
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Gabriella Guelfi | + 3313 word(s) | 3313 | 2021-01-07 07:49:21 | | | |
| 2 | Vivi Li | Meta information modification | 3313 | 2021-01-08 05:08:35 | | |
Video Upload Options
Resilience is conceived as a dynamic developmental process involving the achievement of positive adaptation within the context of significant adversity. Resilience is not a unique ability but rather a set of capacities of a system put in place to absorb a disturbance and to reorganize while trying to retain the same function, structure, and identity. This study describes the characteristics and the molecular mechanisms of resilience to understand the core elements of resilience and its indicators.
1. Understanding Resilience
The term resilience is derived from the Latin word resilire (jump back, bounce), initially applied in physics to indicate the property of some materials to resist shocks without breaking or deforming. It was later used in medicine to indicate the ability to resist and react to adversity. Mammalian resilience is conceived as a dynamic, positive adaptation within a significantly adverse context [1]. Two critical conditions are implicit within this conceptualization of resilience: the exposure to severe adversity; and the acquisition of positive adaptation (Figure 1).
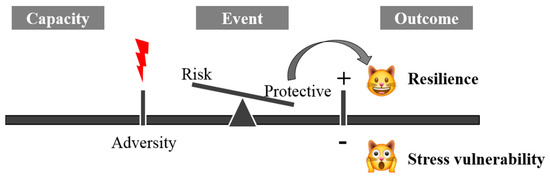
Figure 1. The mechanism of systemic resilience.
Stress is a severe adversity. The term stress is derived from the Latin word stringere, meaning to draw tight. Currently, the literature defines stress as a real or perceived perturbation of the physiological homeostasis or psychological well-being of an organism. To contrast the perturbation and return to normality, an organism uses various behavioral or physiological mechanism. Adaptation in the face of stress is a significant priority for all organisms.
The acquisition of positive adaptation is resilience. The resilience process can be based on three subsequent phases: disturbance, response, and outcome. Based on this theory, Döring et al. [2] defined resilience as the ability of a system, or an individual, to react (respond) to an external force (disturbance) while fulfilling some different conditions at the end of the response (outcome). The disturbance is identified as an external source to the system, leading to stress or resilience. The frequency and severity characterize the disturbance. The frequency of the disturbance is low when the disturbance manifests itself as unexpected or rare.
Conversely, the frequency is high when the disturbing events are repeated. The disturbance severity is difficult to quantify because it is closely related to the ability of organisms to adapt to the system. Disturbances can be ranked as macro- or micro-environmental factors. Macro-environmental factors are features associated with the environment and affect most subjects (e.g., disease pressure, ambient temperature): the response to these factors can be a genetic variance of the entire population. Micro-environmental factors are variations that concern only a minority of the whole population within that macro-environment (e.g., social interactions, nutrition). From a practical point of view, resilience to occasional macro-environmental disturbances-such as disease outbreaks and heatwaves-is less frequent and, therefore, less significant. Döring et al. claimed that the response (outcome) of the resilient system to disturbing events could lead the system to buffer, absorb, tolerate, cope, or adapt to the disturbances. Döring et al. claim that the response of the resilient system to disturbing events can lead the system to buffer, absorb, tolerate, cope, or adapt to the disturbances. The outcome falls within many definitions of resilience; on the one hand, definitions are required as a criterion of resilience, that the system responding to the disturbance succeeds, or at least maintains functionality. On the other hand, the requirement is that the system must neither change its structure nor collapse into a different state [2]. In 2014 the American Psychological Association defined resilience as “the process of adapting well in the face of adversity, trauma, or significant sources of stress” [3].
