Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Miguel Ladero | -- | 3433 | 2024-04-08 11:32:03 | | | |
| 2 | Lindsay Dong | Meta information modification | 3433 | 2024-04-15 07:26:09 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ladero, M.; Reche-Sainz, J.A.; Gallardo, M.E. The Role of Biomaterials in Optic Neuropathies. Encyclopedia. Available online: https://encyclopedia.pub/entry/56566 (accessed on 07 February 2026).
Ladero M, Reche-Sainz JA, Gallardo ME. The Role of Biomaterials in Optic Neuropathies. Encyclopedia. Available at: https://encyclopedia.pub/entry/56566. Accessed February 07, 2026.
Ladero, Miguel, Jose Alberto Reche-Sainz, M. Esther Gallardo. "The Role of Biomaterials in Optic Neuropathies" Encyclopedia, https://encyclopedia.pub/entry/56566 (accessed February 07, 2026).
Ladero, M., Reche-Sainz, J.A., & Gallardo, M.E. (2024, April 08). The Role of Biomaterials in Optic Neuropathies. In Encyclopedia. https://encyclopedia.pub/entry/56566
Ladero, Miguel, et al. "The Role of Biomaterials in Optic Neuropathies." Encyclopedia. Web. 08 April, 2024.
Copy Citation
Hereditary optic neuropathies (HONs) such as dominant optic atrophy (DOA) and Leber Hereditary Optic Neuropathy (LHON) are mitochondrial diseases characterized by a degenerative loss of retinal ganglion cells (RGCs) and are a cause of blindness worldwide. To date, there are only limited disease-modifying treatments for these disorders. The discovery of induced pluripotent stem cell (iPSC) technology has opened several promising opportunities in the field of HON research and the search for therapeutic approaches.
iPSCs
differentiation
transplantation
RGCs
optic neuropathies
1. Introduction
It is foreseen that affections to the retina will grow in the near future, with macular degeneration, diabetic retinopathy, and glaucoma as the most prevalent and leading causes of vision loss and blindness worldwide [1]. Moreover, rare optic neuropathies like Leber Hereditary Optic Neuropathy (LHON) and dominant optic atrophy (DOA) are finding increasing awareness in European society with a clear aim to improve patient access to diagnosis, information, and care. They both share a mitochondrial pathogenesis that leads to the selective loss of retinal ganglion cells (RGCs) and axons [2]. Currently, traditional treatments remain incompetent in terms of restoring visual function. Anatomically, the eye holds the natural advantages of easy accession and operation, immune isolation, and optical transparency, which prioritize ocular diseases for new technology trials, such as stem cell-based treatments [3]. In the last few years, stem cell technologies have revolutionized and hold great promise for the treatment of a wide range of blinding diseases. Among them, induced pluripotent stem cells (iPSCs) are one of the most powerful cell types known. They not only have the possibility of dividing indefinitely, but they have not only the possibility to divide indefinitely; these cells can also potentially differentiate into whatever cell type of the three germ layers [4]. This makes them very useful as models to delve into the physiopathological mechanisms of the diseases, to be used as a platform for drug screenings, or to be applied in cell therapies after being differentiated into the target cell type.
