Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Brandon Lucke-Wold | -- | 3304 | 2024-04-01 15:36:14 | | | |
| 2 | Peter Tang | Meta information modification | 3304 | 2024-04-02 02:53:20 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Diaz, M.J.; Natarelli, N.; Aflatooni, S.; Aleman, S.J.; Neelam, S.; Tran, J.T.; Taneja, K.; Lucke-Wold, B.; Forouzandeh, M. Nanoparticle-Based Treatment Approaches for Skin Cancer. Encyclopedia. Available online: https://encyclopedia.pub/entry/56541 (accessed on 02 March 2026).
Diaz MJ, Natarelli N, Aflatooni S, Aleman SJ, Neelam S, Tran JT, et al. Nanoparticle-Based Treatment Approaches for Skin Cancer. Encyclopedia. Available at: https://encyclopedia.pub/entry/56541. Accessed March 02, 2026.
Diaz, Michael Joseph, Nicole Natarelli, Shaliz Aflatooni, Sarah J. Aleman, Sphurti Neelam, Jasmine Thuy Tran, Kamil Taneja, Brandon Lucke-Wold, Mahtab Forouzandeh. "Nanoparticle-Based Treatment Approaches for Skin Cancer" Encyclopedia, https://encyclopedia.pub/entry/56541 (accessed March 02, 2026).
Diaz, M.J., Natarelli, N., Aflatooni, S., Aleman, S.J., Neelam, S., Tran, J.T., Taneja, K., Lucke-Wold, B., & Forouzandeh, M. (2024, April 01). Nanoparticle-Based Treatment Approaches for Skin Cancer. In Encyclopedia. https://encyclopedia.pub/entry/56541
Diaz, Michael Joseph, et al. "Nanoparticle-Based Treatment Approaches for Skin Cancer." Encyclopedia. Web. 01 April, 2024.
Copy Citation
Nanoparticles (NPs)—defined as particles with one dimension < 100 nm—have emerged as promising drug delivery systems for such antineoplastic drugs, owing to their enhanced targeting, permeability, and retention. NPs have further shown great promise in overcoming multidrug resistance and cytotoxicity barriers intrinsic to current targeted treatment modalities, with considerable variance attributed to their classification.
nanoparticle
skin cancer
drug carriers
organic
inorganic
1. Introduction
The American Cancer Society estimates that north of 95 thousand new melanomas will be diagnosed in 2023, with an expected death toll of nearly 8 thousand [1]. Risk factors for skin cancer development include positive family history, sun and ultraviolet radiation exposure, genodermatoses, and light complexion, among others. Beyond complications and outcomes, skin cancer also imposes significant financial strains: the annual cost of treating skin cancer has been estimated at over USD 8 billion since 2007, compared to total treatment costs of USD 3.6 billion from 2002 to 2006 [2][3]. Per-case treatment costs for basal cell carcinoma and squamous cell carcinomas diagnosed in 2011, secondary to occupational solar radiation exposure, were greater than CAD 5.5 thousand and 10.5 thousand, respectively [4]. Worse yet, accumulating evidence indicates that primary melanoma survivors are at an elevated risk of developing keratinocyte carcinoma, thereby disproportioning these outcomes across the population [5][6].
Although most skin cancers are comfortably excised as localized diseases, therapeutic approaches for locally advanced and metastatic skin cancers are frequently complicated by dysimmune toxicities and limited efficacies [7]. Nanoparticles (NPs)—defined as particles with one dimension < 100 nm—have recently emerged as promising drug delivery systems for such antineoplastic drugs, owing to their enhanced targeting, permeability, and retention [8][9]. NPs have further shown great promise in overcoming multidrug resistance and cytotoxicity barriers intrinsic to current targeted treatment modalities [10][11], with considerable variance attributed to their classification (Figure 1).
