, a form of germ cell tumors, utility in primary testicular tumors was noted; however, a metastatic ovarian teratoma was included in the series with marked remission [6]. A small clinical trial using mithramycin as salvage chemotherapy in 26 patients with advanced metastatic malignancy has also been reported. Two patients had ovarian pathology. While the majority (16/26) of patients did not have a therapeutic response, one ovarian subject had “temporary arrest” of the disease, while the other ovarian subject had quantitative remission. Regarding adverse side effects, they were few and relatively mild [7]. A follow-up clinical trial in 1968, involved 32 subjects with advanced malignancies of multiple primary tumors. There was a single ovarian cancer subject that had progression of disease on therapy. Interestingly, 10/32 subjects had no adverse cytotoxic effects from therapy. Reflecting the highly advanced nature of the subjects’ disease, 10/32 subjects died within two weeks of therapy [8].
2. Development of Mithramycin Analogues
Whilst mithramycin has significant anticancer activity, severe toxicity precludes its use. This issue has prompted the development of novel and more selective mithramycin derivatives. The analogs contain the tricyclic chromophore of mithramycin, which preserves the DNA binding function while differing in the nature of the 3-side chain or the identity of the sugars. The varying side chains can be optimized to interact selectively with specific transcription factors and modulate the toxicity and efficacy of the analogs. [9]. Genetic approaches to alter the mithramycin biosynthesis pathway resulted in the synthesis of “mithralogs”, with altered pharmacological and biochemical properties [10]. Mithramycin analogs are synthesized by semisynthetic techniques using a mutant strain of a bacterial producer that generates a mithramycin precursor or a shunt product, which is then elaborated by standard chemical synthesis [11]. These mithramycin derivatives demonstrate promise with retained ability to inhibit Sp-1 transcription and tumorigenesis while reducing toxicity compared to unaltered mithramycin [12][13][14][15][16][17][18]. Most recently, mithramycin SA analogs, with 3-side chain modifications, showed highly improved selectivity of inhibition of Ewing sarcoma and TMPRSS2-ERG expressing prostate cancer cells over mithramycin [19]. Similarly, mithramycin 2′-oximes displayed improved selectivity against Ewing sarcoma cells in vitro as well as a better pharmacokinetic PK profile and somewhat superior efficacy in mouse Ewing sarcoma xenografts, relative to those of mithramycin [20].
One of the most promising mithralogs, demycarosyl-3D-betat-D-digitoxosyl-mithramycin SK (MTM-DIG-MSK or EC-8042), has demonstrated retained anti-tumor properties, including in vitro ovarian cancer lines, but reduced in vitro toxicity in fibroblasts and mononuclear blood cells, compared to mithramycin [10][14][21][18]. Mithramycin and EC-8042 reduced the viability and invasiveness of malignant melanoma cell lines [22]. EC-8042 inhibited SP1 transcription in sarcoma cell lines with the induction of cell cycle arrest and apoptosis, and interestingly was not a substrate for several drug resistance efflux pumps [23]. Compared to mithramycin SK, another mithramycin analog, EC-8042 had greater anti-transcriptional effects in colon carcinoma cell lines [18]. Additionally, in a prostate cancer mouse model, mithramycin analogs were 4-32x better tolerated than standard mithramycin, and exhibited considerable anti-tumor effects against xenografts without evidence of systemic toxicity [24]. There is also some evidence to suggest a synergistic effect with other chemotherapeutic agents in some cancer cell lines. EC-8042 potentiated the effect of docetaxel in triple-negative breast cancer cells in mouse xenograft models. There was no synergy with carboplatin in that setting [25].
Mithramycin analogs also demonstrated efficacy in pre-clinical ovarian studies. EC-8042 displayed significant anti-tumor activity against ovarian cancer cell lines with significantly lower toxicity to fibroblasts and peripheral blood cells compared to mithramycin; while strongly inhibiting Sp1 transcription and reduction in several genes implicated in tumorigenesis including VEGF, BRCA2, cMyc, and src [13][21]. In one of the most detailed studies involving ovarian cancer and MTM analogs, MTM-SDK and MTM-SK, demonstrated considerable inhibition of Sp1 dependent transcription in vitro. Additionally, a mouse model demonstrated significant growth inhibition of subcutaneous ovarian xenografts for both compounds. In an orthotopic xenograft model, MTM-SDK demonstrated a significant increase in the median time of abdominal distension/ascites and median survival. Importantly, the compound was well tolerated without signs of toxicity in the mouse model [15].