Biomaterials are playing an increasing role in the generation, expansion, differentiation, and transplantation of stem cells and the cells, tissues, and organoids obtained from them as they can provide an adequate environment to maintain stemness during cell self-renewal, guide cell fate during differentiation, and/or maintain the full functionality of target cells and tissues during implantation. Moreover, they should provide the highest accessibility and affordability for a widespread clinical translation of cell-based therapies [5]. Though there are still several needs to be fulfilled, biomaterials are key to creating microenvironments that are identical or similar to the extracellular matrix that surrounds cells, tissues, and organoids. In this regard, decellularized extracellular matrices (dECMs) can provide the adequate physicochemical and biological cues for cell survival, expansion, and differentiation, though decellularization protocols still need optimization, present low productivity, and are specifically dependent on the original tissues that provide them [6]. Matrigel has emerged as a commercial matrix of the dECM type as obtained from Engelbreth–Holm–Swarm mouse sarcoma by Corning Inc. (New York, NY, USA) and is now the substrate of reference for stem cell research. However, due to its origin, this basement membrane extracellular matrix is in contact with diverse cell types (epithelial, endothelial, fat, and smooth muscle cells) and presents a diverse composition from batch to batch that also affects its biochemical properties [7]. As several dECMs, Matrigel presents xenogenic contaminants and a very complex composition of structural proteins, glycoproteins, glycosaminoglycans, and proteoglycans, with 2000 major components and more than 15,000 minor components. Moreover, even if there is a booming market for dECMs from diverse origins (murine, bovine, porcine…), with forecasted compound annual growth rate (CAGR) in the 9.8% range for the period 2023–2030 and a value estimated to be USD 88.73 million by that year, there is still ample space for other natural and synthetic alternatives. In fact, there is a lack of xenogenic-free, highly tuneable, and reproducible materials with a perfectly known chemical composition and physical structure that can act as cell and tissue support and guide and as dECM mimics. In the case of RGCs and neurons, efforts have been directed to synthetic polymers and biopolymers (both mono- and copolymers (polyacrylamide—PAM, polyethyleneglycol—PEG, ethylene–vinylacetate copolymer—EVA, polylactic acid—PLA, poly-ε-caprolactone—PCL, polyglycerolsebacate/poly-ε-caprolactone—PGS/PCL, polylactic-co-glycolic acid/polycaprolactone—PLGA/PCL)) [7][8][9][10] and natural polymers like silk, alginate, hyaluronic acid (HA), chitosan, xanthan gum, and extracellular matrix molecules [11][12][13]. Works reporting on successful differentiation of hiPSCs to RGCs on materials other than Matrigel are scarce [8][9][10]. In these cases, synthetic doped biopolymers seem acceptable, although low final cell production is achieved and cell harvesting from them seems difficult. Knowledge is even more scarce when referring to microbial or crustacean-derived polysaccharides, although their hydrogel structure much more closely resembles extracellular matrix (ECM), making them very promising from a physical perspective [13][14][15]. However, they have a limited capacity for the binding of cells, growth factors, or ECM proteins (except HA) without modification. Moreover, a certain batch-to-batch variability exists.
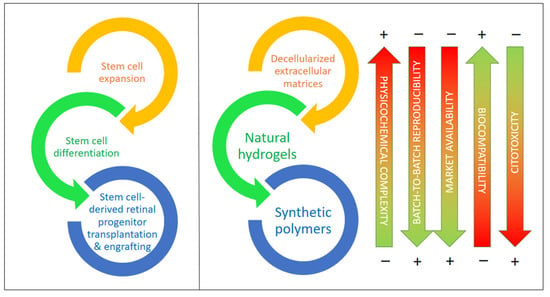
2. The Role of Biomaterials in Optic Neuropathies
According to recent studies, supporting materials and carriers would help in the procurement, transplantation, and adaptation of differentiated cells created from iPSCs and other stem cells by providing physical and chemical cues that guide the differentiation of these and other stem cells and stabilize the target cell types, for example, against oxidations due to reactive oxygen species (ROS), which result in apoptosis in most cases [16]. In the last few years, there has been increasing evidence reported on the importance of decellularized extracellular matrix (dEMC) biomaterials, synthetic polymers, and natural hydrogels based on polysaccharides and/or proteins, either alone or in combination with very active small molecules. These biomaterials are key to the protection of stem cells (iPSCs in particular), helping in their expansion, differentiation to RGCs, and, ultimately, in their successful transplantation into the retinal space [17][18]. Figure 1 compiles the main types of biomaterials employed for the expansion, differentiation, transplantation, and engrafting of stem cells, together with a visual assessment of their main advantages and drawbacks.

Figure 1. Biomaterials for stem cell expansion, differentiation, and transplantation and the in vivo engrafting of stem cells including types and their most relevant features. Biomaterials are ordered from higher to lower physicochemical complexity and higher to lower biocompatibility.
2.1. Extracellular Matrix-Based Biomaterials
A first, and natural, approach to bioscaffolds is the use of the target tissue without cells, that is, the decellularized extracellular matrix (dEMC), as, having relatively low immunogenicity, these materials present the appropriate tridimensional architecture, an adequate chemical composition rich in diverse collagens, elastin, laminin, fibronectin, and other key proteins, as well as a multitude of low-molecular weight peptides and other chemical compounds. Such microenvironments present, therefore, the adequate structural and chemical nature to support stem cells, including iPSCs, and promote their differentiation into functional cells and tissues, helping in the restoration of injured organs, the regeneration of inner tissues, and the replacement of lost tissues and organs [19]. Though the biomimetic identity of dEMC scaffolds is undeniable, serving as a model for any biomaterial, some issues still remain that should be overcome: (1) methods for cell removal still need to be optimized to remove any immunogenic compound and avoid the addition of toxic chemicals; (2) dEMC bioscaffolds should degrade within the proper time, having the appropriate shape and volume for the application; (3) organs and tissues for dEMC construction should be selected to minimize health issues, following a strict quality control process; (4) the inner architecture should be preserved, helping in the innervation and vascularization of tissues and organs for a full functionality; (5) populations of dEMC scaffolds with novel cells and structures still present complexities that need further research; (6) more biological cues of a physical and chemical nature are required to help in the latter issue; and (7) further operational time is required for cellular adhesion, proliferation, and final differentiation and/or the biomaterial transplantation needs of standardization to help in the comparison of in vitro and in vivo results from different research groups to ultimately establish more appropriate conditions for tissue and/or organ regeneration [19][20].