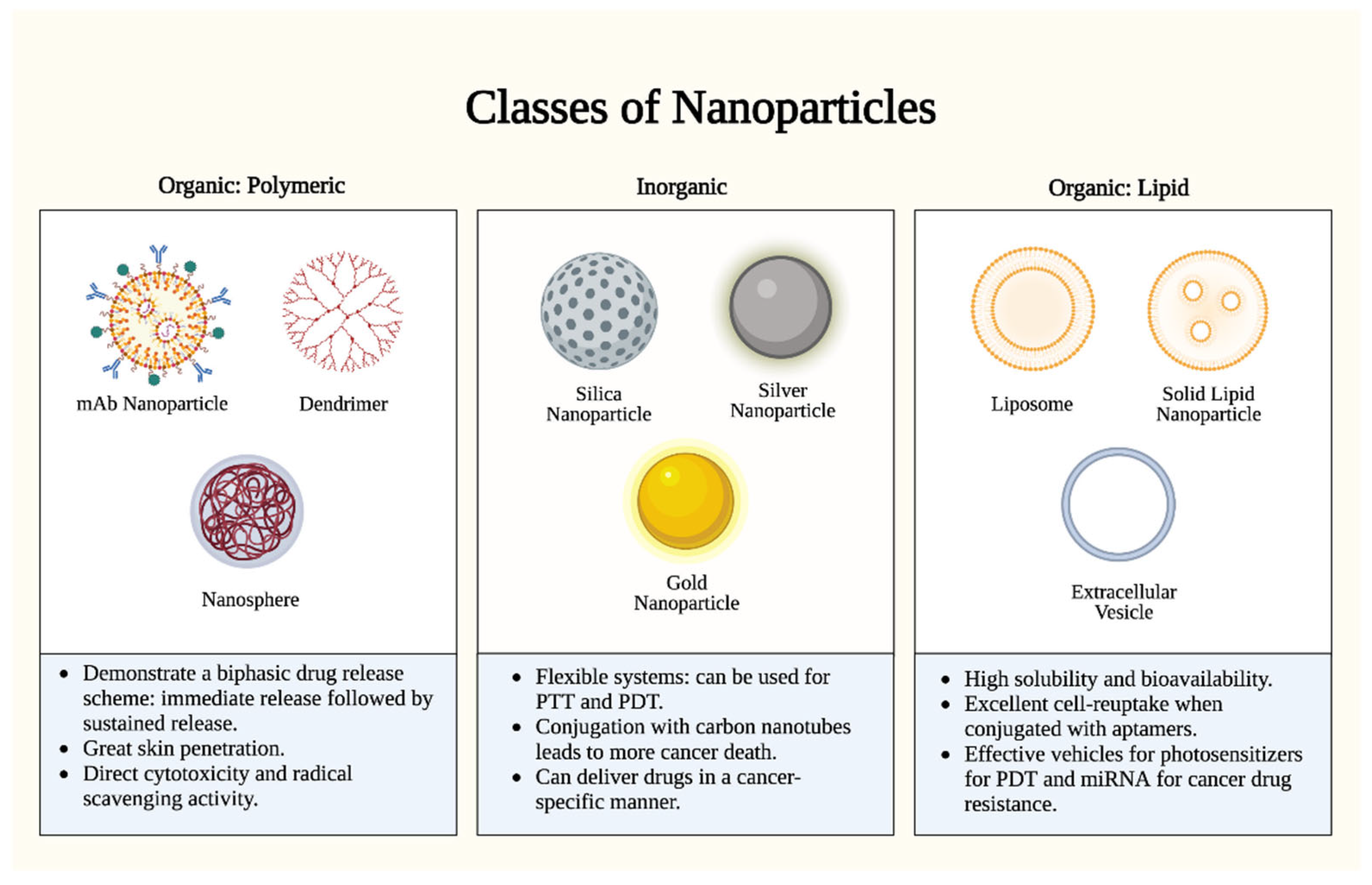
Figure 1. Nanoparticle types. There are two (2) major classes of nanoparticles: organic (polymeric and lipid-based) and inorganic. Each class has specific advantages and mechanisms. Polymeric nanoparticles proffer enhanced bioavailability and a controlled release profile, but they are limited by complex manufacturing and potential toxicity. Inorganic nanoparticles proffer uniquely tunable sizes, shapes, and conjugations, but they are limited by biodegradability concerns and long-term toxicity. Lipid nanoparticles proffer high biocompatibility and biodegradability, but they are limited by reduced payload capacities and stability challenges. PTT: photothermal therapy; PDT: photodynamic therapy. Figure created with Biorender.com.
