1000/1000
Hot
Most Recent

Background: Functional restoration of abdominal wall defects represent one of the fundamental challenges of reconstructive surgery. Synthetic grafts or crosslinked animal-derived biological grafts are characterized by significant adverse reactions, which are mostly observed after their implantation. The aim of this study was to evaluate the efficacy of the decellularization protocol to produce a completely acellular full-thickness abdominal wall scaffold.
Methods: Full-thickness abdominal wall samples were harvested from Wistar rats and submitted to a 3 cylce decellularization process. Histological, analysis was applied to evaluate the effect of the decellularization protocol. Mechanical testing and immunogenicity assessment were also performed.
Results: Histological analysis results showed the efficient decellularization abdominal wall samples after the 3rd cycle. Decellularized abdominal wall scaffolds were characterized by good biomechanical properties
Conclusion: The data presented herein confirm the effective production of rat-derived full-thickness abdominal wall scaffold. Expanding this approach will allow the exploitation of the capacity of the proposed decellularization protocol in producing acellular abdominal wall scaffolds from larger animal models or human cadaveric donors. In this way, the utility of biological scaffolds with preserved in vivo remodelling properties may be one step closer to its application in clinical studies.
Decellularization of Full-Thickness Abdominal Wall For Reconstructive Surgery
George Skepastianos1,2* , Panagiotis Mallis3*, Efstathios Michalopoulos3, Gerasimos Tsourouflis4
1Plastic Surgery Department, EANP Metaxa, National Hospital of Athens, 51 Botatsi Street, 185 37 Pireus Greece, skep-19@hotmail.com (G.S.);
2Center of Experimental Surgery, Biomedical Research Foundation Academy of Athens, 4 Soranou Ephessiou Street, 115 27 Athens, Greece
3Hellenic Cord Blood Bank, Biomedical Research Foundation Academy of Athens, 4 Soranou Ephessiou Street, 115 27 Athens, Greece. , pmallis@bioacademy.gr (P.M.); smichal@bioacademy.gr (S.M.)
4Second Department of Propedeutic Surgery, Medical School, University of Athens, 11527 Athens, Greece, gerasimos.ts@gmail.com (G.T.)
* George Skepastianos and Panagiotis Mallis have equally contributed to this work as first authors.
Summary
Abdominal wall defects resulting from trauma, infection, congenital conditions, complications of abdominal surgeries, and neoplastic diseases are common and necessitate reconstructive surgery [1]. Incisional hernias, occurring after laparotomy, affect 5-20% of patients and pose significant challenges for repair [2]. Annually, over 700,000 abdominal wall reconstruction surgeries are performed in the United States, with more than 20 million performed globally [1-4].
Complications associated with abdominal wall surgery include muscle weakness and alterations in collagen composition [5]. Specifically, reduced collagen I and increased collagen III levels impact abdominal wall tensile strength and mechanical stability [5-8]. To address these challenges, scaffolds with specific biomechanical properties are essential for tissue regeneration and wound healing [9].
Currently, commercially produced synthetic scaffolds play a crucial role in abdominal wall reconstruction [1, 10, 11]. These scaffolds can be made from either biodegradable or non-biodegradable materials. Biodegradable materials like polyglycolic acid (PLGA) and polyglactin 910 (PLGA 910) undergo hydrolysis, allowing tissue-resident macrophages to degrade them into monomers that contribute to ECM regeneration. However, biodegradable materials often lead to scar tissue formation at the injury site [15-17]. Abdominal wall reconstruction often faces challenges related to scar tissue formation and adverse reactions to synthetic materials. To address this, researchers explore alternative strategies, including biological scaffolds derived from natural or animal-derived extracellular matrix (ECM). However, existing approaches have limitations, such as immune responses and suboptimal biomechanical properties. Decellularization—a process that removes cellular components while preserving ECM—offers promise. Our study focuses on rat-derived full-thickness abdominal wall scaffolds. We optimized a cost-effective, perfusion-free decellularization method involving three cycles. Histological analysis, biochemical assays, and DNA quantification confirmed successful decellularization. Biomechanical testing revealed improved properties post-decellularization.These findings contribute to the development of large-scale, full-thickness abdominal wall scaffolds for enhanced tissue reconstruction. Future work may refine protocols and explore clinical applications.
Histological and biochemical evaluation of decellularized whole rat-derived abdominal wall.
To assess the effectiveness of the decellularization process for rat-derived abdominal wall tissue, we have conducted histological and biochemical analyses. In this study, tissue samples were examined under a microscope along both circumferential and longitudinal axes. The goal was to verify successful removal of cellular components while preserving the extracellular matrix (ECM). Biochemical assessment involved analyzing DNA, RNA, proteins, and other molecules to understand tissue composition. The absence of resident cells after decellularization was confirmed through biochemical markers. (Figure 1 and Figure 2).