Depending on the dECM origin (organ/tissue or cell culture), diverse techniques can be employed to remove cells, either physical, chemical, or enzymatic and alone or in combination. Procedures that are too mild result in incomplete cell removal, while techniques that are too harsh destroy the innate structure of the tissue, removing key structural cues that are needed in cell repopulation and/or leaving behind toxic compounds. Thus, each dECM has its own optimal fabrication procedure. Regarding the optic nerve, there are only a few works focused on obtaining dECMs [20][21]. Mild procedures based on non-ionic surfactants, like Triton X-100, and their mixture with sodium dodecylsulfate (SDS), an anionic surfactant, provide a decellularized fully integral tissue showing an almost complete absence of cytotoxicity, while SDS alone proved to be deleterious to the optic nerve tissue structure [21]. A progressive protocol using these surfactants on murine and porcine eyes was also successful in obtaining dECM structures that were later on repopulated with retinal pigment epithelial cells (RPECs) and/or with ocular progenitor cells (OPCs) obtained from hiPSCs [22]. Their chemical complexity was evidenced by identifying up to 3837 proteins in the murine eye. In the case of RPECs, they successfully differentiated into a cellular multilayered structure containing well-defined regions containing pigmented cells, photoreceptors, Müller glia, astrocytes, and ganglion cells.
In the case of the optic nerve, to guide and protect RGC axons, providing the adequate support for synaptic connections, two types of dECM biosupports have been developed: an injectable ECM hydrogel that can be placed around or inside the ocular globe and a hybrid electrospun PEUU polymer–ECM tubular sheet [20]. Both materials are suitable for traumatic optic neuropathies (TONs) but, evidently, can be employed in the onset of other types of neuropathies, including HONs. Physical and chemical guiding of RGCs after transplantation can be provided by these supports, as they can deliver and/or contain neurotrophins, stem cells, and inmunotherapeutics. In fact, direct RGC injection usually results in an unordered cell growth and in synaptogenesis failure, as axons have to be directed towards the optic nerve. In particular, the presence of tenascins R and C is key to the correct development of RGC axons, their cellular boundary, and their synaptic connections [23]. Tenascins R and C, together with laminin, fibronectin, and chondroitinsulfate proteoglycans, are of great importance in the preservation of optic nerve structures and stiffness. As reviewed recently by Zhang et al. [24], stiffness is of paramount importance in the transmission and transduction of force signals in all ocular cell types. On the other side, collagen mimetic peptides play a role in the protection of RGCs by restoring collagen fibrillar organization in the ECM [25] peptides obtained from the neural retina and the retinal pigment epithelium (RPE) certainly helps in the maturation of photoreceptors, axon connectivity in the synapsis, and response to light when working with organoids derived from stem cells [17]. Recently, and considering RGCs in particular, a roadmap has been set by the RReSTORe Consortium [26], showing that advances in five key areas are needed to restore the visual pathway damaged by diverse optic neuropathies. These five areas are as follows: (1) RGC development and differentiation, (2) transplantation methods and models, (3) RGC survival, maturation, and host interactions, (4) inner retinal wiring, and (5) eye-to-brain connectivity. Significant progress in these areas needs, at least, the joined efforts of professionals of several fields in medicine, biology, chemistry, material science, and medical engineering.
