
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Leila Rashki Ghaleno | + 1870 word(s) | 1870 | 2021-01-26 09:42:00 | | | |
| 2 | Vicky Zhou | Meta information modification | 1870 | 2021-01-27 04:04:31 | | |
Video Upload Options
Sperm DNA Oxidation has destructive effects on sperm structures and functions, thus can result in male infertility. The particular composition of the sperm membrane, rich in polyunsaturated fatty acids, and the easy access of sperm DNA to oxidative damage due to sperm cell specific cytologic and metabolic features (no cytoplasm left and cells unable to mount stress responses) make it the cell type in metazoans most susceptible to oxidative damage. In particular, oxidative damage to the spermatozoa genome is an important issue and a cause of male infertility, usually associated with single- or double-strand paternal DNA breaks.
1. Introduction
The increasing prevalence of infertility in couples is a worldwide health problem affecting roughly 15% of couples. Infertility is defined as the inability of a couple to conceive after at least one year of unprotected intercourse. According to WHO statistics, the inability to procreate has been increasing in recent years, and many couples of childbearing age suffer from this problem [1]. Numerous studies have shown that women and men share equal responsibility in that situation, i.e., about 30% to 40% for each partner [2], the remaining share representing cases for which the origin of infertility cannot be attributed to one or the other partner.
Fragmentation of sperm DNA, whether single-stranded or double-stranded, is the most recognized form of DNA damage to the sperm nucleus. High sperm nucleus fragmentation has been clearly associated with an increased risk of miscarriage, poor embryonic quality, and implantation failure [3]. These last years, sperm DNA fragmentation (SDF) has been the subject of intense study, and there appears today to be a consensus on the fact that the degree of sperm nucleus fragmentation is a reliable indicator of reproductive success, although there is not yet a consensus regarding the technique to be used to diagnose it.
A long-lived misconception that generates a lot of confusion in both the scientific and clinical communities is the thinking that SDF is a read-out of sperm DNA oxidative alterations. In fact, SDF is partly attributable to oxidative stress as it can also result from unresolved meiotic breaks, the remnant of apoptotic cells not fully evacuated during spermatogenesis, or mechanical breaks in the sperm nucleus especially upon chromatin remodeling and histone to protamine replacement at the onset of spermiogenesis. When oxidative stress is important SDF is most often associated, however, sperm DNA oxidation is a condition that can occur alone (i.e., not associated with SDF) and must be considered on its own.
Sperm DNA oxidative stress studies over the past decade have shown that nucleosides, particularly guanosine and adenosine, are very sensitive to oxidation. In addition, it appears that oxidative stress may also affect the sperm epigenome, with possible adverse effects on embryo development [4]. In this context, the level of oxidation of the spermatic nucleus is a parameter that must be seriously considered both in the development of tools to measure this type of damage and in understanding the consequences it may have. A better knowledge of these aspects could certainly contribute to obtaining improved results in assisted reproduction techniques (ARTs) for couples concerned with male factor-related infertility.
2. Sperm DNA Oxidation: A Hidden Danger
Since oxidation of the sperm DNA, although it may accompany nucleus fragmentation, can frequently exist independently of it, the question is then, what are the consequences of this situation when it exists alone? This has been clearly demonstrated in transgenic animal models in which epididymal luminal antioxidant protection has been lowered, resulting in a significant increase in the number of oxidized bases on the sperm nucleus independently of any other alterations [5][6]. Indeed, in this model, the oxidation generated was not sufficient to induce sperm nucleus fragmentation and the epididymis of transgenic mice found ways to compensate for sperm membrane lipid peroxidation [6][7]. The sperm nucleus of these transgenic mice was shown to contain a high level of oxidized guanosine residues, the so-called 8-oxodG or 8-OHdG, the most susceptible nucleoside to oxidation [5]. The presence of these oxidized bases was correlated with chromosomal domains still associated with nucleosomes and particularly to those inter-toroid DNA linker strands associated with the sperm nuclear matrix, most likely because of their peripheral nuclear localization [5][8]. It should be noted that if guanosine is the most susceptible to oxidation, adenosine is next and the two other bases (cytosine and thymidine) may get oxidized too, although to a lesser extent. Therefore, by detecting solely 8-OHdG residues one gets only a part of the real picture of the sperm DNA oxidative alteration. Interestingly, not all mouse chromosomes were found to be equally susceptible to DNA oxidation. In the mouse model, a gradient of damage was found with smaller chromosomes being more susceptible to oxidative damage than longer ones [5][8]. The rationale for this was that in the mouse sperm nucleus, small chromosomes are more peripheral than longer ones [5][8]. A parallel situation was found in humans with histone-enriched regions of chromosomes and, again, especially DNA linker strands in nucleosomal organization connecting protamine toroids, being susceptible to oxidation [9]. In contrast with the mouse situation, in the human sperm nucleus, the number of oxidized 8-OHdG bases found on chromosomes followed a linear relation with their respective length [9]. This discrepancy was explained by the fact that the human sperm nucleus is a lot less condensed than the mouse sperm nucleus, authorizing ROS to penetrate deeper inside it, readily contacting each chromosome. This lower level of nuclear compaction of the human sperm nucleus is due to its higher content in persisting histones approximately 10 times more represented than in the mouse sperm nucleus [10][9]. In the mouse and the human, some special hot spots for sperm DNA oxidation were identified [5][9]. It is interesting to note that human chromosome 15 is by far the most sensitive to DNA oxidation in a region known to bear loci involved in syndromes occurring more frequently in children from aging couples [9].