In 2015, the concept of “preclusion” defined resilience as “the ability of an individual to limit or preclude the detrimental effects of a stressor” [4]. That same year, resilience acquired multifaceted meanings, showing conceptual similarities with other notions such as adaptation, homeostasis, allostasis, and invulnerability. But these notions, although similar, show conceptual differences. The concept of adaptation is limited to a specific stress, but it does not, by itself, indicate flexibility in successful adaptation to all new challenges over a lifetime. While resilience allows the system to make significant changes, it is not limited to specific stress. The term homeostasis defines the self-regulatory capacity of living beings, which is very important to keep the internal environment constant despite variations in the external environment. However, while homeostasis is based on achieving a status quo because of feedback mechanisms, resilience, through dynamic and complex processes, allows active adaptation to new conditions. Allostasis is the ability to maintain the physiological stability of systems through change: this definition is very similar to the concept of resilience, but it does not focus on recovery after diseases [2].
Over the past decade, scientific studies on resilience in stressful situations have developed in many areas, especially in mammals, and resilience has been examined across a range of contexts, such as physiological (e.g., disease, temperature stress) or psychological (e.g., novel environment, social stressor, interaction). The new concept of resilience is a “general” combined trait, consisting of different resilience types depending on the nature of the disturbance [5].
To date, the research on animal resilience investigates mainly two aspects: animal productivity and animal welfare [6]. Resilience might be measured based on deviations from expected production levels over a period of time. However, detecting specific and sensible resilience indicators might provide the opportunity to include resilience in the breeding goals. The advantage of genetic selection, in contrast to management improvements, is that it affects all subsequent generations of livestock. Resilient animals are animals that need little/less attention time: increasing resilience is, therefore, desired.
Referred behavioral and physiological responses to stressful stimuli have been termed coping resources. Importantly, coping styles do not equate with success in coping with stressors [5]: the term describes the ongoing strategy or processes an animal employs when reacting to stressors rather than the coping response outcome [7]. Coping is a series of continually changing cognitive and behavioral efforts to manage specific external and internal demands. Coping strategies are essential to minimize the impact of stress and determine the degree of resilience or susceptibility. Coping is active when a subject tries to deal with a challenge, deals with fears, participates in problem-solving, and seeks social support. It also engages positive reassessment of aversive experiences that can produce long-term resilience.
2. Stress Vulnerability and Resilience
Essential to understanding the precise meaning of resilience is to introduce the concept of stress because the features of stressful events define if and when an animal will go back to a pre-crisis status. Stress is defined as any factor of different nature (physical, chemical, behavioral, social) that changes or threatens homeostasis, thereby eliciting specific response mechanisms. Two contrasting hypotheses have explained the impact of stress. The cumulative stress models claim that the build-up of stress across the life span or adversity never has any beneficial effect; instead, the risk of disease gradually increases [8]. The match/mismatch model, which explains the concept of stress/epigenetic changes, includes adaptation to early-life stressors (even significant cumulative stressors) for specific individuals, thus including the concept of resilience. Early-life exposure to stressors can bring about epigenetic changes to match an organism to its environment and decrease the risk of vulnerability. A mismatch between the phenotypic outcome of the epigenetic changes and the ability to cope with current environmental stressors increases the risk of vulnerability [9].
The way an organism perceives and responds to stressors changes based on previous stress exposures: in general, exposure to recurring sources of stress induces chronic stress, associated with negative consequences for both physical and behavioral health. However, it is also essential to understand that the evolution process did not select the biological response to stress to harm or kill the animal, but rather improve survival [10]. A psycho-physiological stress response is one of the fundamental survival mechanisms of nature, e.g., without a fight-or-flight stress response, the gazelle has no chance of escaping, just as a lion has no chance of catching a gazelle [11]. Thus, during short-term stress, multiple physiological systems are activated to enable survival. Mild or moderate exposure to stress is much less likely to result in adverse health consequences: on the contrary, it may be beneficial to development [12]. Numerous studies have shown that both in humans and animals, short-term stress experienced at the time of immune activation induces a significant enhancement of the ensuing immune response [13][14]. Dhabhar et al. [10] first proposed that the short-term stress response prepares the cardiovascular, musculoskeletal, and neuroendocrine systems for fight or flight or prepare the brain for the challenges (e.g., figuring out an escape route) with a model of spectrum stress. On one side, we have good stress or eustress, which involves a rapid biological response mounted in the presence of the stressor, followed by a rapid, responsive shut-down on cessation of the stressor: such responses induce physiological conditions that are likely to enhance protective immunity, mental and physical performance, and overall health. The opposite end of the spectrum is characterized by bad stress or distress, which involves chronic or long-term biological changes that are likely to result in deregulation or suppression of immune function, a decrease in mental and physical performance, and an increased likelihood of disease: the deregulation of these latter processes persists long after the stressor has ceased (Figure 2).