2. Primer on Nanoparticle Utility for Skin Cancer Treatment
The use of NPs in anti-skin cancer therapy has gained popularity due to their unique physicochemical properties that increase the efficacy of cancer treatments. NPs have shown promising results in skin cancer therapy through various mechanisms of action, such as encapsulating therapeutic moieties in photodynamic therapy (PDT), conducting heat-induced damage in photothermal therapy (PTT), inducing activation and phenotype alteration in immunomodulation, and enhancing drug delivery and penetration in chemotherapy [12][13][14][15]. Various treatment types are illustrated in Figure 2.
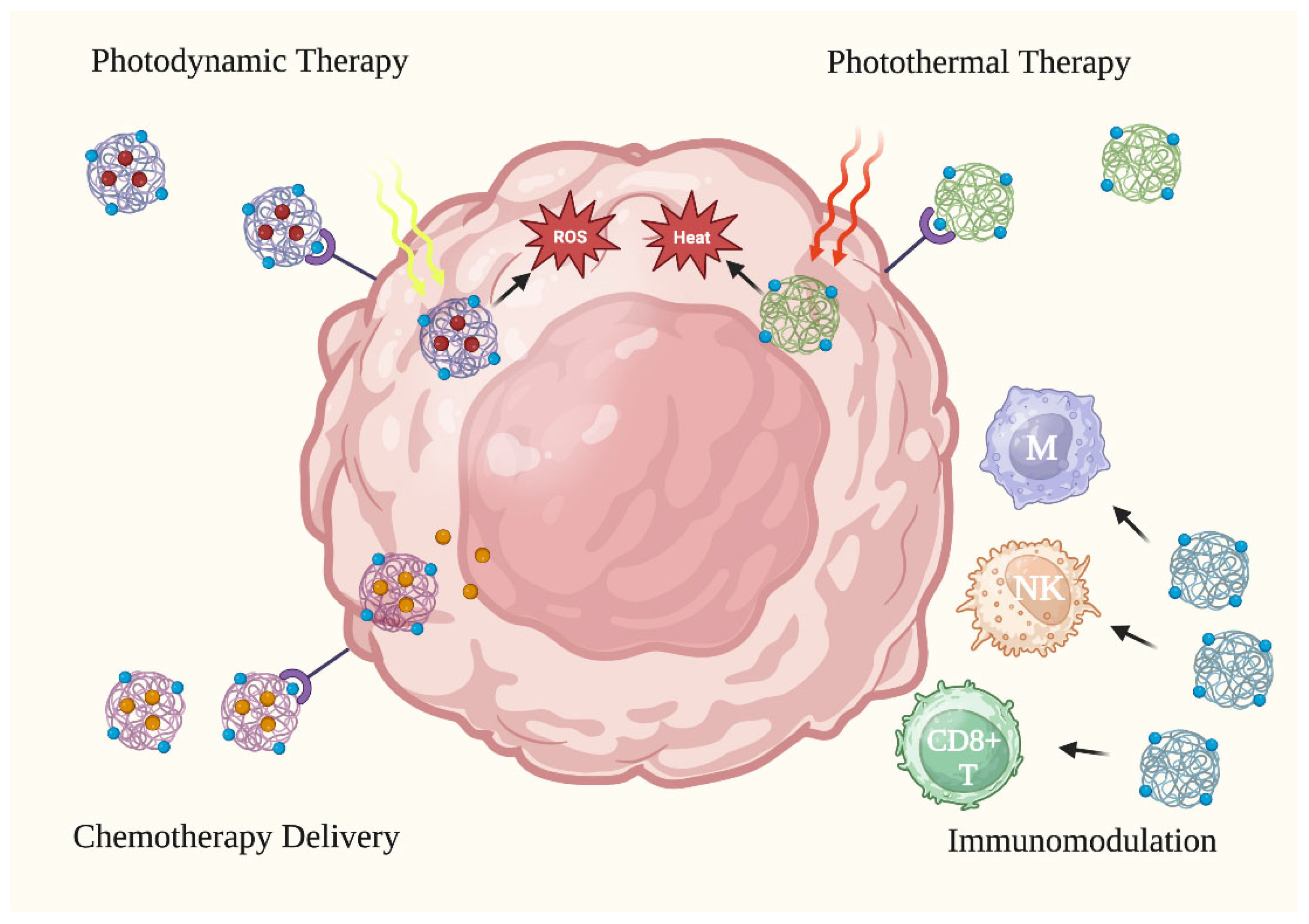
Figure 2. Anti-cancer applications of nanoparticles. Nanoparticles have been tested in skin cancer treatment via four (4) primary treatment methods: photodynamic therapy, photothermal therapy, activating the immune system to attack cancer cells, and improving the delivery of chemotherapy to cancer cells. CD8+ T: CD8+ T cell. M: macrophage. NK: natural killer cell. ROS: reactive oxygen species. Figure created with Biorender.com.