Interestingly, mating transgenic males with an oxidized sperm nucleus with wildtype females of proven fertility revealed that there was no impact on fertilization success rates but that it was associated with impaired developmental processes [6]. In particular, higher miscarriage rates, higher abnormal developments and higher perinatal mortality were recorded when compared to control mating with wild-type males [6]. In humans, hotspots for sperm DNA oxidation were investigated in fertile and infertile cohorts and it was observed that they were significantly more oxidized in infertile males when compared to fertile ones [9].
Undisputable Consequences of Sperm DNA Oxidation
Due to their specific features, spermatozoa are quasi-silent cells devoid of most of the functional attributes of any other cell type. In particular and of utmost importance, mature spermatozoa do not have a functional DNA repair system, which makes these cells unable to correct DNA base alterations. Classically, the base excision repair (BER) pathway oversees the removal of all damaged nucleotides as a result of oxidation or deamination. In the case of oxidized guanosine and adenosine residues, by far the most frequent situations due to the susceptibility of these bases to oxidation, the BER system involves the activity of the 8-oxoguanine glycosylase (OGG1), which cuts off the oxidized guanine so-called 8-oxo-guanine or 8-hydroxyguanine (8-oxo-7,8-dihydroguanine = 8-OHdG) creating an open apyrimidine site (AP site). Next, the apyrimidinic/apurinic endonuclease 1 (APE1), together with the scaffolding protein known as X-ray repair cross-complementing (XRCC1) that interacts with DNA ligase and polymerases, completes the BER pathway and fills in the AP site with an unaltered residue (Figure 1). Unfortunately, spermatozoa have been shown to lack a complete BER system. They only possess OGG1 activity but lack the APE1 and XRCC1 activities [11]. Oxidized guanine residues in the sperm DNA will thus be readily processed by the sperm OGG1, creating AP sites that will be carried out in the oocyte upon fertilization. It will be the task of the maternal APE1 and XRCC1 activities to complete the BER pathway in the hours that follow fertilization during the complex events leading the sperm nucleus back to a somatic like configuration allowing the fusion of the parental genetic materials. This gender shared process is a nice example of the complementary roles played by the two gametes in the process of generating a new individual. It also brings forward the major role played by the oocyte in repairing the paternal genetic material.
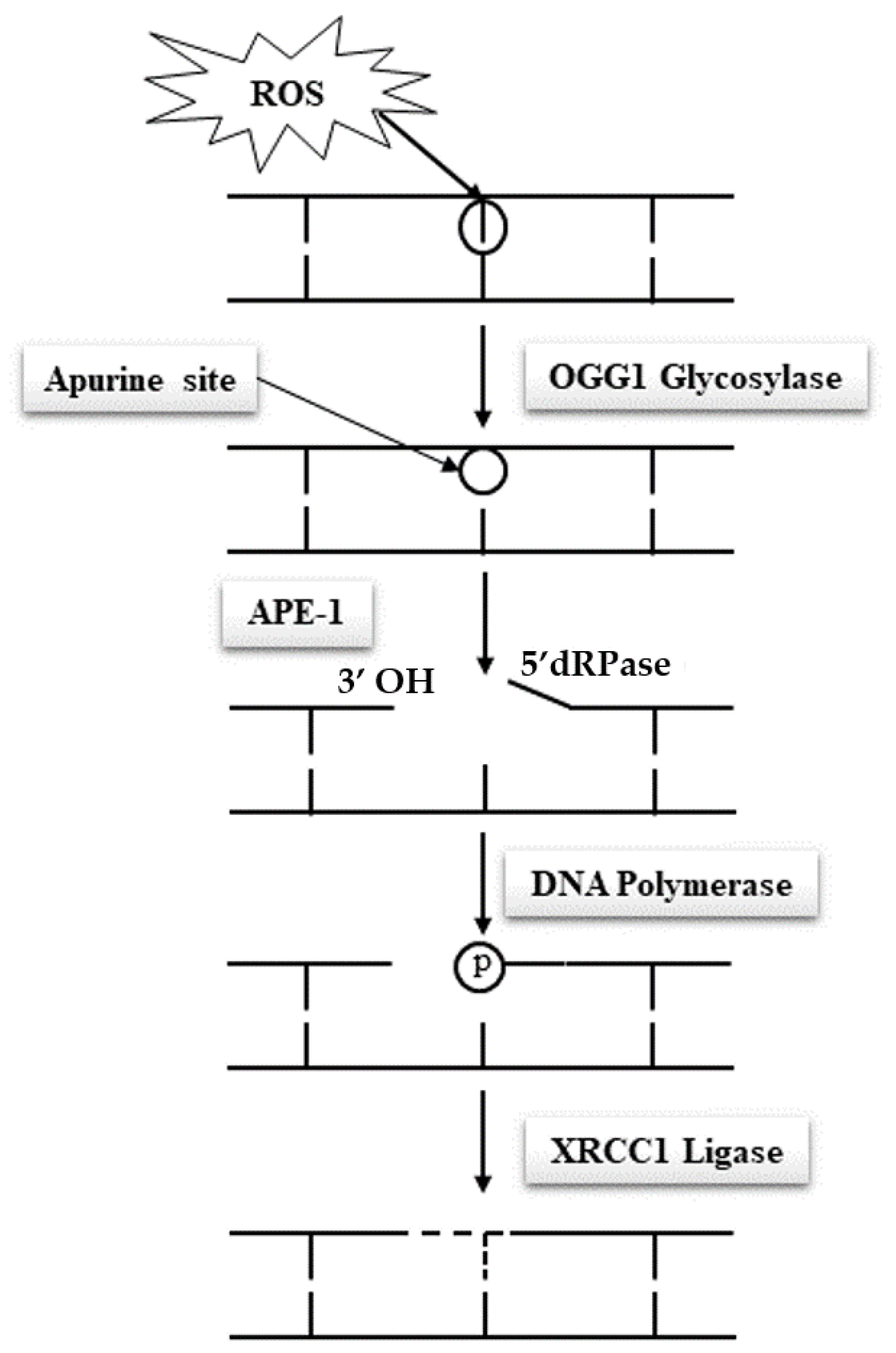