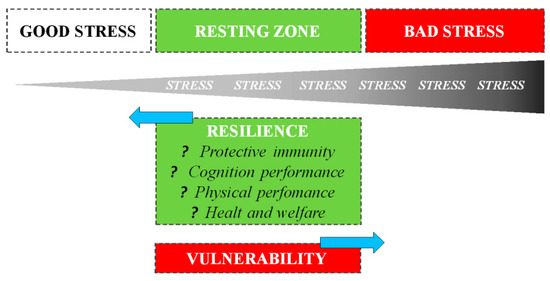
Figure 2. Staying on the good side of the stress spectrum to maintain health, one needs to optimize GOOD stress, maximize the RESTING ZONE, and minimize BAD stress. A resting zone exists between bad (harmful) and good (beneficial) stress, and the efficiency with which an organism returns to its resting zone following stress depends on its resilience.
The stress spectrum also describes the resting zone of low/no stress representing a state of health maintenance/restoration: after stressing, the amplitude, speed, and efficiency with which an organism returns to a resting state depends on its resilience.
To reconcile the differences between the cumulative stress model [15] that does not allow the subject to adapt to epigenetic changes and the match/mismatch model [9] that includes the concept of adaptation to early-life stressors, the three-hit concept of vulnerability and resilience arises [16]. This model underlines the importance of gene-environment interactions during critical phases of early life brain development to switch from vulnerability to resilience outcome. Daskalakis et al. [16] consider following the three-hit model: the interaction of genetic factors (Hit 1, epigenetic factors) with early life experiences (Hit 2, experiences) causes altered endocrine regulations and epigenetic changes during brain development, programming gene expression patterns relevant for an evolving phenotype. These programmed phenotypes have different susceptibility to challenges later in life (Hit 3, phenotype), promoting resilience or vulnerability. If the subjects are exposed to the latter type of later-life environment, they will develop the vulnerability phenotype, but when exposed to another type of later-life environment, the same phenotype will result in resilience outcomes [12].
Epigenetics modifications refer to relevant gene expression changes (with subsequent changes in cellular phenotype) that result from mechanisms other than from changes in DNA nucleotide sequence. Such modifications are induced by environmental events that directly allow the genome to adapt during delicate developmental periods and possibly to a lesser extent-in adulthood, leading to changes in gene expression and neural function [17]. In recent years, fostered by unprecedented biomolecular developments, a new way was conceived of considering the response of the animal to stress and adversity as an individual feature strategy resulting from the interaction between environmental signals and genome: the epigenome. Unlike the genome sequence, epigenome marks are less stable and can change in response to various environmental stimuli. However, epigenetic marks, sensitive to environmental exposure, transform the local chromatin environment, affect DNA accessibility, and regulate gene transcription or interfere in the mRNA translation through non-coding RNA. Epigenetic marks can disrupt regular gene expression and protein expression profiles [18]. Stress factors can impact the levels and the turnover of epigenetic factors either directly or indirectly. Whereas, with the same mechanism, protective factors ameliorate or alter the response of the subject to environmental stress. Examples of protective factors are maternal care, emotional relationships, and social support [19]. The protective factors that can increase a resilient behavior are contrasted by the risk factors representing those conditions, which increase the probability of experiencing a specific pathology. They can be linked to genetic factors and lifestyles, for example, obesity, illness, brief primary maternal care, and any other stressors [20].