2.2. Synthetic Polymers and Copolymers
Although extracellular matrices of diverse origin and nature, though mainly murine, porcine or bovine, are envisaged as the natural type of structures for cell/tissue/organ production and/or transplantation, they have a natural physicochemical complexity that hinders massive usage at a clinical level. Several reasons and challenges have been mentioned before, though a still notable market growth for dECM is expected in the years to come (9.8% CAGR in the 2023–2030 period). Even with this bountiful prospect, it is evident that less complex, easier to produce 3D cell bioscaffolds are wanted; scaffolds that will mimic dECM structures and, partially, chemical complexities at a fraction of the cost are pursued for scientific and technical reasons. On one side, less complex ECM mimics allow us to understand the diverse roles of physical structures and chemical components in cell–ECM interaction and, ultimately, how it determines the fate of stem cells. On the other hand, they are prone to massive production with relatively simple purification (synthetic polymers) or a more complex downstream but a higher similarity to ECMs (polysaccharide and/or protein-based hydrogels) [27]. Synthetic polymers present the advantage of higher control over their physicochemical properties, while their interaction with cells is inexistent and has to be constructed by grafting diverse biopolymers/biomonomers.
In general, synthetic polymers should demonstrate a local and systemic compatibility if they are to be used as transplantation supports. In this regard, PCL shows promise [28]. Electrospray and fiber structures in these polymers have been proven to be very effective in guiding cell proliferation [29]. Moreover, polyester biomaterials with a fiber structure, such as PCL either alone or mixed with poly(glycerol sebacate) (PGS) or with poly-l-lactide (PLL) or PLGA, have been used as supports for retinal progenitor cells (RPCs) to promote their attachment and expansion [10]. All materials were fabricated with the same electrospinning method and conditions, showing that PGS/PCL scaffolds mixed at a 2:1 weight ratio were superior to the other copolymers and the pure PCL, as indicated by the positive expression of RAX (retina and anterior neural fold homeobox) and NESTIN (neuroectodermal stem cell) markers, as well as RPC attachment and the proliferation rate. Focusing on hiPSC differentiation into RGCs, Chen et al. developed a biomimetic polybenzyl glutamate scaffold. They proceeded from hiPSC-derived neural spheres, observing the outgrowth of neurites and a distinct differentiation to neurons via RNA-seq. Moreover, the ontological and gene network analyses showed that the expressed genes were linked to differentiation into RGCs. Still, a complete RGC characterization was absent [30].
In fact, to facilitate RGC regeneration, a growth stimulation microenvironment should be created. Sluch et al. showed that the combination of PCL nanofiber scaffolds and forskolin, an activator of adenylate cyclase, added in the early stage of iPSC differentiation into RGCs improves this process [31]. Laugther et al. fabricated poly(serinol hexamethylene urea) (PSHU), functionalized it with poly-N-isopropylacrylamide (PNIPAAm), and subsequently grafted an RGD motif containing a short peptide via carbodiimide chemistry. After RGD encapsulation into the gel, evident axon growth was perceived by confocal microscopy [32]. Electrospinning has been shown to be a promising fabrication technology for hiPSC expansion and differentiation, but it can be combined with 3D printing to provide axon guidance together with precise positioning of RGCs in the scaffold. This allows for better cell viability and electrophysiology while guiding neurite pathfinding, a key aspect in the eye–brain connection [33]. This creation of long, well-aligned, and organized axons in RGCs has also been observed in an EVA scaffold with parallel grooves created by thermal nano-imprinted lithography [8]. These RGCs demonstrated higher functionality than a control without any topographical cue.
In addition, the presence of chemical and electronic cues also guides axon growth. Chemical neurotrophic factors and guidance cues are commonly used in solution as components of cell culture media. In fact, to increase their stability and create a rich microenvironment, they can be immobilized. Kador et al. immobilized netrin-1 onto electrospun PLA, observing that a netrin-1 gradient along the fibers helped the seeded RGCs to acquire a polarized form, which ultimately led to more mature RGCs, as shown by the developed dendrite form and the electrophysiological function [34]. Electrical stimulation of RGCs on aligned polypyrrole/graphene nanofibers also resulted in better viability with a higher resistance to apoptosis and necrosis, and axon length doubled from 50 to 107 μm [35]. Combining organic photovoltaic materials with adequate electron donors, a photocurrent can be created by laser illumination, simulating an endogenous electric field [36]. This current, again, can increase the growth and maturation of RGCs derived from hiPSCs, stimulating the differentiation of RPCs into RGCs.