Many situations may affect the efficacy by which the oocyte BER pathway works. Fertilization with spermatozoa bearing a high level of oxidized residues that will potentially overwhelm the oocyte repair capacity is one of these situations. On the female side, oocytes coming from aging women or oocytes that have been stressed following for example hormonal stimulation during ART, chemo or radiation therapies or cryoconservation, all situations that are known to reduce egg quality, represent other classical cases that could lead to incomplete sperm nuclear repair, potentially leading to adverse developmental consequences including an increased mutational load in the next generation [12][13][14][15][16][17]. Any weakness in the spermatozoa’s ability to create abasic sites via its OGG1 activity when facing excessive oxidation of guanine residues, in addition to any weakness in the oocyte ability to complete the BER pathway after fertilization, will therefore promote a classical Hoogsteen-type base pair mismatch, which will lead to de novo G to T transversion mutations that will be carried on to any cell of the developing embryo [10][18].
It is interesting to note that 80% of the 40,000 or so de novo mutations (not present in any of the parents) one records in offspring are of paternal origin, with most of them being of the transversion type. This observation underlines the very important role of the paternal DNA in the transmission of genetic alterations potentially involved in increasing miscarriage rates and infertility, abnormal embryonic development, and the increased susceptibility of the next generations to disease. It is also interesting to note that the number of mutations attributable to sperm increases by 1.51 per year with the father’s age, while the number of mutations attributable to oocyte increases by 0.37 mutations per year with the mother’s age, again emphasizing the paternal share in the offspring mutational load [19].
In this context, one understands how important the criteria of gamete quality and sperm nuclear integrity are. This should prompt the clinical community to a more critical perception of our current approach of evaluating the male partner of infertile couples. Despite undisputable data associating sperm DNA/nuclear loss of integrity, among which sperm DNA oxidation is by far the most common, with reproductive failures there is still no routine evaluation of the integrity of the paternal nucleus and DNA. Considering the dual role of oxidation on spermatozoa being both detrimental when in excess and serving physiological processes when optimal, several parameters should be evaluated to get a precise view of the sperm nuclear oxidative status. In our opinion a complete view of the sperm nuclear oxidative status could be given by the monitoring of three distinct parameters: sperm nuclear condensation, sperm nuclear fragmentation and sperm DNA oxidation.
References
- Agarwal, A.; Majzoub, A.; Parekh, N.; Henkel, R. A Schematic Overview of the Current Status of Male Infertility Practice. World J. Mens Health 2020, 38, 308–322.
- Mayorga-Torres, B.J.M.; Camargo, M.; Cadavid, Á.P.; Du Plessis, S.S.; Cardona-Maya, W. Are oxidative stress markers associated with unexplained male infertility? Andrologia 2017, 49, e12659.