3. Resilience in Response to Epigenetics Remodeling
Nature (genetics) and nurture (life experience) through genetic and epigenetic mechanisms control the gene expressions in order to imprint a response of susceptibility or resilience to stress [21]. The term epigenetics derives from the Greek prefix “epi” which means upon or over, and genetics, which refers to the sequence of genomic DNA. Epigenetic transcriptional regulatory mechanisms include DNA methylation, post-translational modifications of histones, and changes in the position of the secondary structures formed by DNA and histones called nucleosomes, all of which are collectively referred to as the epigenome [22]. Another epigenetic mechanism includes non-coding R.N.A. (ncRNA) such as microRNA (miRNA) and large non-coding R.N.A. (lncRNA) [23]. LncRNAs are associated with chromatin modification complexes such as repressive complexes, recruiting and targeting them in specific genomic regions. These mechanisms can act separately or in synergy to modulate chromatin structure and its accessibility to the transcriptional machinery. Epigenetic mechanisms are highly dynamic and can be influenced by environmental factors such as diet, social/familial relationships, and stress [21].
The epigenome has the potential to encode a molecular memory of past events that can influence gene expression, neuronal function, and future behaviors. Epigenetic regulation of chromatin plays a role in determining the adaptive or maladaptive nature of neural and behavioral responses to environmental stressors [22]. Animal models of the epigenetics of stress responses have been most valuable for establishing the molecular and cellular mechanisms by which stressor-induced changes in chromatin regulation impact behavioral stress responses [22]. Stressors are among the environmental stimuli that can change DNA methylation patterns in the brain, and various stress paradigms have shown that they decrease methylation of multiple genes encoding intermediaries in the HPA axis. Neural plasticity genes, such as the BDNF, are also targets of regulation by DNA methylation [22].
For example, in rodents, the axis reactivity is reduced following high maternal care or a short adult separation, while the hyperactivity of the axis is associated with prenatal stress or prolonged maternal separation during growth [22]. The homeostatic regulation of the HPA axis is also affected by epigenetic regulation and DNA methylation of its multiple genes. A newborn rat exposure to a rodent abuse model with daily disruptive caregiving during the first postnatal week is associated with an increase in DNA methylation of BDNF exon IV and IX. BDNF methylation led to a down-regulating of the corresponding mRNA [22]. On the other hand, the exposure of adult rats to predator stress and social instability leads to increased DNA methylation and decreased mRNA expression of BDNF in the hippocampus, but not in the prefrontal cortex [24]. The latter suggests that BDNF methylation changes induced by environmental stress could contribute to brain plasticity and determine different behavioral responses to stressors [22]. In the presence of environmental stressors, the methylation or demethylation of DNA cytosine occurs through writers and erasers of enzyme regulation. Many of the proteins regulating methylation processes are subjected to stress-dependent expression changes as the Gadd45 scaffold family, the Aid/APOBEC family of cytosine deaminase, the methyl-DNA binding protein Mbd4, and the Tet protein family [22]. In mice, ten days of chronic social defeat stress caused a significant increase of DNMT3A mRNA expression in N-acetylcysteine to stimulate depressive behavior [25] which in turn gave rise, in the hypothalamic paraventricular nucleus (PVN), to the reduction of the Corticotrophin-Releasing Factor (CRF) promoter gene methylation [26]. CRF secreted from PVN neurons is a crucial regulator of the HPA axis after chronic social defeat stress exposure, and DNA methylation induction has a pro-depressive function.
DNA methylation was also associated with resilience to a different experimental stressor paradigm called chronic ultra-mild stress (CUMS). CUMS is induced by a series of mild environmental and social stressors that bring about depressive-like behaviors if protracted over time. Interestingly, after CUMS induction, susceptible and stress-resilient mouse strains showed an enhancement of methylation Glial-Derived Neurotrophic Factor (GDNF) promoter gene. GDNF gene DNA methylation is correlated with animal behavior. Susceptible strain methylation increase was associated with GDNF expression decrease. Whereas, in resistant strain methylation, the increase was associated with GDNF mRNA increase. This strain difference appears to arise from various proteins recruited to promoter methylation sites [26].