2.3. Natural Hydrogels Based on Polysaccharides and/or Proteins
Synthetic hydrogels present the advantage of their well-known and tailor-made chemical composition, as well as controllable physical behavior and structure. However, they lack a natural affinity for cells and there are problems that arise due to their low biodegradability/integrability when this factor is critical (for example, for tissue engineering). Their combination with natural hydrogel ingredients (proteins, polysaccharides) or the use of hydrogels based on these polymers can overcome these barriers [37][38]. These natural polymers are biocompatible, biodegradable, and abundant and, if properly chosen and/or modified, they present low immunogenicity. Proteins are very adequate for diverse crosslinking approaches, being also critical for hydrogel–cell interactions. Depending on the selected crosslinking chemistry, their hydrogels or hydrogels containing them and/or synthetic polymers and polysaccharides will present mechanical, structural, and chemical features much resembling those of ECMs [37]. On their side, polysaccharides can present low immunogenicity and proper physical features when crosslinked via physical interactions or through covalent bonds [38]. Immunogenicity is linked to foreign-body response (FBR): once the hydrogel is transplanted, a number of proteins are adsorbed, mediating the interactions with diverse cells of the body. Regulating FBR is critical for cell transplant survival and aims at low protein adsorption, but polysaccharide and protein purity (absence of cytotoxic compounds) is still a costly and difficult-to-achieve aim. As for the cellular cargo within these hydrogels, their ultimate fate is also controlled by the hydrogel 3D macrostructure (stiffness/viscoelasticity) and the presence of cell adhesion motifs, such as peptides with an RGD sequence [38].
To obtain RGCs from stem cells, 2D and 3D methodologies are being developed. Hunt et al. employed alginate-based hydrogels activated at several concentrations with peptides with an RGD motif (GRGDSP-) to encapsulate hiPSC- and hESC-derived embryoid bodies. They also studied hydrogels of hyaluronic acid and hyaluronic acid/gelatin. Results of qRT-PCR indicated that, in the case of the alginate hydrogel with 0.5% w/v RGD peptides, apart from other markers related to RPCs showing the retinal differentiation of pigmented RPCs and OVs (optic vesicles), the higher expression of the marker MATH5, related to RGCs, is evident compared to the control differentiation in liquid media [39]. Apart from hESCs and hiPSCs, dental pulp stem cells (DPSCs) can be differentiated to neuronal lineages and RGCs. In this sense, Roozafzoon et al. studied the creation of RGCs out of DPSCs using both a 2D classical method and a 3D strategy by encapsulating DPSCs from rats in a fibrin hydrogel. In this latter condition, the expression of the markers Pax6, Atoh7, and Brn3b was dramatically higher (2.307-fold, 1.624-fold, and 3.14-fold, respectively) in 3D fibrin hydrogels [40]. The authors concluded that the hydrogels provide a non-toxic 3D microenvironment with structural and mechanical properties similar to those of ECMs, which promotes differentiation to RGCs. A usual combination is alginate and gelatin for the fabrication of hydrogels. Haghighat et al. proved that its combination with retinol promoted the expression of Nestin, RPE65, and Rhodopsin genes and, therefore, the differentiation of mouse MSCs (mesenchymal stem cells) to RGCs [41].
In RPC or RGC transplantation, cell viability and phenotype maintenance are the first objectives, while the second target is cell grafting into the target tissue. It is relatively usual in clinical and research practice to inject cells suspended in buffered liquid media (e.g., PBS) in the vitreal [42] or in the subretinal regions [43]. However, the shear stress that these cells suffer reduces their viability, as shown by Dromel et al. [44]. These authors compared the viability of hRPCs when injected with a 31-gauge needle after being suspended in PBS and after their encapsulation in a hydroxyphenyl propionic acid–gelatin gel (Gtn-HPA) that was enzymatically crosslinked in vivo, on site, after transplantation. Both the suspended and encapsulated RPCs were injected into the sub-retinal eye space of several rats, with a higher number of living engrafted cells and less immune response observed when delivered using the Gtn-HPA gel. This protection also resulted in higher cell viability and proliferation, lower apoptosis, and phenotype maintenance. Gtn-HPA gels appear to be fine vehicles for RPC transplantation as these gels can be crosslinked in situ through the use of small quantities of horseradish peroxidase (HRP) and hydrogen peroxide, showing similar cell survival rates when compared to 2D in vitro controls [44]. Gtn-HPA and HA-Tyr (hyaluronic acid-tyramine) hydrogels, when mixed, are physically and chemically similar to the vitreous and can be crosslinked by HRP-H2O2 chemistry in situ after injection, facilitating RGC attachment to the inner limiting membrane (ILM) of the retina. Dromel et al. compared the pure gels and their combinations at diverse rates, creating interpenetrating Gtn-HPA/HA-Tyr networks that helped RGCs to engraft to the ILM and connect to the optic nerve by extending long axons. Still, even though the Evans rats used in the study remained healthy, functional and behavioral tests need to be performed.