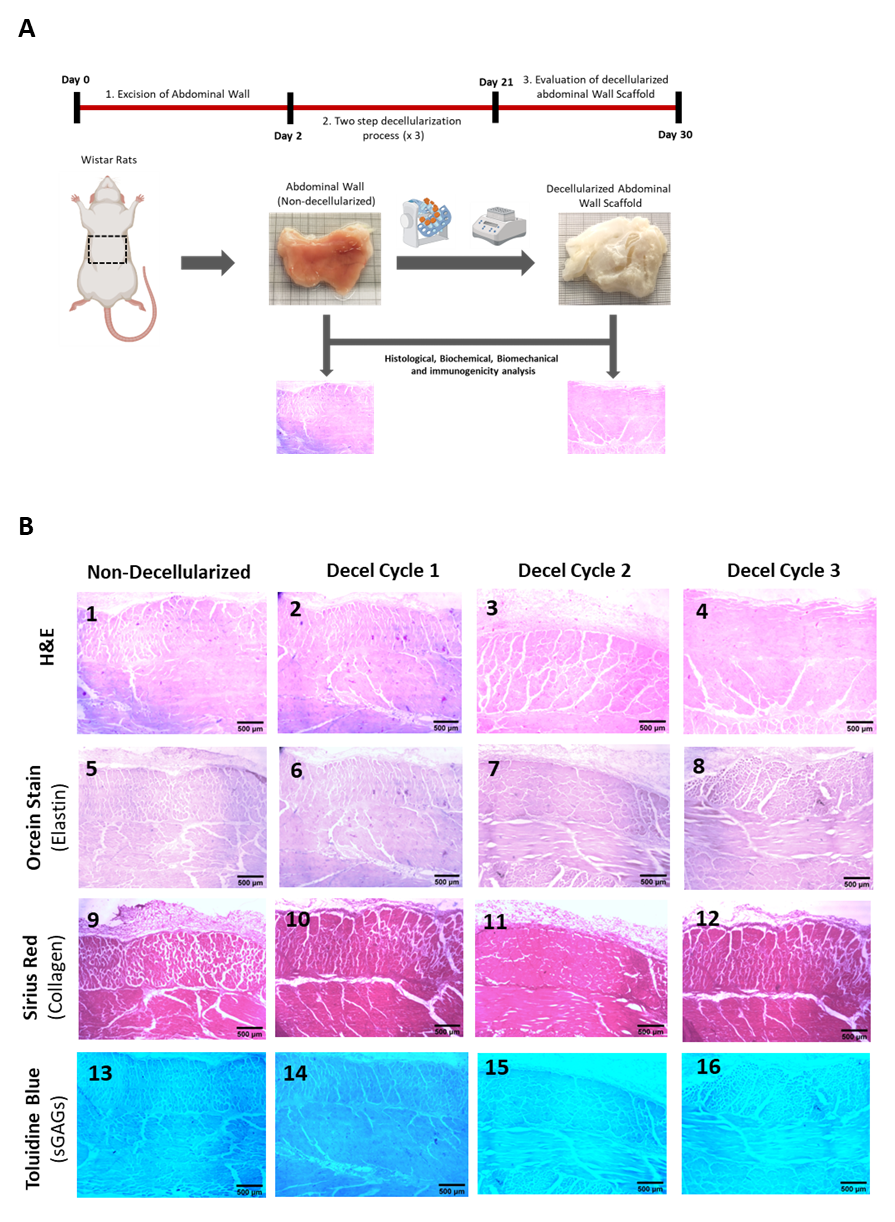
Figure 1. Assessment of decellularization of the full-thickness rat-derived abdominal wall. Overview of the applied decellularization protocol for producing acellular full-thickness abdominal wall scaffolds (A). Histological analysis involving the performance of H&E (B1-B4), Orcein stain (B5-B8), Sirius red (B9-b12) and Tolouidine blue (B13-B16) of non-decellularizaed and decellularized full-thickness abdominal wall samples. All images are presented with original magnification 2.5x and scale bars 500 μm.