An extensively studied genetic target, subjected to epigenetic regulation, is the NR3C1 gene encoding glucocorticoid receptor (GR). Evidence suggests that the methylation of the NR3C1 promoter regions are related to vulnerability or stress resilience [27]. Hippocampal NR3C1 is upregulated in rats exposed to high maternal care during the early postnatal days: this mediates enhanced glucocorticoid feedback, long term decreased HPA axis responsivity and stress-resilient phenotypes during adulthood. In contrast, early life stress or low maternal care decreases hippocampal NR3C1 expression, increases HPA responsivity, and predisposes adults to stress vulnerable phenotypes. In both cases, the mRNA expression encoding the GR is inversely correlated with DNA methylation of CpG residues in the NR3C1promoter regions [22]. NR3C1 serves as a binding site for the transcription of nerve growth factor-inducible protein A (NGFI-A). During a critical period in the first week of life, high maternal care is thought to determine the set-point of HPA axis responsivity in adulthood through NR3C1 promoter demethylation that permits NGFI-A-dependent GR expression [28][29] (Figure 8).
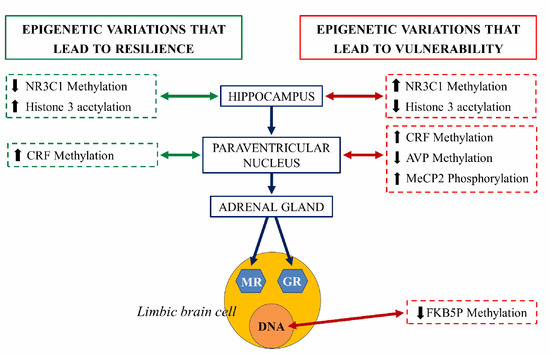
Figure 8. Schematic representation of the epigenetic regulation of the hypothalamus-hypophysis-adrenal axis (grey axis). The right side of the figure illustrates the epigenetic changes that lead to vulnerability to stress and risk at each structure (red arrow). The left side represents epigenetic variations that lead to resilience at each structure (green arrow). NR3C1: steroid receptor gene, pMeCP2: phosphorylated protein related to methylation of histones, CRF, corticotropin-releasing factor gene, AVP: arginine vasopressin gene, FKBP5: gene coding for chaperones for the expression of glucocorticoid receptors (GR) and mineralocorticoid receptors (MR) [30].
Adverse early-life stress (ELS) induces, in mice, long-lasting alterations in passive stress coping and memory. This phenotype was accompanied by a persistent increase in the HPA neurons of arginine vasopressin (AVP) expression associated with DNA hypomethylation of methyl CpG-binding protein 2 (MeCP2) epigenetic marking. Thus, ELS controls DNA methylation in neurons to generate stable changes in AVP expression, which triggers neuroendocrine and behavioral alterations, features that are frequently identified in depression [31].
Recent studies on transgenerational inheritance in mammals suggest potential inheritance of epigenetic patterns via multiple mechanisms conferred by paternal sperm and maternal germline, potentially reflecting ancestral stress and impacting anxiety-related mental health in offspring by shaping endocrine programming, brain development, and ways to cope with stress [32]. Perturbed maternal behaviors by unpredictable separation and maternal stress widely affect methylation in the brain and cause hypomethylation or hypermethylation in the offspring of different genes altering the gene expression. Strikingly, the aberrant methylation is perpetuated across successive generations and is present in the germline of first-generation males and the brain and germline of second-generation progeny: this progeny and the following show multiple stress-related symptoms such as depressive-like behaviors and social anxiety. Due to disrupted maternal care, aberrant DNA methylation affects several tissues; it can subsist after meiosis in male germ cells and is trans-generationally transmitted, suggesting a powerful potential way of maintenance inheritance of the effects of early chronic stress [8]. Like sperm cells, oocytes may also carry epigenetic anomalies resulting from stress exposure since the trans-generational inheritance of stress-induced symptoms occurs through females independently of maternal care [33].
