
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Predrag Putnik | -- | 2660 | 2024-05-12 13:55:36 | | | |
| 2 | Jason Zhu | Meta information modification | 2660 | 2024-05-13 08:07:51 | | |
Video Upload Options
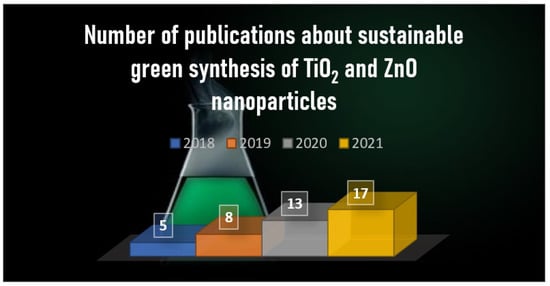
Possible eco-friendly synthesis of TiO2, ZnO, as well as a few special nanomaterials, using different plant extracts instead of harmful organic solutions. The main role of the biomolecules in the synthesis of different NPs is the reduction of metal salts, as well as capping and stabilizing them. Hence, the plant-mediated nanomaterials have a variety of different shapes and sizes comparing to the general, chemical-based synthesis processes. Furthermore, these bio-compounds not only reduce the metal salts, but also functionalize the surface of the newly synthesized NPs, which includes synergistic effects for various applications. Furthermore, the pure plant extracts can also act as catalysts. Even though, that these phytochemicals are mainly used as reduction agents, they can also possess photoactivity and can be used as photocatalysts.
1. Introduction
2. Synthesis TiO2/ZnO Nanoparticles Based on Plant Extracts
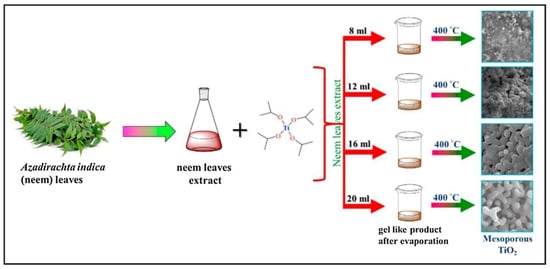
One example for the green approaches in the synthesis of TiO2 nanoparticles is explained in the study by authors Dash et al. [1]. In their work the leaf extract of Azadirachta indica was applied. For the preparation of the plant extract, firstly, the fresh, green leaves were collected and washed with tap water. After that, they were also washed using cetyltrimethylammonium bromide (CTAB) solution as well as with distilled water and with 2 M NaOH solution. After washing the leaves, they were dried at ambient temperature. When the drying process was finished, 3 g of the finely cut leaves were mixed with 200 mL distilled water and boiled until the amount of water decreased to 60 mL. Finally, the mixture was filtered and the extract was stored for further use. In order to prepare a mesoporous form of TiO2, different amounts of plant extract (8, 12, 16 and 20 mL) were mixed with 0.4 mL of titanium tetraisopropoxide. The reaction mixtures were continuously stirred for 12 h at 35 °C. Then, the temperature was step-by-step increased to 70 °C to eliminate the water from the solution. Finally, after evaporating the water, the obtained product was calcined at 400 °C for 3 h. The formation of white powder proved the successful synthesis of TiO2. The newly synthesized NPs, depending on the amount of the plant extract (8, 12, 16 and 20 mL) were named as MTO-8, MTO-12, MTO-16 and MTO-20, respectively (Figure 2).