Several studies have demonstrated the efficacy of NPs in improving photodynamic therapy. PDT is a non-invasive technique that is used in dermatology, predominantly, for the treatment of actinic keratoses and non-melanoma skin cancers. More recently, their use in cutaneous melanomas has been described. PDT requires three components—the photosensitizer, which must penetrate the skin, excitation light, and oxygen [15]. The photosensitizer is placed inside the targeted cancer cell or in the tumor tissue. When the light is administered, it excites and activates the photosensitizer and generates reactive oxygen species (ROS), which can lead to the apoptosis of the cancer cells and the destruction of tumor tissues [15]. At the same time, however, PDT lacks selectivity, and the ROS can damage surrounding healthy tissues as well [15]. Another issue with PDT is the low bioavailability and delivery of naturally occurring photosensitizers, such as protoporphyrin IX (PphIX). A nanoparticle-based delivery system can encapsulate PphIX into NPs that penetrate the epidermal barrier and allow for targeted delivery to tumor cells [16]. To increase the specificity of nanoparticle delivery, the properties of NPs can be altered to increase interactions with cancer cells. Cancer cells often overexpress integrin receptors (αvβ3) on their cell surface [16]. Targeted delivery of TiO2 NPs, which have shown cytotoxicity against melanoma cells, conjugated to a Arg-Gly-Asp (RGD) motif, would thus make PDT more selective, promoting binding with integrin αvβ3 [16][17]. TiO2 NPs, conjugated to RGD, exhibit a cytotoxic effect in αvβ3 integrin-expressing mice melanoma cells but not in the normal cells lacking this integrin [16]. In addition to TiO2-based PDT, ultra-small hollow silica nanocarriers (HSdots) (~10 nm) can serve as nanocarriers for the targeted topical delivery of photosensitizer zinc phthalocyanine (ZnPc) [18]. ZnPC is a porphyrin that is excited by near-infrared light. When ZnPC-loaded HSdots are conjugated to folic acid, they selectively target squamous cell carcinoma (SCC) regions due to the high number of folic acid receptors in SCC tissues [18]. Photosensitizers can also be loaded into solid lipid nanocarriers for more effective drug delivery and increased selectivity in tumor cells [15]. Phthalocyanine aluminum chloride (AlPc), another example of a photosensitizer, absorbs light between 660 and 770 nm.
Photothermal therapy is similar to PDT but without the need for ROS to interact with target cells or tissues. PTT requires the conversion of near-infrared light into heat that can damage cancer cells. PTT can lead to the risk of recurrence or metastasis, however, due to the incomplete elimination of tumor cells [19]. The introduction of NPs into the tumor sites can allow for more efficient destruction of cancer cells through the excitation of NPs, inducing a moderate temperature increase and inducing irreversible cell damage to cancerous cells while minimizing harm to non-target tissues [19]. Magnetite (Fe3O4) NPs are one such type of NP that demonstrates high absorptivity at near-infrared wavelengths. When Fe3O4 NPs are activated with near-infrared irradiation, they efficiently convert light into heat and induce apoptosis [19]. NPs can also be utilized in combination therapy involving PTT and immunotherapy. When both types of skin cancer therapies are used together, it can stimulate further tumor shrinkage and reduce the risk of recurrence and metastasis [20]. This was demonstrated in a study that synthesized polydopamine-coated Al2O3 NPs and injected the NPs directly into B16F10 melanoma allografts in mice for PTT. Then, CpG, a potent stimulator of Th1-type cells, was injected into the mice, so tumor volumes and the number of living mice were recorded. The Al2O3 within the NP worked with the CpG to trigger a robust cell-mediated immune response that allowed for increased elimination of residual tumor cells. After the combined treatment, 50% of the mice successfully achieved the goal of tumor eradication and survived for 120 days [20]. Gold and silver nanoparticle-assisted PTT, or plasmonic photothermal therapy (PPTT) represents another route. When gold and silver NPs are irradiated, electrons are excited; then, they relax and emit strong localized heat that can destroy nearby surrounding cancer cells [21]. Gold and silver NPs can be combined with carbon nanotubes, which have high thermal conductivity after laser excitation, as effective agents for PPTT [21].