While the recent clinical trials involving mithramycin were underwhelming, there have been no recent trials investigating mithramycin analogs or combination therapies with other agents. Novel drug delivery vehicles for mithramycin have been explored with reported success. It has been demonstrated that polymeric nanoparticles of mithramycin can be formulated by techniques suitable for amphiphilic compounds [26]. Via a unique delivery approach, mithramycin loaded nanoparticles have demonstrated considerable efficacy against pancreatic cancer xenografts [27]. Mithramycin analogs MTM-SK and MTM-SDK were also placed in polymer micelles in order to increase bioavailability and drug targeting, with improved cytotoxicity compared to free drug in non-small cell lung cancer lines [28]. Of note, there have been no investigations of such novel drug delivery mechanisms for mithramycin or analogs in ovarian cancer.
References
- National Center for Biotechnology Information. PubChem Compound Summary for CID 230076, Mithramycin a. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Mithramycin-a (accessed on 1 August 2020).
- Lopez-Martinez, D.; Liang, C.-C.; Cohn, M.A. Cellular response to DNA interstrand crosslinks: The Fanconi anemia pathway. Cell. Mol. Life Sci. 2016, 73, 3097–3114.
- Grohar, P.J.; Glod, J.; Peer, C.J.; Sissung, T.M.; Arnaldez, F.I.; Long, L.; Figg, W.D.; Whitcomb, P.; Helman, L.J.; Widemann, B.C. A phase I/II trial and pharmacokinetic study of mithramycin in children and adults with refractory Ewing sarcoma and EWS-FLI1 fusion transcript. Cancer Chemother. Pharmacol. 2017, 80, 645–652.
- Grohar, P.J.; Woldemichael, G.M.; Griffin, L.B.; Mendoza, A.; Chen, Q.-R.; Yeung, C.; Currier, D.G.; Davis, S.; Khanna, C.; Khan, J.; et al. Identification of an Inhibitor of the EWS-FLI1 Oncogenic Transcription Factor by High-Throughput Screening. J. Natl. Cancer Inst. 2011, 103, 962–978.
- Wohlert, S.E.; Künzel, E.; Machinek, R.; Méndez, C.; Salas, J.A.; Rohr, J. The Structure of Mithramycin Reinvestigated. J. Nat. Prod. 1999, 62, 119–121.
- Kofman, S.; Medrek, T.J.; Alexander, R.W. Mithramycin in the treatment of embryonal cancer. Cancer 1964, 17, 938–948.
- Sewell, I.A.; Ellis, H. A trial of mithramycin in the treatment of advanced malignant disease. Br. J. Cancer 1966, 20, 256–263.
- Baum, M. A clinical trial of mithramycin in the treatment of advanced malignant disease. Br. J. Cancer 1968, 22, 176–183.
- Hou, C.; Mandal, A.; Rohr, J.; Tsodikov, O. Allosteric Interference in Oncogenic FLI1 and ERG Transactions by Mithramycins. Structure 2020.
- Albertini, V.; Jain, A.; Vignati, S.; Napoli, S.; Rinaldi, A.; Kwee, I.; Nur-e-Alam, M.; Bergant, J.; Bertoni, F.; Carbone, G.M.; et al. Novel GC-rich DNA-binding compound produced by a genetically engineered mutant of the mithra-mycin producer Streptomyces argillaceus exhibits improved transcriptional repressor activity: Implications for cancer therapy. Nucleic Acids Res. 2006, 34, 1721–1734.
- Méndez, C.; González-Sabín, J.; Morís, F.; Salas, J.A. Expanding the Chemical Diversity of the Antitumoral Compound Mithramycin by Combinatorial Bio-synthesis and Biocatalysis: The Quest for Mithralogs with Improved Therapeutic Window. Planta Med. 2015, 81, 1326–1338.
- Weidenbach, S.; Hou, C.; Chen, J.-M.; Tsodikov, O.; Rohr, J. Dimerization and DNA recognition rules of mithramycin and its analogues. J. Inorg. Biochem. 2016, 156, 40–47.
- Fernández-Guizán, A.; Mansilla, S.; Barceló, F.; Vizcaíno, C.; Núñez, L.-E.; Morís, F.; González, L.E.N.; Portugal, J. The activity of a novel mithramycin analog is related to its binding to DNA, cellular accumulation, and inhibition of Sp1-driven gene transcription. Chem. Interactions 2014, 219, 123–132.
- Núñez, L.E.; Nybo, S.E.; González-Sabín, J.; Pérez, M.; Menéndez, N.; Braña, A.F.; Shaaban, K.A.; He, M.; Morís, F.; Salas, J.A.; et al. A Novel Mithramycin Analogue with High Antitumor Activity and Less Toxicity Generated by Com-binatorial Biosynthesis. J. Med. Chem. 2012, 55, 5813–5825.
- Previdi, S.; Malek, A.; Albertini, V.; Riva, C.; Capella, C.; Broggini, M.; Carbone, G.M.; Rohr, J.; Catapano, C.V. Inhibition of Sp1-dependent transcription and antitumor activity of the new aureolic acid analogues mithramycin SDK and SK in human ovarian cancer xenografts. Gynecol. Oncol. 2010, 118, 182–188.