References
- Li, Z.; Keel, S.; Liu, C.; He, M. Can artificial intelligence make screening faster, more accurate, and more accessible? Asia-Pacific J. Ophthalmol. 2018, 7, 436–441.
- Chen, B.S.; Harvey, J.P.; Gilhooley, M.J.; Jurkute, N.; Yu-Wai-Man, P. Mitochondria and the eye—manifestations of mitochondrial diseases and their management. Eye 2023, 37, 2416–2425.
- Zhang, C.J.; Jin, K.; Jin, Z.B. Stem cells and genetic engineering empower therapeutic development for blinding eye diseases. Eye 2023, 4, 139–170.
- Ortuño-Costela, M.D.C.; Cerrada, V.; García-López, M.; Gallardo, M.E. The challenge of bringing iPSCs to the patient. Int. J. Mol. Sci. 2019, 20, 6305.
- Kharbikar, B.N.; Mohindra, P.; Desai, T.A. Biomaterials to enhance stem cell transplantation. Cell Stem Cell 2022, 29, 692–721.
- Kim, S.; Min, S.; Choi, Y.S.; Jo, S.H.; Jung, J.H.; Han, K.; Kim, J.; An, S.; Ji, Y.W.; Kim, Y.G.; et al. Tissue extracellular matrix hydrogels as alternatives to Matrigel for culturing gastrointestinal organoids. Nat. Commun. 2022, 13, 1692.
- Aisenbrey, E.A.; Murphy, W.L. Synthetic alternatives to Matrigel. Nat. Rev. Mater. 2020, 5, 539–551.
- Yang, T.C.; Chuang, J.H.; Buddhakosai, W.; Wu, W.J.; Lee, C.J.; Chen, W.S.; Yang, Y.P.; Li, M.C.; Peng, C.H.; Chen, S.J. Elongation of axon extension for human ipsc-derived retinal ganglion cells by a nano-imprinted scaffold. Int. J. Mol. Sci. 2017, 18, 2013.
- Behtaj, S.; Karamali, F.; Najafian, S.; Masaeli, E.; Esfahani, M.H.N.; Rybachuk, M. The role of PGS/PCL scaffolds in promoting differentiation of human embryonic stem cells into retinal ganglion cells. Acta Biomater. 2021, 126, 238–248.
- Behtaj, S.; Karamali, F.; Masaeli, E.; Anissimov, Y.G.; Rybachuk, M. Electrospun PGS/PCL, PLLA/PCL, PLGA/PCL and pure PCL scaffolds for retinal progenitor cell cultivation. Biochem. Eng. J. 2021, 166, 107846.
- Hunt, N.C.; Hallam, D.; Chichagova, V.; Steel, D.H.; Lako, M. The application of biomaterials to tissue engineering neural retina and retinal pigment epithelium. Adv. Healthc. Mater. 2018, 7, 1800226.
- Glaser, T.; Bueno, V.B.; Cornejo, D.R.; Petri, D.F.S.; Ulrich, H. Neuronal adhesion, proliferation and differentiation of embryonic stem cells on hybrid scaffolds made of xanthan and magnetite nanoparticles. Biomed. Mater. 2015, 10, 045002.
- Ojeda-Hernández, D.D.; Canales-Aguirre, A.A.; Matias-Guiu, J.; Gomez-Pinedo, U.; Mateos-Díaz, J.C. Potential of chitosan and its derivatives for biomedical applications in the central nervous system. Front. Bioeng. Biotechnol. 2020, 8, 389.
- Mohammadinejad, R.; Kumar, A.; Ranjbar-Mohammadi, M.; Ashrafizadeh, M.; Han, S.S.; Khang, G.; Roveimiab, Z. Recent advances in natural gum-based biomaterials for tissue engineering and regenerative medicine: A review. Polymers (Basel) 2020, 12, 176.
- Habibi, H.; Khosravi-Darani, K. Effective variables on production and structure of xanthan gum and its food applications: A review. Biocatal. Agric. Biotechnol. 2017, 10, 130–140.
- García-López, M.; Arenas, J.; Gallardo, M.E. Hereditary optic neuropathies: Induced pluripotent stem cell-based 2D/3D approaches. Genes (Basel) 2021, 12, 112.
