Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Farhid Hemmatzadeh | -- | 1505 | 2024-04-05 04:28:22 | | | |
| 2 | Camila Xu | Meta information modification | 1505 | 2024-04-07 10:04:31 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Chalak, M.; Hesaraki, M.; Mirbahari, S.N.; Yeganeh, M.; Abdi, S.; Rajabi, S.; Hemmatzadeh, F. The Application of Immortalized Cell Lines. Encyclopedia. Available online: https://encyclopedia.pub/entry/56558 (accessed on 01 March 2026).
Chalak M, Hesaraki M, Mirbahari SN, Yeganeh M, Abdi S, Rajabi S, et al. The Application of Immortalized Cell Lines. Encyclopedia. Available at: https://encyclopedia.pub/entry/56558. Accessed March 01, 2026.
Chalak, Mahla, Mahdi Hesaraki, Seyedeh Nasim Mirbahari, Meghdad Yeganeh, Shaghayegh Abdi, Sarah Rajabi, Farhid Hemmatzadeh. "The Application of Immortalized Cell Lines" Encyclopedia, https://encyclopedia.pub/entry/56558 (accessed March 01, 2026).
Chalak, M., Hesaraki, M., Mirbahari, S.N., Yeganeh, M., Abdi, S., Rajabi, S., & Hemmatzadeh, F. (2024, April 05). The Application of Immortalized Cell Lines. In Encyclopedia. https://encyclopedia.pub/entry/56558
Chalak, Mahla, et al. "The Application of Immortalized Cell Lines." Encyclopedia. Web. 05 April, 2024.
Copy Citation
Immortalized cell lines are a powerful tool for biological, biochemical, and biological growth, differentiation, and aging studies. They are also used in immunology, hematology, cancer biology, and toxicology research.
immortalization
cell division
telomeres
hTERT
cell line
1. Introduction
In 1965, Leonard Hayflick introduced the idea that cells possess a mechanism for keeping track of the number of times they divide, a concept later termed the Hayflick limit, which aligns with the number of replication cycles [1]. The molecular basis of this phenomenon is based on the gradual shortening of telomeric DNA (Figure 1). The shortening of telomeres occurs with each cell division, ultimately causing cells to reach their Hayflick limit, whereby growth stops after approximately 60 times population doubling [2][3][4][5]. When telomeres become too short and cannot function naturally, cellular senescence in the M1 phase (a cellular growth arrest, also called the M1 stage) occurs [5][6][7]. Thus, very short telomeres are identified by cells as double-stranded breaks and create DNA damage responses that include cellular apoptosis and replicative senescence mechanisms [4].
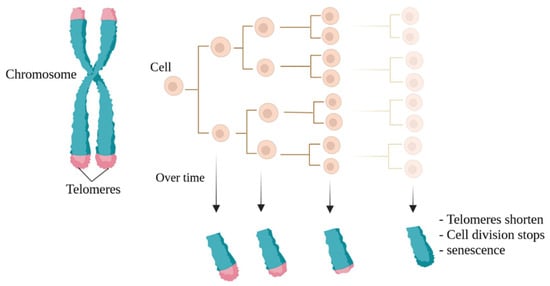
Figure 1. The Hayflick limit. Naturally, with each cell division, the telomeres at the ends of chromosomes become shorter, a phenomenon known as the Hayflick limit. The final stage is also known as cellular aging.
Therefore, due to the short lifespan of cells in laboratory conditions, a continuous supply of these cells from their specialized sources seems necessary for scientific research. However, the repeated procurement of cells requires much skill and experience if the donor is available, as mature cells from their origin tissues (such as vascular tissue) are difficult to obtain [8].
Furthermore, the preparation of primary human and animal tissues for the extraction and isolation of various cell types is fraught with significant challenges, including the potentially invasive nature of the process, an increased risk of infection, pain, ethical issues, and the difficulty of the process itself. In some cases, some patients may not consent to the use of excess samples separated from their organs, which can delay access to cellular resources in research. In addition to all these problems, obtaining cells from various sources and their availability for conducting a study can lead to errors and changes in the interpretation and results of research. For example, as human umbilical vein endothelial cells (HUVECs) are obtained from the umbilical cord, they are not only influenced by maternal hormones but also affected by embryonic gonads. In other words, even the fetal sex from which HUVEC cells are isolated can play a significant role in the reproducibility of the results. In fact, various studies have shown sexual differences in the umbilical cord between men and women in terms of gene expression, protein expression, cell survival, tubule formation capacity, autophagy, cellular ATP and metabolite levels, oxidative stress, and angiogenesis [9][10][11][12]. This challenge can also apply to other cells. Therefore, researchers need immortalized cells that do not enter the senescence phase after several cell divisions, considering the limitations of obtaining more adaptable cells over time.