NPs can suppress tumor growth through targeted immunomodulation. Studies have demonstrated NPs altering macrophage polarization towards an M1-like phenotype and increasing CH8+ T cell density [22][23]. Among them, one study utilized nanosized membrane vesicles, known as extracellular vesicles (EVs), which were isolated and purified from the ginseng root, known for their anticancer properties [24]. Mice with B16F10 melanoma were treated with ginseng-derived NPs (GDNPs) therapy [24]. GDNP treatment significantly suppressed melanoma growth in tumor-bearing mice by increasing the presence of M1 macrophages detected in tumor tissue [24]. A separate study found that chitosan-poly(acrylic acid) NPs (CS-PAA), loaded with R848 and MnCl2 (R-M@CS-PAA NPs), can also exert an anti-tumor effect by promoting the M1 phenotype [22]. R848 is a toll-like receptor (TLR)7/8 agonist that is known to effectively drive the M1 polarization of tumor-associated macrophages [22]. Administration of R848 alone, however, can cause adverse side effects. Mn2+ can also enhance the activation of CD8+ T cells and natural killer cells [22]. R-M@CS-PAA NPs enhanced the polarization of macrophages into the M1 phenotype, and they inhibited the proliferation of B16F10 cells [22]. Another study utilized the immunogenic NPs formulated in micron-sized crystals [23]. Cucumber mosaic virus-like particles, containing tetanus toxin peptide (CuMVTT) NPs covered in a microcrystalline tyrosine (MCT) adjuvant, were injected into tumor sites. CD8+ T cell density was increased in the B16F10 melanoma tumors treated with CuMVTT + MCT [23]. Further, a study sought to enhance immune checkpoint inhibition therapy through antigen delivery by using an E2 protein nanoparticle conjugated to a CpG adjuvant and an MHC-I restricted glycoprotein 100 epitope (gp100). It was found that immunization with CpG-gp-E2 NPs significantly increased CD8+ T cell percentage at the tumor site. The group that received the combination treatment showed a striking increase in survival compared to groups receiving CpG-gp-E2 alone (p < 0.001) or anti-PD1 alone (p < 0.001) [25]. The surface characteristics of the nanoparticle can also be adjusted to significantly affect the cell entry and intracellular behaviors of NPs to enhance immunomodulation. A new, highly specific inhibitor JQ-1 was shown to be effective in the internalization and reduction in expression of PD-L1 in cancer cells, dendritic cells, and tumor-associated macrophages. A silica core, with etched polydopamine NPs loaded with JQ-1, allows for a sustained release pattern of the drug, reducing the expression of PD-L1 on cancer cells and, simultaneously, activating the immune system, as well as reducing the risk of tumor recurrence and metastasis [14]. The increased roughness of NPs exhibited elevated cellular uptake, allowing the effective entry of JQ-1 into the residual tumor cells. Further, the use of NPs coated with sucrose can prevent aggregation and promote favorable interaction with the tumor microenvironment [26]. When silver NPs were coated with sucrose (S-AgNPs), stability was increased in an aqueous solution, making them suitable intravenous agents. S-AgNPs also enhanced the antitumor activity of anti-PD-1 treatment and significantly increased tumor-infiltrating CD8+ T cells [26].