- Dorgau, B.; Felemban, M.; Hilgen, G.; Kiening, M.; Zerti, D.; Hunt, N.C.; Doherty, M.; Whitfield, P.; Hallam, D.; White, K.; et al. Decellularised extracellular matrix-derived peptides from neural retina and retinal pigment epithelium enhance the expression of synaptic markers and light responsiveness of human pluripotent stem cell derived retinal organoids. Biomaterials 2019, 199, 63–75.
- Behtaj, S.; Öchsner, A.; Anissimov, Y.G.; Rybachuk, M. Retinal tissue bioengineering, materials and methods for the treatment of glaucoma. Tissue Eng. Regen. Med. 2020, 17, 253–269.
- Zhang, X.; Chen, X.; Hong, H.; Hu, R.; Liu, J.; Liu, C. Decellularized extracellular matrix scaffolds: Recent trends and emerging strategies in tissue engineering. Bioact. Mater. 2022, 10, 15–31.
- Ren, T.; van der Merwe, Y.; Steketee, M.B. Developing extracellular matrix technology to treat retinal or optic nerve injury. eNeuro 2015, 2, 77–92.
- Topuz, B.; Aydin, H.M. Preparation of decellularized optic nerve grafts. Artif. Organs 2022, 46, 618–632.
- Maqueda, M.; Mosquera, J.L.; García-Arumí, J.; Veiga, A.; Duarri, A. Repopulation of decellularized retinas with hiPSC-derived retinal pigment epithelial and ocular progenitor cells shows cell engraftment, organization and differentiation. Biomaterials 2021, 276, 121049.
- Reinhard, J.; Roll, L.; Faissner, A. Tenascins in retinal and optic nerve neurodegeneration. Front. Integr. Neurosci. 2017, 11, 30.
- Zhang, R.; Li, B.; Li, H. Extracellular-matrix mechanics regulate the ocular physiological and pathological activities. J. Ophthalmol. 2023, 2023, 7626920.
- McGrady, N.R.; Pasini, S.; Baratta, R.O.; Del Buono, B.J.; Schlumpf, E.; Calkins, D.J. Restoring the extracellular matrix: A neuroprotective role for collagen mimetic peptides in experimental glaucoma. Front. Pharmacol. 2021, 12, 764709.
- Soucy, J.R.; Aguzzi, E.A.; Cho, J.; Gilhooley, M.J.; Keuthan, C.; Luo, Z.; Monavarfeshani, A.; Saleem, M.A.; Wang, X.-W.; Wohlschlegel, J.; et al. Retinal ganglion cell repopulation for vision restoration in optic neuropathy: A roadmap from the RReSTORe Consortium. Mol. Neurodegener. 2023, 18, 64.
- Nicolas, J.; Magli, S.; Rabbachin, L.; Sampaolesi, S.; Nicotra, F.; Russo, L. 3D extracellular matrix mimics: Fundamental concepts and role of materials chemistry to influence stem cell fate. Biomacromolecules 2020, 21, 1968–1994.
- Han, I.C.; Bohrer, L.R.; Gibson-Corley, K.N.; Wiley, L.A.; Shrestha, A.; Harman, B.E.; Jiao, C.; Sohn, E.H.; Wendland, R.; Allen, B.N.; et al. Biocompatibility of human induced pluripotent stem cell–derived retinal progenitor cell grafts in immunocompromised rats. Cell Transplant. 2022, 31, 09636897221104451.
- Xue, J.; Wu, T.; Qiu, J.; Xia, Y. Accelerating cell migration along radially aligned nanofibers through the addition of electrosprayed nanoparticles in a radial density gradient. Part. Part. Syst. Charact. 2022, 39, 2100280.
- Chen, T.C.; She, P.Y.; Chen, D.F.; Lu, J.H.; Yang, C.H.; Huang, D.S.; Chen, P.Y.; Lu, C.Y.; Cho, K.S.; Chen, H.F.; et al. Polybenzyl glutamate biocompatible scaffold promotes the efficiency of retinal differentiation toward retinal ganglion cell lineage from human-induced pluripotent stem cells. Int. J. Mol. Sci. 2019, 20, 178.
- Sluch, V.M.; Davis, C.H.O.; Ranganathan, V.; Kerr, J.M.; Krick, K.; Martin, R.; Berlinicke, C.A.; Marsh-Armstrong, N.; Diamond, J.S.; Mao, H.Q.; et al. Differentiation of human ESCs to retinal ganglion cells using a CRISPR engineered reporter cell line. Sci. Rep. 2015, 5, 16595.