Immortalized cell lines are modified cells that can be grown indefinitely [13]. Ideally, immortalized cells are genetically and phenotypically similar or identical to their source tissue and can reproduce and be cultured in the long term. Immortalized cell lines can be used in most research instead of primary cells because they offer several advantages, including cost-effectiveness, ease of use, unlimited availability of materials, and the bypassing of ethical concerns associated with animal and human tissue use [14][15][16].
In the late 1950s, George Otto Gey, head of tissue culture research at Johns Hopkins, initiated the process of immortalization and established a non-aging cell line called HeLa, derived from a rare adenocarcinoma found in the cervix of a young woman named Henrietta Lacks. This marked the first instance of such a phenomenon [17][18]. Prior to this, cells derived from other human cells could only survive for a few days in culture, but the behavior of Lacks’s tumor cells was different. This was the first successful attempt to immortalize and maintain human cells in vitro because the ability of Hela cells made them useful in various biological studies [19][20][21][22][23].
In fact, HeLa cells have an active version of telomerase during cell division [24], which copies telomeres repeatedly. This prevents the gradual shortening of telomeres, which plays a role in cell aging and, ultimately, cell death. Thus, cells evade the Hayflick limit, resulting in unlimited cell division and immortality. Major discoveries have been made using this cell line, including the development of the polio vaccine in 1953 [25], the link between human papillomavirus (HPV) and cervical cancer, and the role of telomerase in chromosome maintenance [26].
Generally, various cell lines have revolutionized scientific investigations and have found applications in vaccine development, drug testing for metabolism and toxicity, generating antibodies, exploring gene functions, creating artificial tissues like synthetic skin, and producing biologic substances such as therapeutic proteins [16][19][26].
2. The Application of Immortalized Cell Lines
Immortalized cell lines are a powerful tool for biological, biochemical, and biological growth, differentiation, and aging studies. They are also used in immunology, hematology, cancer biology, and toxicology research. Additionally, for therapeutic purposes, studying immortalized cells will be useful in achieving better results for regenerative medicine [27]. Cell line immortalization can also help cell biologists achieve their goals of treating diseases and improving health factors. For example, they have used immortalized cell lines as model systems for studying neuronal development and performance recovery in neurological disease models, such as Huntington’s disease [28][29].
In general, the use of these cell lines can be useful for clinical development related to the treatment of Huntington’s disease patients [29]. Another example is human vocal fold epithelial cells, which are a valuable tool for studying epithelial–fibroblast cell interactions that dictate the disease and health of this specialized tissue [30]. The RPE-1 human retinal pigment epithelial cell line has also been widely used to study physiological events in human cellular culture systems [31]. Pig endothelial cells can be also used as a laboratory model to study the properties of the blood–brain barrier and some hemorrhagic diseases [32]. Encapsulated cell technology is also a useful approach for the continuous and local delivery of genetically modified therapeutic proteins. However, the clinical development of encapsulated cell technology to deliver therapeutic proteins from macro-capsules is still limited due to the lack of a compatible allogeneic cell line for therapeutic purposes in humans [33].
In this regard, it is possible to create appropriate immortal cell lines, such as the human myoblast cell line created in the study by Lathuiliere A. and colleagues. Valuable results have also been achieved using enclosed cell technology. As an example, non-immortalized human lung epithelial cells created by hTERT overexpression without the use of viral oncogenes have been used to investigate various aspects of lung cancer, such as epithelial-to-mesenchymal transition and the cancer stem cell theory. The use of non-immortalized lung epithelial cells has improved researchers’ understanding of lung cancer pathogenesis, and these models can be valuable research tools [34]. Additionally, creating cell lines that preserve genetic information and drug responses can enable more drug screening and mechanistic studies [35].