| Type of Catalyst | Applied Plant Extract in Experiments | Method of Synthesis | Size of the Newly Synthesized Particles | Structure of the Newly Synthesized Particles | Type of Pollutant in the Photocatalytic Experiments | Applied Irradiation | Efficiency of the Photocatalytic Degradation (%) | Reaction Rate Constant | Study |
|---|---|---|---|---|---|---|---|---|---|
| TiO2 | Leaf extract of Azadirachta indica | Plant-mediated synthesis | Average crystal size in the range of 12.7–16.8 nm | Mesoporous structure of TiO2 | Rhodamine 6 G | UV irradiation | 64% after 57 min of irradiation | 0.0321 min−1 | [1] |
| TiO2 | Aloe Vera gel from the plant leaf | Hydrothermal synthesis | Size of pure TiO2 57 nm, while the Ag@TiO2 38 nm | Combination of anatase and rutile phase | Picric acid | Visible irradiation | After 50 min of irradiation a decent amount of PA was removed | Not mentioned | [6] |
| TiO2 | Leaf extract of Cinnamomum camphora | Synthesis under ambient conditions | 12.6 ± 1.7 nm | Spherical shape and anatase phase of the Au-Ag/TiO2 | methyl orange (MO), rhodamine B and methylene blue | UV-Vis irradiation (Xe lamp) | 89.4% of MO after 60 min of irradiation; Complete degradation in the case of mixture dyes after 60 min of irradiation | 0.0356 min−1 in the case of MO degradation; For the mixture the constant was not mentioned | [3] |
| TiO2 | Leaf extract of Deinbollia pinnata | Sol–gel method | Average crystal size in the range of 19–21 nm | Aggregated, semi-spherical shape with anatase phase | Methyl orange | UV irradiation | 97.53% after 150 min of irradiation | Not mentioned | [7] |
| TiO2 | Leaf extract of Euphorbia hirta | Plant-mediated synthesis | Avarage crystal size in the range of 20–50 nm | Spherical shape and cubic phase of TiO2 | Methylene blue (MB), MO, alizarin red (AR) and crystal violet (CV) | Direct sunlight | 86.8% (CV); 81.3% (AR); 77.5% (MO) after 6 h of irradiation | Not mentioned | [4]. |
| ZnO | Leaf extract of Syzygium Cumini | Not mentioned | 11.35 nm | Agglomerated, well-crystallized hexagonal wurtzite structure | Methylene blue | Sunlight irradiation | 91.4% after 180 min of irradiation | Not mentioned | [8] |
| ZnO | Pullulan, product of Aureobasidium pullulans fungus | Precipitation method | Average particle size 110.86 nm | Flower-like strucutre | Methyl orange | UV irradiation | 97% after 300 min of irradiation | Not mentioned | [9][10] |
| ZnO | Leaf extract of Cinnamomum tamala | Plant-mediated synthesis | Average particle size 35 nm | Hexagonal wurtzite crystallite structure | Methylene blue | Direct sunlight | 98.07% after 90 min of irradiation | Not mentioned | [11] |
| ZnO | Plant extract of Gynostemma pentaphyllum | Co-precipitation method | 35.41 nm | Hexagonal structure of crystalline nanoparticles | Malachite green | UV irradiation | 89% after 180 min of irradiation | Not mentioned | [12] |
| ZnO | Peel extract of Cavendish bananas | Plant-mediated synthesis | 15.3 nm | Triangular and spherical shaped particles with hexagonal wurtzite structure | BB9 organic dye; Crystal violet (CV) and Congo red (CR) | UV-Vis irradiation (xenon lamp) | 100% of BB9 after 90 min of irradiation; 97.79% of CV and 81.70% of CR after 420 min of irradiation | 0.5254 h−1 for CV and 0.2837 h−1 for CR | [13] |
| ZnO | Leaf extract of Alchornea laxiflora | Plant-mediated synthesis | 29–38 nm, depending on the volume of leaf extract | Quasi-hexagonal shape with hexagonal crystallographic phase | Congo red | Direct sunlight | 87% after 60 min of irradiation | 0.0401 min−1 | [14] |
| ZnO | Peel extract of banana | Plant-mediated synthesis | 18.86–20.72 depending on the type of banana | Nanocrystalline ZnO | Not mentioned | Not mentioned | Believed to be effective in the photodegradation | Not mentioned | [5] |
| ZnO | Jujube fruit extract | Plant-mediated synthesis | 19 nm | Highly spherical shape with hexagonal wurtzite structure | Methylene blue (MB) and Eriochrome black-T (ECBT) | Direct sunlight | 85% of both dyes after 300 min of irradiation | 0.0087 min−1 for MB and 0.0067 min−1 for ECBT | [15] |