Evaluation of biomechanical properties
The evaluation of the decellularization process for the production of acellular whole abdominal wall scaffolds involved the uniaxial testing of non-decellularized and decellularized samples (after 1st, 2nd and 3rd cycles, Figure 3). Specifically, the σΤrans for the non-decellularized and decellularized samples of 1st, 2nd and 3rd cycles were 105 ± 15, 184 ± 15, 208 ± 25 and 218 ± 28 kPa, respectively (Figure 3). The εTrans for the non-decellularized and decellularized samples of 1st, 2nd and 3rd cycles was 309 ± 20, 312 ± 47, 312 ± 45 and 313 ± 36, respectively (Figure 3). The σUTS for the non-decellularized and decellularized samples of 1st, 2nd and 3rd cycles was 899 ± 51, 1026 ± 107, 1028 ± 160 and 1035 ± 136 kPa (Figure 3). The εUTS for the non-decellularized and decellularized samples of 1st, 2nd and 3rd cycles was 681 ± 66, 859 ± 98, 860 ± 55 and 916 ± 160 (Figure 3). The El-E for the non-decellularized and decellularized samples of 1st, 2nd and 3rd cycles was 88.2 ± 8, 101 ±16, 113 ± 22 and 115 ± 14 kPa (Figure 3). The Col-E for the non-decellularized and decellularized samples of 1st, 2nd and 3rd cycles was 2840 ± 352, 3139 ± 375, 3156 ± 377 and 3161 ± 444 kPa, respectively (Figure 3). Statistically significant differences regarding the σΤrans (p< 0.001), εUTS (p< 0.01) and El-E (p< 0.001), were observed between non-decellularized and decellularized samples.
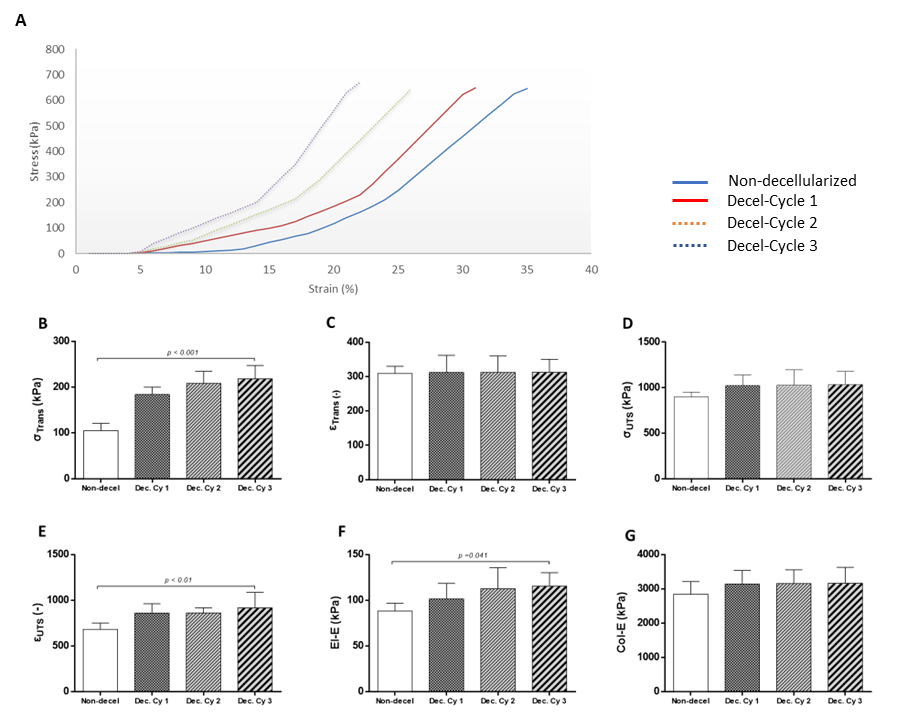
Figure 2. Biomechanical evaluation of non-decellularized and decellularized whole abdominal wall scaffolds. Representative stress-strain curves of non-decellularized and decellularized samples after the performance of 1st, 2nd and 3rd cycles. The biomechanical analysis involved the determination of σΤrans (B), εΤrans (C), σUTS (D), εUTS (E), El-E (F) and Col-E (G). Statistically significant differences were observed only for the σΤrans (p< 0.001), εUTS (p< 0.01) and El-E (p< 0.001), between non-decellularized and decellularized samples.
Conclusion
The proposed decellularization protocol has significant potential for producing abdominal wall scaffolds. These scaffolds can be derived from large animal models (such as porcine origin) or human cadaveric donors. Notably, this study explored the creation of non-immunogenic acellular meshes with excellent tissue integrity and mechanical properties. These meshes could serve as transplants for repairing abdominal wall defects.Compared to current gold-standard methods (such as synthetic scaffolds), these acellular biologic scaffolds offer advantages due to their in vivo tissue remodeling properties. However, comprehensive evaluation of the decellularized biologic scaffolds is necessary in the future to ensure FDA clearance. Once validated, clinicians can use these scaffolds more efficiently, bringing us closer to their clinical application.
References