The immortalized NTera2 (NT2) cell line has undergone a thorough examination for its potential in brain grafting. Notably, these cells have been safely employed in clinical trials focused on treating brain injuries [36][37]. Lately, a method involving the lentiviral gene transfection of c-MYC has been employed to create immortalized megakaryocyte cell lines, regulated by the TET-on system, using blood cell precursor cells derived from iPSCs (induced pluripotent stem cells). These cells have the capability to produce platelets for use in clinical trials [38]. Cell lines that are not immortalized can serve as valuable tools for investigating the effectiveness and potential harm of drugs. For instance, human cell lines have been extensively employed in pharmacogenomic research concerning cancer, aiding in the forecast of clinical responses, contributing to the development of pharmacogenomic theories for subsequent experiments, and uncovering fresh insights into the various factors influencing drug responses. Within the category of model system cell lines, immortalized types like EBV-transformed lymphoblastoid cell lines (LCLs) are frequently utilized to assess how genetic diversity impacts the effectiveness and safety of drugs [39].
Additionally, if aim to apply this technique in a different research area, it would pertain to the creation of immortalized cell lines for food production, a significant hurdle in the field of cell agriculture. This field is dedicated to generating meat and other animal-derived products via tissue engineering and synthetic biology. Specifically, these cultured meat cell lines must meet criteria for food safety, be capable of large-scale propagation and differentiation in an efficient manner, and exhibit taste, texture, and nutritional qualities that appeal to consumers [40].
In this context, utilizing primary cells for cultured meat production necessitates the maintenance of donor animal herds for sample preparation, with regular and approved sample collection for food production. In contrast to primary cell cultures, immortalized cell lines do not undergo senescence and can undergo limitless divisions. As a result, they are more straightforward to investigate and enable the production of cultured meats that are both safer and more consistently achieved (Figure 2), eliminating the requirement for animal biopsies [27].
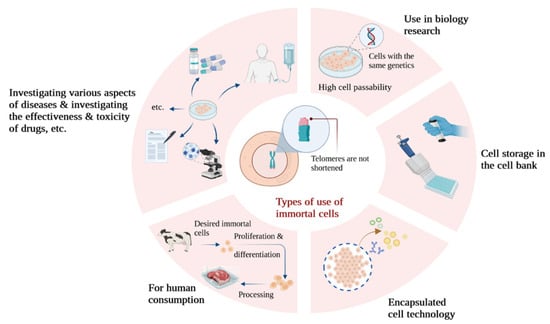
Figure 2. A look at some of the applications of immortalized cells in different types of research.
References
- Hayflick, L. The Limited In Vitro Lifetime of Human Diploid Cell Strains. Exp. Cell Res. 1965, 37, 614–636.
- Hayflick, L.; Moorhead, P.S. The Serial Cultivation of Human Diploid Cell strains. Exp. Cell Res. 1961, 25, 585–621.
- Pérez-Mancera, P.A.; Young, A.R.; Narita, M. Inside and out: The activities of senescence in cancer. Nat. Rev. Cancer 2014, 14, 547–558.
- Blackburn, E.H. Telomeres: No end in sight. Cell 1994, 77, 621–623.
- Feng, J.; Funk, W.D.; Wang, S.-S.; Weinrich, S.L.; Avilion, A.A.; Chiu, C.-P.; Adams, R.R.; Chang, E.; Allsopp, R.C.; Yu, J. The RNA component of human telomerase. Science 1995, 269, 1236–1241.
- Harley, C.B.; Futcher, A.B.; Greider, C.W. Telomeres shorten during ageing of human fibroblasts. Nature 1990, 345, 458–460.
- Shay, J.W.; Wright, W.E. Senescence and immortalization: Role of telomeres and telomerase. Carcinogenesis 2005, 26, 867–874.
- Bajpai, V.K.; Andreadis, S.T. Stem cell sources for vascular tissue engineering and regeneration. Tissue Eng. Part. B Rev. 2012, 18, 405–425.
- Addis, R.; Campesi, I.; Fois, M.; Capobianco, G.; Dessole, S.; Fenu, G.; Montella, A.; Cattaneo, M.G.; Vicentini, L.M.; Franconi, F. Human umbilical endothelial cells (HUVECs) have a sex: Characterisation of the phenotype of male and female cells. Biol. Sex. Differ. 2014, 5, 18.
- Lorenz, M.; Blaschke, B.; Benn, A.; Hammer, E.; Witt, E.; Kirwan, J.; Fritsche-Guenther, R.; Gloaguen, Y.; Bartsch, C.; Vietzke, A. Sex-specific metabolic and functional differences in human umbilical vein endothelial cells from twin pairs. Atherosclerosis 2019, 291, 99–106.