| ZnO | Leaf extract of Prunus cerasifera | Plant-mediated synthesis | Average crystal size 12 nm | Aggregated spheroidal shape with wurtzite hexagonal phase | Bromocresol green (BG), Bromophenol Blue (BB), Methyl red (MR) and Methyl blue (MB) | Direct sunlight | 93.12% of BG; 90.54% of BB; 88.49% of MR and 76.76% of MB after 10 min of irradiation | Not mentioned | [16] |
| ZnO | Leaf extract of Becium grandiflorum | Biological approach | Average crystal size of 20 nm | Hexagonal wurtzite structure | Methylene blue | UV irradiation | 69% after 200 min of irradiation | 0.0019 min−1 | [17] |
| ZnO | Root extract of Codonopsis lanceolata | Modified co-precipitation method | 500 nm | Spherical, flower-like shape with hexagonal wurtzite structure of ZnO | Methylene blue | UV irradiation | 90.3% after 40 min of irradiation | 0.057 min−1 | [18] |
| ZnO | Leef extract of Peltophorum pterocarpum | Plant-mediated synthesis | 11.64 nm | Flowershaped particles with hexagonal wurtzite phase of ZnO | Methylene blue | Sunlight irradiation | 95% after 120 min of irradiation | 0.021 min−1 | [19] |
| ZnO | Husk extract of Zea mays (Z-ZnO) and peel extract of Artocarpus heterophyllus (A-ZnO) and Punica granatum (P-ZnO) | Co-precipitation method under low temperature | 28 (Z-ZnO), 55 (A-ZnO) and 25 (P-ZnO) nm | Z-ZnO flower-like; A-ZnO cauliflower-like and P-ZnO small nanoflower structure with hexagonal ZnO wurtzite phase | Antibacterial activity | Visible light irradiation | 93.2% (Z-ZnO), 85.7% (A-ZnO) and 99.2% (P-ZnO) after 180 min of irradiation | 0.0130 (Z-ZnO), 0.0091 (A-ZnO) and 0.0280 (p-ZnO) min−1 | [20] |
| ZnO | Leaf extract of Sapindus mukorossi | Plant-mediated synthesis | 10–1000 nm | Spherical-spiral shape | Methylene blue | Sunlight irradiation | 99% (ZnO-PMMA); 98% (Ni2O3-PMMA); 93% (CuO-PMMA); 90% (Fe3O4-PMMA) after 130 min of irradiation | 0.1349 (ZnO-PMMA); 0.1321 (Ni2O3-PMMA); 0.1263 (CuO-PMMA); 0.1231 (Fe3O4-PMMA) min−1 | [21] |
| ZnO | Leaf extract of curry with coconut water | Plant-mediated synthesis | 1.80, 1.62 and 1.88 nm with respect to 10-, 15- and 20-mL concentration of extract | Agglomerated, irregular spherical shape | Methylene blue | Sunlight irradiation | 98.45% after 60 min of irradiation | 0.0579 min−1 | [22] |
| ZnO | Leaf extract of Stevia rebaudiana | Co-precipitation method | Average crystallite size 4.71 nm | Agglomerated flower-like shape with hexagonal wurtzite structure of the ZnO | Methylene blue | UV irradiation | 76% after 30 min of irradiation | Not mentioned | [23] |
| ZnO | Root extract of Saponaria officinalis | Precipitation method | 42–5500 nm | Sowrd-like shapes with hexagonal wurtzite phase of ZnO | Methylene blue | Visible light irradiation | 15–42% depending on the applied catalyst, after 40 min of irradiation | Lower than the used reference value (i.e. lower than 0.0344 min−1) | [24] |
| ZnO | Leaf extract of Amaranthus dubius | Plant-mediated synthesis | 82–250 nm for ZnO and 71–280 nm for 1% Fe-ZnO | Spherical cubic phase | Naphthalene | UV irradiation | 63.5% (ZnO) and 71.7% (Fe-ZnO) after 240 min of irradiation | 0.0045 (ZnO) and 0.0054 (Fe-ZnO) min−1 | [25] |
| ZnO | Leaf extract of Rosemary | Plant-mediated synthesis | Average crystalline size 28.946 ± 0.002 nm | Quasi-hexagonal structure with high degree of agglomeration | Textile effluent | Visible light irradiation | 63% after 100 min of irradiation | 0.0111 s−1 | [26] |
| ZnO | Leaf extract of Solanum lycopersicum | Plant-mediated synthesis | Average crystalline size 33 nm | Agglomerated spherical shape with hexagonal wurtzite structure of ZnO | Congo red | Sunlight irradiation | 80% after 300 min of irradiation | Not mentioned | [27] |
References
- Dash, L.; Biswas, R.; Ghosh, R.; Kaur, V.; Banerjee, B.; Sen, T.; Patil, R.A.; Ma, Y.-R.; Haldar, K.K. Fabrication of mesoporous titanium dioxide using azadirachta indica leaves extract towards visible-light-driven photocatalytic dye degradation. J. Photochem. Photobiol. A Chem. 2020, 400, 112682.