- Laughter, M.R.; Ammar, D.A.; Bardill, J.R.; Pena, B.; Kahook, M.Y.; Lee, D.J.; Park, D. A self-assembling injectable biomimetic microenvironment encourages retinal ganglion cell axon extension in vitro. ACS Appl. Mater. Interfaces 2016, 8, 20540–20548.
- Kador, K.E.; Grogan, S.P.; Dorthé, E.W.; Venugopalan, P.; Malek, M.F.; Goldberg, J.L.; D’Lima, D.D. Control of retinal ganglion cell positioning and neurite growth: Combining 3D printing with radial electrospun scaffolds. Tissue Eng. Part A 2016, 22, 286–294.
- Kador, K.E.; Alsehli, H.S.; Zindell, A.N.; Lau, L.W.; Andreopoulos, F.M.; Watson, B.D.; Goldberg, J.L. Retinal ganglion cell polarization using immobilized guidance cues on a tissue-engineered scaffold. Acta Biomater. 2014, 10, 4939–4946.
- Yan, L.; Zhao, B.; Liu, X.; Li, X.; Zeng, C.; Shi, H.; Xu, X.; Lin, T.; Dai, L.; Liu, Y. Aligned nanofibers from polypyrrole/graphene as electrodes for regeneration of optic nerve via electrical stimulation. ACS Appl. Mater. Interfaces 2016, 8, 6834–6840.
- Hsu, C.C.; Lin, Y.Y.; Yang, T.C.; Yarmishyn, A.A.; Lin, T.W.; Chang, Y.L.; Hwang, D.K.; Wang, C.Y.; Liu, Y.Y.; Lo, W.L.; et al. P3HT:Bebq2-based photovoltaic device enhances differentiation of hiPSC-derived retinal ganglion cells. Int. J. Mol. Sci. 2019, 20, 2661.
- Davari, N.; Bakhtiary, N.; Khajehmohammadi, M.; Sarkari, S.; Tolabi, H.; Ghorbani, F.; Ghalandari, B. Protein-based hydrogels: Promising materials for tissue engineering. Polymers 2022, 14, 986.
- Yang, Q.; Peng, J.; Xiao, H.; Xu, X.; Qian, Z. Polysaccharide hydrogels: Functionalization, construction and served as scaffold for tissue engineering. Carbohydr. Polym. 2022, 278, 118952.
- Hunt, N.C.; Hallam, D.; Karimi, A.; Mellough, C.B.; Chen, J.; Steel, D.H.W.; Lako, M. 3D culture of human pluripotent stem cells in RGD-alginate hydrogel improves retinal tissue development. Acta Biomater. 2017, 49, 329–343.
- Roozafzoon, R.; Lashay, A.; Vasei, M.; Ai, J.; Khoshzaban, A.; Keshel, S.H.; Barabadi, Z.; Bahrami, H. Dental pulp stem cells differentiation into retinal ganglion-like cells in a three dimensional network. Biochem. Biophys. Res. Commun. 2015, 457, 154–160.
- Zhu, D.; Gao, J.; Tang, C.; Xu, Z.; Sun, T. Evaluation of the potential effects of retinol and alginate/gelatin-based scaffolds on differentiation capacity of mouse mesenchymal stem cells (MSCs) into retinal cells. Int. J. Stem Cells 2021, 15, 183–194.
- Wang, J.J.; Wang, T.Z.; Guan, B.; Liu, X.X.; Gong, Z.; Li, Y.; Li, L.L.; Ke, L.N.; Nan, K.H. Implantable patches assembled with mesenchymal stem cells and gelatin/silk fibroin composite microspheres for the treatment of traumatic optic neuropathy. Appl. Mater. Today 2022, 26, 101278.
- Park, J.; Baranov, P.; Aydin, A.; Abdelgawad, H.; Singh, D.; Niu, W.; Kurisawa, M.; Spector, M.; Young, M.J. In situ cross-linking hydrogel as a vehicle for retinal progenitor cell transplantation. Cell Transplant. 2019, 28, 596–606.
- Colombe Dromel, P.; Singh, D.; Alexander-Katz, A.; Kurisawa, M.; Spector, M.; Young, M. Injectable gelatin hydroxyphenyl propionic acid hydrogel protects human retinal progenitor cells (hRPCs) from shear stress applied during small-bore needle injection. Appl. Mater. Today 2020, 19, 100602.
More
Information
Subjects:
Cell & Tissue Engineering
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
963
Revisions:
2 times
(View History)
Update Date:
15 Apr 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