The interaction between genes and the environment is necessary for the animals’ life, and environmental enrichment changes the epigenetic nature of an organism, enhancing neural plasticity, resilience to stressors, and repair [34]. After more than six decades of work on environmental enrichment, we laud the advances in understanding relevant biological parameters and critical mechanisms; however, further research will have to identify the stage of life and how long it takes enrichment to activate benefits, promote plasticity and improve resilience.
References
- Rutter, M. Implications of Resilience Concepts for Scientific Understanding. Ann. N. Y. Acad. Sci. 2006, 1094, 1–12.
- Döring, T.F.; Vieweger, A.; Pautasso, M.; Vaarst, M.; Finckh, M.R.; Wolfe, M.S. Resilience as a Universal Criterion of Health. J. Sci. Food Agric. 2015, 95, 455–465.
- Building Your Resilience. Available online: https://www.apa.org/topics/resilience (accessed on 10 October 2020).
- Dantzer, R.; Cohen, S.; Russo, S.J.; Dinan, T.G. Resilience and Immunity. Brain Behav. Immun. 2018, 74, 28–42.
- Colditz, I.G.; Hine, B.C. Resilience in Farm Animals: Biology, Management, Breeding and Implications for Animal Welfare. Anim. Prod. Sci. 2016, 56, 1961.
- Fletcher, D.; Sarkar, M. Psychological Resilience: A Review and Critique of Definitions, Concepts, and Theory. Eur. Psychol. 2013, 18, 12–23.
- Von Holst, D. The Concept of Stress and Its Relevance for Animal Behavior. In Advances in the Study of Behavior; Elsevier: Amsterdam, The Netherlands, 1998; Volume 27, pp. 1–131. ISBN 978-0-12-004527-3.
- Nederhof, E.; Schmidt, M.V. Mismatch or Cumulative Stress: Toward an Integrated Hypothesis of Programming Effects. Physiol. Behav. 2012, 106, 691–700.
- Gluckman, P.D.; Hanson, M.A.; Buklijas, T.; Low, F.M.; Beedle, A.S. Epigenetic Mechanisms That Underpin Metabolic and Cardiovascular Diseases. Nat. Rev. Endocrinol. 2009, 5, 401–408.
- Dhabhar, F.S. The Power of Positive Stress—A Complementary Commentary. Stress 2019, 22, 526–529.
- Dhabhar, F.S. The Short-Term Stress Response—Mother Nature’s Mechanism for Enhancing Protection and Performance under Conditions of Threat, Challenge, and Opportunity. Front. Neuroendocrinol. 2018, 49, 175–192.
- Hornor, G. Resilience. J. Pediatr. Health Care 2017, 31, 384–390.
- Dhabhar, F.S. Acute Stress Enhances While Chronic Stress Suppresses Skin Immunity: The Role of Stress Hormones and Leukocyte Trafficking. Ann. N. Y. Acad. Sci. 2006, 917, 876–893.
- Dhabhar, F.S. Effects of Stress on Immune Function: The Good, the Bad, and the Beautiful. Immunol Res 2014, 58, 193–210.
- McEWEN, B.S. Stress, Adaptation, and Disease: Allostasis and Allostatic Load. Ann. N. Y. Acad. Sci. 1998, 840, 33–44.
- Daskalakis, N.P.; Bagot, R.C.; Parker, K.J.; Vinkers, C.H.; de Kloet, E.R. The Three-Hit Concept of Vulnerability and Resilience: Toward Understanding Adaptation to Early-Life Adversity Outcome. Psychoneuroendocrinology 2013, 38, 1858–1873.
- Fagiolini, M.; Jensen, C.L.; Champagne, F.A. Epigenetic Influences on Brain Development and Plasticity. Curr. Opin. Neurobiol. 2009, 19, 207–212.