- Verma, R.; Pathak, S.; Srivastava, A.K.; Prawer, S.; Tomljenovic-Hanic, S. ZnO nanomaterials: Green synthesis, toxicity evaluation and new insights in biomedical applications. J. Alloys Compd. 2021, 876, 16017.
- Jiang, X.; Wang, Z.; Zhang, X.; Jiang, G.; Peng, Y.; Xu, S.; Cao, M.; Dai, X.; Liu, Z.; Ma, J. Enhanced photocatalytic activity of biosynthesized Au-Ag/TiO2 catalyst by removing excess anchored biomolecules. J. Nanopart. Res. 2019, 21, 211.
- Udayabhanu, J.; Kannan, V.; Tiwari, M.; Natesan, G.; Giovanni, B.; Perumal, V. Nanotitania crystals induced efficient photocatalytic color degradation, antimicrobial and larvicidal activity. J. Photochem. Photobiol. B 2018, 178, 496–504.
- Fernanda, O.D.; Putri, T.F.; Marfuah, S.; Aliyatulmuna, A.; Fajaroh, F. Utilization of banana peels extracts in the synthesis of ZnO nanoparticles. In Proceedings of the 4th International Conference on Mathematics and Science Education (ICOMSE) 2020: Innovative Research in Science and Mathematics Education in The Disruptive Era, Malang, Indonesia, 25–26 August 2020.
- Hariharan, D.; Thangamuniyandi, P.; Jegatha Christy, A.; Vasantharaja, R.; Selvakumar, P.; Sagadevan, S.; Pugazhendhi, A.; Nehru, L.C. Enhanced photocatalysis and anticancer activity of green hydrothermal synthesized Ag@TiO2 nanoparticles. J. Photochem. Photobiol. B 2020, 202, 111636.
- Rufai, Y.; Chandren, S.; Basar, N. Influence of Solvents’ Polarity on the Physicochemical Properties and Photocatalytic Activity of Titania Synthesized Using Deinbollia pinnata Leaves. Front. Chem. 2020, 8, 597980.
- Sadiq, H.; Sher, F.; Sehar, S.; Lima, E.C.; Zhang, S.; Iqbal, H.M.N.; Zafar, F.; Nuhanović, M. Green synthesis of ZnO nanoparticles from Syzygium Cumini leaves extract with robust photocatalysis applications. J. Mol. Liquids 2021, 335.
- Mohamed Isa, E.D.; Shameli, K.; Ch’ng, H.J.; Che Jusoh, N.W.; Hazan, R. Photocatalytic degradation of selected pharmaceuticals using green fabricated zinc oxide nanoparticles. Adv. Powder Technol. 2021, 32, 2398–2409.
- Mohamed Isa, E.D.; Che Jusoh, N.W.; Hazan, R.; Shameli, K. Photocatalytic degradation of methyl orange using pullulan-mediated porous zinc oxide microflowers. Environ. Sci. Pollut. Res. Int. 2021, 28, 5774–5785.
- Narath, S.; Koroth, S.K.; Shankar, S.S.; George, B.; Mutta, V.; Waclawek, S.; Cernik, M.; Padil, V.V.T.; Varma, R.S. Cinnamomum tamala Leaf Extract Stabilized Zinc Oxide Nanoparticles: A Promising Photocatalyst for Methylene Blue Degradation. Nanomaterials 2021, 11, 1558.
- Park, J.K.; Rupa, E.J.; Arif, M.H.; Li, J.F.; Anandapadmanaban, G.; Kang, J.P.; Kim, M.; Ahn, J.C.; Akter, R.; Yang, D.C.; et al. Synthesis of zinc oxide nanoparticles from Gynostemma pentaphyllum extracts and assessment of photocatalytic properties through malachite green dye decolorization under UV illumination-A Green Approach. Optik 2021, 239, 166249.
- Abdullah, F.H.; Abu Bakar, N.H.H.; Abu Bakar, M. Comparative study of chemically synthesized and low temperature bio-inspired Musa acuminata peel extract mediated zinc oxide nanoparticles for enhanced visible-photocatalytic degradation of organic contaminants in wastewater treatment. J. Hazard. Mater. 2021, 406, 124779.