Moreover, organic NPs have been evidenced to deliver chemotherapy drugs, in a targeted way, to prevent toxicity to healthy cells, enhance drug penetration depth, and provide targeted drug delivery [12][13]. Fe2O3 NPs can be conjugated to L-cysteine (L-cys) to increase stability, and then, they can be bound to doxorubicin (Dox). Binding Dox to L-cys-coated Fe2O3 NPs allowed for efficient Dox delivery after internalization into melanoma cells. After the rapid uptake of Fe3O4-L-Cys-Dox NPs in melanoma cells, within 3 h of treatment, there were noticeable apoptotic effects detectable at 48 h post-exposure [12]. Another study demonstrated the preparation of chitosan NPs to enhance the tumor penetration capability of 10-hydroxycamptothecin (HCPT) [13]. Chitosan is a cationic polysaccharide that can interact with negatively charged biological membranes by electrostatic interaction [13]. Thus, when HCPT is encapsulated into the core of chitosan-coated NPs, the charge interaction with biomembranes allows for penetration deep into the tumor and promotes internalization by tumor cells [13]. Further, in vitro analysis displayed sustained release patterns, whereas HCPT, alone, exhibited a very rapid release rate [13].
3. Inorganic Nanoparticles
Inorganic nanoparticles, such as titanium dioxide [16], zinc oxide [19], carbon nanotubes, gold nanoparticles, silver nanoparticles, and silica nanoparticles have been extensively tested as therapeutic drug delivery systems for skin cancer prevention (i.e., sun protection) and treatment.
3.1. Gold Nanoparticles (AuNPs)
AuNPs have been shown to penetrate and accumulate effectively in tumoral tissue due to their high biocompatibility, customizable surface properties, and their ability to be conjugated to other molecules [27][28]. AuNPs effectively absorb photon energy following laser exposure and convert it to heat, which can dissipate and evoke damage to nearby cancer cells, making them effective to utilize in photothermal therapy (PTT) [28]. PTT experiments using AuNPs have consistently shown prolonged survival in melanoma tumor models, as well as effective tumor regression due to the cell death of skin cancer cells, with limited damage to surrounding healthy tissue [28][29][30][31]. AuNPs have also proven efficacious in stabilizing photosensitizers in photodynamic therapy (PDT) and providing enhanced cellular uptake, leading to increased amounts of skin cancer cell apoptosis and singlet oxygen generation [32]. The function of AuNPs has been enhanced by coating them with other materials or conjugating them to other molecules [27][30][31][33][34]. Coating AuNPs with materials, such as red blood cell membranes, has allowed for a significant reduction in the rapid physiological clearance of NPs by the monocyte–macrophage system [33]. The conjugation to cell-penetrating peptides, such as tumor-targeting adaptor folic acid, has allowed for enhanced cellular uptake and elevated PTT effects [30]; conjugation to cell-targeting molecules, such as anti-HER2 and melanoma-associated antigen antibodies, allows for selective killing and uptake via melanoma cells [31][34]; conjugation to other antitumor therapies, such as betulin, has resulted in increased growth inhibition and the proliferation of melanoma cells in vitro [27]. Utilizing AuNPs with other anti-skin cancer therapies has proven efficacious due to the properties that allow them to selectively enter into tumor cells and inhibit the growth of cancer cells. Further exploration of the use of AuNPs with varying therapies may continue to prove beneficial.
3.2. Silver Nanoparticles (AgNPs)
AgNPs exhibit high biocompatibility, resistance to oxidation, and a wide array of antimicrobial and anti-inflammatory activities [35][36]. AgNPs, when compared to AuNPs, have been found to have a greater photodynamic effect in PDT and generate more cytotoxic reactive oxygen species following irradiation, resulting in higher extinction coefficients in tumor cells, higher ratios of scattering to extinction, and higher field enhancement [35]. AgNPs, similarly to AuNPs, exhibit good optical absorbance and low toxicity towards normal cells, so they are a viable material for use in PTT as well [21][37]. AgNPs used alone in PTT, against a murine model of melanoma, have been shown to invoke up to 45% necrosis of tumor cells, and when conjugated to carbon nanotubes, they can invoke up to 70% necrosis [21]. AgNPs coated with bovine serum albumin have also been utilized in PTT, and they are able to invoke nearly complete tumor cell death at temperatures above 45 °C while also proving to have inhibitory effects on the angiogenesis of tumor cells [37]. AgNPs have also shown greater anti-tumor effects upon optimization with other materials or when synthesized in different manners. A study has shown that AgNPs synthesized from Fusarium incarnatum fungal extracts have an ability to inhibit tyrosinase activity (the main enzyme in the biosynthesis of melanin), in melanoma cells, in a dose-dependent manner [36]. In addition to maximizing their cytotoxic effects, it is equally as important to maximize the ability of AgNPs to reach cancer cells. This has been done by coating AgNPs with materials that make them more likely to be taken up into cancer cells, such as polyvinylpyrrolidone (PVP). PVP-AgNPs have been shown to decrease the genotoxic effects of AgNP therapy, as well as allow for an enhancement in the rates of cancer cell apoptosis [38]. New and improved methods of enhancing the use of AgNPs with other cancer therapies, new methods of synthesis, or ways to coat the molecules are being reported in the literature, and they may lead to the development of new and improved methods of treating human skin cancer.