- Zhang, Y.; Kutateladze, T. Diet and the Epigenome. Nat. Commun. 2018, 9, 3375.
- Rutter, M. Resilience in the Face of Adversity: Protective Factors and Resistance to Psychiatric Disorder. Br. J. Psychiatry 1985, 147, 598–611.
- Willett, W.C. Balancing Life-Style and Genomics Research for Disease Prevention. Science 2002, 296, 695–698.
- Franklin, T.B.; Saab, B.J.; Mansuy, I.M. Neural Mechanisms of Stress Resilience and Vulnerability. Neuron 2012, 75, 747–761.
- Zannas, A.S.; West, A.E. Epigenetics and the regulation of stress vulnerability and resilience. Neuroscience 2014, 264, 157–170.
- Dudley, K.J.; Li, X.; Kobor, M.S.; Kippin, T.E.; Bredy, T.W. Epigenetic mechanisms mediating vulnerability and resilience to psychiatric disorders. Neurosci. Biobehav. Rev. 2011, 35, 1544–1551.
- Roth, T.L.; Zoladz, P.R.; Sweatt, J.D.; Diamond, D.M. Epigenetic modification of hippocampal Bdnf D.N.A. in adult rats in an animal model of post-traumatic stress disorder. J. Psychiatr. Res. 2011, 45, 919–926.
- Elliott, E.; Ezra-Nevo, G.; Regev, L.; Neufeld-Cohen, A.; Chen, A. Resilience to social stress coincides with functional DNA methylation of the Crf gene in adult mice. Nat. Neurosci. 2010, 13, 1351–1353.
- Uchida, S.; Hara, K.; Kobayashi, A.; Otsuki, K.; Yamagata, H.; Hobara, T.; Suzuki, T.; Miyata, N.; Watanabe, Y. Epigenetic status of Gdnf in the ventral striatum determines susceptibility and adaptation to daily stressful events. Neuron 2011, 69, 359–372.
- Vitellius, G.; Trabado, S.; Bouligand, J.; Delemer, B.; Lombès, M. Pathophysiology of Glucocorticoid Signaling. Ann. Endocrinol. 2018, 79, 98–106.
- Weaver, I.C.G.; Cervoni, N.; Champagne, F.A.; D’Alessio, A.C.; Sharma, S.; Seckl, J.R.; Dymov, S.; Szyf, M.; Meaney, M.J. Epigenetic programming by maternal behavior. Nat. Neurosci. 2004, 7, 847–854.
- Van Bodegom, M.; Homberg, J.R.; Henckens, M.J.A.G. Modulation of the Hypothalamic-Pituitary-Adrenal Axis by Early Life Stress Exposure. Front. Cell. Neurosci. 2017, 11.
- Zapata-Martín del Campo, C.; Martínez-Rosas, M.; Guarner-Lans, V. Epigenetic programming of synthesis, release, and/or receptor expression of common mediators participating in the risk/resilience for comorbid stress-related disorders and coronary artery disease. Int. J. Mol. Sci. 2018, 19, 1224.
- Murgatroyd, C.; Patchev, A.V.; Wu, Y.; Micale, V.; Bockmühl, Y.; Fischer, D.; Holsboer, F.; Wotjak, C.T.; Almeida, O.F.X.; Spengler, D. Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nat. Neurosci. 2009, 12, 1559–1566.
- Schiele, M.A.; Domschke, K. Epigenetics at the crossroads between genes, environment and resilience in anxiety disorders. Genes Brain Behav. 2018, 17, e12423.
- Weiss, I.C.; Franklin, T.B.; Vizi, S.; Mansuy, I.M. Inheritable effect of unpredictable maternal separation on behavioral responses in mice. Front. Behav. Neurosci. 2011, 5.
- Kentner, A.C.; Lambert, K.G.; Hannan, A.J.; Donaldson, S.T. Editorial: Environmental enrichment: Enhancing neural plasticity, resilience, and repair. Front. Behav. Neurosci. 2019, 13, 75.