- Ribas-Maynou, J.; Benet, J. Single and Double Strand Sperm DNA Damage: Different Reproductive Effects on Male Fertility. Genes 2019, 10, 105.
- Drevet, J.R.; Aitken, R.J. Oxidative Damage to Sperm DNA: Attack and Defense. Adv. Exp. Med. Biol. 2019, 1166, 107–117.
- Kocer, A.; Henry-Berger, J.; Noblanc, A.; Champroux, A.; Pogorelcnik, R.; Guiton, R.; Janny, L.; Pons-Rejraji, H.; Saez, F.; Johnson, G.D.; et al. Oxidative DNA damage in mouse sperm chromosomes: Size matters. Free Radic. Biol. Med. 2015, 89, 993–1002.
- Chabory, E.; Damon, C.; Lenoir, A.; Kauselmann, G.; Kern, H.; Zevnik, B.; Garrel, C.; Saez, F.; Cadet, R.; Henry-Berger, J.; et al. Epididymis seleno-independent glutathione peroxidase 5 maintains sperm DNA integrity in mice. J. Clin. Investig. 2009, 119, 2074–2085.
- Noblanc, A.; Damon-Soubeyrand, C.; Karrich, B.; Henry-Berger, J.; Cadet, R.; Saez, F.; Guiton, R.; Janny, L.; Rejraji, H.; Alvarez, J.G.; et al. DNA oxidative damage in mammalian spermatozoa: Where and why is the male nucleus affected? Free Radic. Biol. Med. 2013, 65, 719–723.
- Champroux, A.; Cocquet, J.; Henry-Berger, J.; Drevet, J.R.; Kocer, A. A Decade of Exploring the Mammalian Sperm Epigenome: Paternal Epigenetic and Transgenerational Inheritance. Front. Cell Dev. Biol. 2018, 6, 50.
- Xavier, M.; Nixon, B.; Roman, S.; Scott, R.; Drevet, J.; Aitken, R. Paternal impacts on development: Identification of genomic re-gions vulnerable to oxidative DNA damage in human spermatozoa. J. Hum. Reprod. 2019, 34, 1876–1890.
- Drevet, J.R.; Aitken, R.J. Oxidation of Sperm Nucleus in Mammals: A Physiological Necessity to Some Extent with Adverse Impacts on Oocyte and Offspring. Antioxidant 2020, 9, 95.
- Smith, T.B.; Dun, M.D.; Smith, N.D.; Curry, B.J.; Connaughton, H.S.; Aitken, R.J. The presence of a truncated base excision repair pathway in human spermatozoa that is mediated by OGG1. J. Cell Sci. 2013, 126, 1488–1497.
- Lord, T.; Aitken, R.J. Fertilization stimulates 8-hydroxy-2′-deoxyguanosine repair and antioxidant activity to prevent mutagenesis in the embryo. Dev. Biol. 2015, 406, 1–13.
- Horta, F.; Catt, S.; Ramachandran, P.; Vollenhoven, B.; Temple-Smith, P. Female ageing affects the DNA repair capacity of oocytes in IVF using a controlled model of sperm DNA damage in mice. Hum. Reprod. 2020, 35, 529–544.
- Cozzubbo, T.; Neri, Q.; Rosenwaks, Z.; Palermo, G. To what extent can oocytes repair sperm DNA fragmentation? Fertil. Steril. 2014, 102, e61.
- Winship, A.L.; Stringer, J.M.; Liew, S.H.; Hutt, K.J. The importance of DNA repair for maintaining oocyte quality in response to anti-cancer treatments, environmental toxins and maternal ageing. Hum. Reprod. Updat. 2018, 24, 119–134.
- Taheri, F.; Mehrizi, A.A.; Khalili, M.A.; Halvaei, I. The influence of ovarian hyperstimulation drugs on morphometry and mor-phology of human oocytes in ICSI program. Taiwan. J. Obstet. Gynecol. 2018, 57, 205–210.
- Bosch, E.; Labarta, E.; Kolibianakis, E.; Rosen, M.; Meldrum, D. Regimen of ovarian stimulation affects oocyte and therefore em-bryo quality. J. Fertil. Steril. 2016, 105, 560–570.
- Aitken, R.J.; Drevet, J.R. The importance of oxidative stress in determining the functionality of mammalian spermatozoa: A two-edged sword. Antioxidants 2020, 9, 111.
- Jónsson, H.; Sulem, P.; Kehr, B.; Kristmundsdottir, S.; Zink, F.; Hjartarson, E.; Hardarson, M.T.; Hjorleifsson, K.E.; Eggertsson, H.P.; Gudjonsson, S.A.; et al. Parental influence on human germline de novo mutations in 1548 trios from Iceland. Nature 2017, 549, 519–522.