- Lorenz, M.; Koschate, J.; Kaufmann, K.; Kreye, C.; Mertens, M.; Kuebler, W.M.; Baumann, G.; Gossing, G.; Marki, A.; Zakrzewicz, A. Does cellular sex matter? Dimorphic transcriptional differences between female and male endothelial cells. Atherosclerosis 2015, 240, 61–72.
- Zhang, Y.; Lingappan, K. Differential sex-specific effects of oxygen toxicity in human umbilical vein endothelial cells. Biochem. Biophys. Res. Commun. 2017, 486, 431–437.
- Kulshreshtha, A.; Agrawal, A. Regulation by non-coding RNAs in respiratory disorders. In RNA-Based Regulation in Human Health and Disease; Elsevier: Amsterdam, The Netherlands, 2020; pp. 233–249.
- Gomez-Lechon, M.; Donato, M.; Castell, J.; Jover, R. Human hepatocytes as a tool for studying toxicity and drug metabolism. Curr. Drug Metab. 2003, 4, 292–312.
- MacDonald, C. Development of new cell lines for animal cell biotechnology. Crit. Rev. Biotechnol. 1990, 10, 155–178.
- Schurr, M.J.; Foster, K.N.; Centanni, J.M.; Comer, A.R.; Wicks, A.; Gibson, A.L.; Thomas-Virnig, C.L.; Schlosser, S.J.; Faucher, L.D.; Lokuta, M.A. Phase I/II clinical evaluation of StrataGraft: A consistent, pathogen-free human skin substitute. J. Trauma 2009, 66, 866.
- Masters, J.R. HeLa cells 50 years on: The good, the bad and the ugly. Nat. Rev. Cancer 2002, 2, 315–319.
- Svalastog, A.L.; Martinelli, L. Representing life as opposed to being: The bio-objectification process of the HeLa cells and its relation to personalized medicine. Croat. Med. J. 2013, 54, 397–402.
- Adey, A.; Burton, J.N.; Kitzman, J.O.; Hiatt, J.B.; Lewis, A.P.; Martin, B.K.; Qiu, R.; Lee, C.; Shendure, J. The haplotype-resolved genome and epigenome of the aneuploid HeLa cancer cell line. Nature 2013, 500, 207–211.
- Robin, T.; Bairoch, A.; Müller, M.; Lisacek, F.; Lane, L. Large-Scale Reanalysis of Publicly Available HeLa Cell Proteomics Data in the Context of the Human Proteome Project. J. Proteome Res. 2018, 17, 4160–4170.
- Zhang, P.; Zhao, X.; Zhang, W.; He, A.; Lei, B.; Zhang, W.; Chen, Y. Leukemia-associated gene MLAA-34 reduces arsenic trioxide-induced apoptosis in HeLa cells via activation of the Wnt/β-catenin signaling pathway. PLoS ONE 2017, 12, e0186868.
- Sağlam Metiner, P.; Can, H.; Ayyıldız Tamiş, D.; Karakavuk, M.; Kımız Geboloğlu, I.; Gülçe İz, S.; Atalay Şahar, E.; Değirmenci Döşkaya, A.; Gürüz, Y.; Deliloğlu Gürhan, S.; et al. The use of Toxoplasma gondii tachyzoites produced in HeLa cells adhered to Cytodex 1 microcarriers as antigen in serological assays: An application of microcarrier technology. Cytotechnology 2019, 71, 91–105.
- Olea-Flores, M.; Kan, J.; Carlson, A.; Syed, S.A.; McCann, C.; Mondal, V.; Szady, C.; Ricker, H.M.; McQueen, A.; Navea, J.G.; et al. ZIP11 Regulates Nuclear Zinc Homeostasis in HeLa Cells and Is Required for Proliferation and Establishment of the Carcinogenic Phenotype. Front. Cell Dev. Biol. 2022, 10, 895433.
- Ivanković, M.; Cukusić, A.; Gotić, I.; Skrobot, N.; Matijasić, M.; Polancec, D.; Rubelj, I. Telomerase activity in HeLa cervical carcinoma cell line proliferation. Biogerontology 2007, 8, 163–172.
- Scherer, W.F.; Syverton, J.T.; Gey, G.O. Studies on the propagation in vitro of poliomyelitis viruses. IV. Viral multiplication in a stable strain of human malignant epithelial cells (strain HeLa) derived from an epidermoid carcinoma of the cervix. J. Exp. Med. 1953, 97, 695–710.