- Ekennia, A.; Uduagwu, D.; Olowu, O.; Nwanji, O.; Oje, O.; Daniel, B.; Mgbii, S.; Emma-Uba, C. Biosynthesis of zinc oxide nanoparticles using leaf extracts of Alchornea laxiflora and its tyrosinase inhibition and catalytic studies. Micron 2021, 141, 102964.
- Golmohammadi, M.; Honarmand, M.; Ghanbari, S. A green approach to synthesis of ZnO nanoparticles using jujube fruit extract and their application in photocatalytic degradation of organic dyes. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 229, 117961.
- Jaffri, S.B.; Ahmad, K.S. Neoteric environmental detoxification of organic pollutants and pathogenic microbes via green synthesized ZnO nanoparticles. Environ. Technol. 2019, 40, 3745–3761.
- Kahsay, M.H. Synthesis and characterization of ZnO nanoparticles using aqueous extract of Becium grandiflorum for antimicrobial activity and adsorption of methylene blue. Appl. Water Sci. 2021, 11, 45.
- Lu, J.; Ali, H.; Hurh, J.; Han, Y.; Batjikh, I.; Rupa, E.J.; Anandapadmanaban, G.; Park, J.K.; Yang, D.-C. The assessment of photocatalytic activity of zinc oxide nanoparticles from the roots of Codonopsis lanceolata synthesized by one-pot green synthesis method. Optik 2019, 184, 82–89.
- Pai, S.; Sridevi, H.; Varadavenkatesan, T.; Vinayagam, R.; Selvaraj, R. Photocatalytic zinc oxide nanoparticles synthesis using Peltophorum pterocarpum leaf extract and their characterization. Optik 2019, 185, 248–255.
- Quek, J.-A.; Sin, J.-C.; Lam, S.-M.; Mohamed, A.R.; Zeng, H. Bioinspired green synthesis of ZnO structures with enhanced visible light photocatalytic activity. J. Mater. Sci. Mater. Electron. 2019, 31, 1144–1158.
- Rani, M.; Shanker, U. Sun-light driven rapid photocatalytic degradation of methylene blue by poly(methyl methacrylate)/metal oxide nanocomposites. Coll. Surf. A Physicochem. Eng. Aspects 2018, 559, 136–147.
- Satheshkumar, M.; Anand, B.; Muthuvel, A.; Rajarajan, M.; Mohana, V.; Sundaramanickam, A. Enhanced photocatalytic dye degradation and antibacterial activity of biosynthesized ZnO-NPs using curry leaves extract with coconut water. Nanotechnol. Environ. Eng. 2020, 5.
- Hoon Seo, K.; Markus, J.; Soshnikova, V.; Oh, K.H.; Anandapadmanaban, G.; Elizabeth Jimenez Perez, Z.; Mathiyalagan, R.; Kim, Y.J.; Yang, D.C. Facile and green synthesis of zinc oxide particles by Stevia Rebaudiana and its in vitro photocatalytic activity. Inorg. Nano-Metal. Chem. 2019, 49, 1–6.
- Tănase, M.A.; Marinescu, M.; Oancea, P.; Răducan, A.; Mihaescu, C.I.; Alexandrescu, E.; Nistor, C.L.; Jinga, L.-I.; Diţu, L.M.; Petcu, C.; et al. Antibacterial and Photocatalytic Properties of ZnO Nanoparticles Obtained from Chemical versus Saponaria officinalis Extract-Mediated Synthesis. Molecules 2021, 26, 2072.
- Muthukumar, H.; Gire, A.; Kumari, M.; Manickam, M. Biogenic synthesis of nano-biomaterial for toxic naphthalene photocatalytic degradation optimization and kinetics studies. Int. Biodeterior. Biodegradat. 2017, 119, 587–594.
- Noukelag, S.K.; Razanamahandry, L.C.; Ntwampe, S.K.O.; Arendse, C.J.; Maaza, M. Industrial dye removal using bio-synthesized Ag-doped ZnO nanoparticles. Environ. Nanotechnol. Monitor. Manag. 2021, 16, 100463.
- Preethi, S.; Abarna, K.; Nithyasri, M.; Kishore, P.; Deepika, K.; Ranjithkumar, R.; Bhuvaneshwari, V.; Bharathi, D. Synthesis and characterization of chitosan/zinc oxide nanocomposite for antibacterial activity onto cotton fabrics and dye degradation applications. Int. J. Biol. Macromol. 2020, 164, 2779–2787.