- Remsing, L.L.; González, A.M.; Nur-e-Alam, M.; Fernández-Lozano, M.J.; Braña, A.F.; Rix, U.; Oliveira, M.A.; Méndez, C.; Salas, J.A.; Rohr, J. Mithramycin SK, a novel antitumor drug with improved therapeutic index, mithramycin SA, and demycarosyl-mithramycin SK: Three new products generated in the mithramycin producer Streptomyces argillaceus through combinatorial biosynthesis. J. Am. Chem. Soc. 2003, 125, 5745–5753.
- Scott, D.; Chen, J.-M.; Bae, Y.; Rohr, J. Semi-synthetic mithramycin SA derivatives with improved anticancer activity. Chem. Biol. Drug Des. 2013, 81, 615–624.
- Vizcaíno, C.; Mansilla, S.; Núñez, L.-E.; Méndez, C.; Salas, J.A.; Morís, F.; Portugal, J. Novel mithramycins abrogate the involvement of protein factors in the transcription of cell cycle control genes. Biochem. Pharmacol. 2012, 84, 1133–1142.
- Mitra, P.; Eckenrode, J.M.; Mandal, A.; Jha, A.M.; Salem, S.M.; Leggas, M.; Rohr, J. Development of Mithramycin Analogues with Increased Selectivity toward ETS Transcription Factor Ex-pressing Cancers. J. Med. Chem. 2018, 61, 8001–8016.
- Liu, Y.; Eckenrode, J.M.; Zhang, Y.; Zhang, J.; Hayden, R.C.; Kyomuhangi, A.; Ponomareva, L.V.; Cui, Z.; Rohr, J.; Tsodikov, O.V.; et al. Mithramycin 2’-Oximes with Improved Selectivity, Pharmacokinetics, and Ewing Sarcoma Antitumor Effi-cacy. J. Med. Chem. 2020, 63, 14067–14086.
- Fernández-Guizán, A.; López-Soto, A.; Acebes-Huerta, A.; Huergo-Zapico, L.; Villa-Álvarez, M.; Núñez, L.-E.; Morís, F.; Gonzales, S. Pleiotropic Anti-Angiogenic and Anti-Oncogenic Activities of the Novel Mithralog Demycarosyl-3D-β-d-Digitoxosyl-Mithramycin SK (EC-8042). PLoS ONE 2015, 10, e0140786.
- Federico, A.; Steinfass, T.; Larribére, L.; Novak, D.; Morís, F.; Núñez, L.-E.; Umansky, V.; Utikal, J. Mithramycin A and Mithralog EC-8042 Inhibit SETDB1 Expression and Its Oncogenic Activity in Ma-lignant Melanoma. Mol. Ther. Oncolytics 2020, 18, 83–99.
- Tornin, J.; Martinez-Cruzado, L.; Santos, L.; Rodriguez, A.; Núñez, L.-E.; Oro, P.; Hermosilla, M.A.; Allonca, E.; Fernández-García, M.T.; Astudillo, A.; et al. Inhibition of SP1 by the mithramycin analog EC-8042 efficiently targets tumor initiating cells in sarcoma. Oncotarget 2016, 7, 30935–30950.
- Malek, A.; Núñez, L.-E.; Magistri, M.; Brambilla, L.; Jović, S.; Carbone, G.M.; Morís, F.; Catapano, C.V. Modulation of the Activity of Sp Transcription Factors by Mithramycin Analogues as a New Strategy for Treatment of Metastatic Prostate Cancer. PLoS ONE 2012, 7, e35130.
- Pandiella, A.; Morís, F.; Ocaña, A.; Núñez, L.-E.; Montero, J.C. Antitumoral activity of the mithralog EC-8042 in triple negative breast cancer linked to cell cycle arrest in G2. Oncotarget 2015, 6, 32856–32867.
- Cohen-Sela, E.; Teitlboim, S.; Chorny, M.; Koroukhov, N.; Danenberg, H.D.; Gao, J.; Golomb, G. Single and Double Emulsion Manufacturing Techniques of an Amphiphilic Drug in PLGA Nanoparticles: Formulations of Mithramycin and Bioactivity. J. Pharm. Sci. 2009, 98, 1452–1462.
- Liu, X.J.; Li, L.; Li, Y.; Zhao, C.-Y.; Wang, R.-Q.; Zhen, Y.-S. Mithramycin-loaded mPEG-PLGA nanoparticles exert potent antitumor efficacy against pancreatic carci-noma. Int. J. Nanomed. 2017, 12, 5255–5269.
- Scott, D.; Rohr, J.; Bae, Y. Nanoparticulate formulations of mithramycin analogs for enhanced cytotoxicity. Int. J. Nanomed. 2011, 6, 2757–2767.