- Landry, J.J.; Pyl, P.T.; Rausch, T.; Zichner, T.; Tekkedil, M.M.; Stütz, A.M.; Jauch, A.; Aiyar, R.S.; Pau, G.; Delhomme, N.; et al. The genomic and transcriptomic landscape of a HeLa cell line. G3 2013, 3, 1213–1224.
- Irfan Maqsood, M.; Matin, M.M.; Bahrami, A.R.; Ghasroldasht, M.M. Immortality of cell lines: Challenges and advantages of establishment. Cell Biol. Int. 2013, 37, 1038–1045.
- Geller, H.M.; Quiñones-Jenab, V.; Poltorak, M.; Freed, W.J. Applications of immortalized cells in basic and clinical neurology. J. Cell Biochem. 1991, 45, 279–283.
- Yoon, Y.; Kim, H.S.; Jeon, I.; Noh, J.-E.; Park, H.J.; Lee, S.; Park, I.-H.; Stevanato, L.; Hicks, C.; Corteling, R. Implantation of the clinical-grade human neural stem cell line, CTX0E03, rescues the behavioral and pathological deficits in the quinolinic acid-lesioned rodent model of Huntington’s disease. Stem Cells 2020, 38, 936–947.
- Chen, X.; Lungova, V.; Zhang, H.; Mohanty, C.; Kendziorski, C.; Thibeault, S.L. Novel immortalized human vocal fold epithelial cell line: In vitro tool for mucosal biology. FASEB J. 2021, 35, e21243.
- Hindul, N.L.; Jhita, A.; Oprea, D.G.; Hussain, T.A.; Gonchar, O.; Campillo, M.A.M.; O’Regan, L.; Kanemaki, M.T.; Fry, A.M.; Hirota, K. Construction of a human hTERT RPE-1 cell line with inducible Cre for editing of endogenous genes. Biol. Open 2022, 11, bio059056.
- Hong, H.X.; Zhang, Y.M.; Xu, H.; Su, Z.Y.; Sun, P. Immortalization of swine umbilical vein endothelial cells with human telomerase reverse transcriptase. Mol. Cells 2007, 24, 358–363.
- Lathuiliere, A.; Vernet, R.; Charrier, E.; Urwyler, M.; Von Rohr, O.; Belkouch, M.-C.; Saingier, V.; Bouvarel, T.; Guillarme, D.; Engel, A. Immortalized human myoblast cell lines for the delivery of therapeutic proteins using encapsulated cell technology. Mol. Ther. Methods Clin. Dev. 2022, 26, 441–458.
- Sato, M.; Shay, J.W.; Minna, J.D. Immortalized normal human lung epithelial cell models for studying lung cancer biology. Respir. Investig. 2020, 58, 344–354.
- Zhuang, Y.; Grainger, J.M.; Vedell, P.T.; Yu, J.; Moyer, A.M.; Gao, H.; Fan, X.Y.; Qin, S.; Liu, D.; Kalari, K.R.; et al. Establishment and characterization of immortalized human breast cancer cell lines from breast cancer patient-derived xenografts (PDX). NPJ Breast Cancer 2021, 7, 79.
- Richardson, R.M.; Nguyen, B.; Holt, S.E.; Broaddus, W.C.; Fillmore, H.L. Ectopic telomerase expression inhibits neuronal differentiation of NT2 neural progenitor cells. Neurosci. Lett. 2007, 421, 168–172.
- Hara, K.; Yasuhara, T.; Maki, M.; Matsukawa, N.; Masuda, T.; Yu, S.J.; Ali, M.; Yu, G.; Xu, L.; Kim, S.U. Neural progenitor NT2N cell lines from teratocarcinoma for transplantation therapy in stroke. Prog. Neurobiol. 2008, 85, 318–334.
- Nakamura, S.; Sugimoto, N.; Eto, K. Ex vivo generation of platelet products from human iPS cells. Inflamm. Regen. 2020, 40, 30.
- Niu, N.; Wang, L. In vitro human cell line models to predict clinical response to anticancer drugs. Pharmacogenomics 2015, 16, 273–285.
- Soice, E.; Johnston, J. Immortalizing Cells for Human Consumption. Int. J. Mol. Sci. 2021, 22, 11660.
More
Information
Subjects:
Cell Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Revisions:
2 times
(View History)
Update Date:
07 Apr 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