3.3. Silica Nanoparticles (SiNPs)
SiNPs are silica core polyethylene glycol shell NPs with the ability to function as drug delivery molecules and circumvent the dose-limiting toxicities posed by many anti-skin cancer therapies. They are cleared by the kidneys and have low tissue uptake in most organs, making them an efficacious adjunct therapy in the treatment of skin cancer [39]. SiNPs exhibit favorable pharmacokinetics and low tissue accumulation, so they have been optimized with cell-targeting molecules to directly target cancer cells [39][40]. There is a method that has been shown to be efficacious is conjugating SiNPs to melanocortin-1 receptor, targeting alpha melanocyte-stimulating hormones. This method was found to exhibit effective tumor penetration and distribution in vivo, as well as accumulation and retention of SiNPs in melanoma tumors in vivo [40]. Another method, utilizing the same alpha melanocyte-stimulating hormone functionalization, has shown enhanced efficacy of targeted radiotherapy in melanoma models via efficient internalization of the NPs, as well as favorable tumor uptake and retention. Melanoma-bearing mice treated with this therapy were found to exhibit higher lengths of survival compared to control groups [39]. SiNPs have also been utilized as a system of drug delivery via loading the NPs with anti-cancer drugs, such as verteporfin, cisplatin, or resveratrol [41][42][43]. SiNPs loaded with verteporfin were found to nearly abolish the appearance of lung micrometastases, and they showed reduced lymphangiogenesis of a murine model of melanoma [41]. SiNPs loaded with cisplatin led to reduced toxicity in healthy cells when compared to cisplatin therapy alone, and they are effective at successfully inhibiting tumor growth in in vitro and in vivo studies [43]. SiNPs loaded with resveratrol led to the increased bioavailability and solubility of resveratrol, leading to efficiency cytotoxicity in the cells of two melanoma cancer lines [42]. The potential drawback of loading SiNPs with other anti-cancer therapies is that resveratrol, in the previous study, was found to crystallize in the pores of the NPs, potentially preventing total release of the drug [42]. Overall, SiNPs provide favorable pharmacological characteristics that may be utilized alongside other therapies to enhance their efficacy and improve outcomes. Further evaluation may provide better insight into how to best exploit their advantageous characteristics.
4. Organic Nanoparticles
The use of organic nanoparticles, such as liposomes, solid lipid nanoparticles, polymeric nanoparticles, dendrimers, mAb nanoparticles, and extracellular vesicles have also shown unique promise in skin cancer research. Compared to inorganic NPs, the organic NPs report greater functionalization with cancer-targeting ligands and increased drug loading versality. Noteworthy findings from studies on lipid and polymeric NP-based treatment in the context of skin cancer have been summarized below.
4.1. Lipid Nanoparticles (LNPs)
LNPs are a drug delivery system composed of ionizable lipids, allowing enhanced solubility and bioavailability while reducing toxicity. The search strategy yielded nine studies evaluating LNP use in the treatment of melanoma. LNPs loaded with temozolomide (TMZ), anti-parasitic benzimidazole, plumbagin, Zataria multiflora essential oil, and Mentha longiflora and Mentha pulegium essential oils have demonstrated cytotoxicity against melanoma cells in vitro. Mentha longiflora/Mentha pulegium-LNPs and Zataria multiflora-LNPs reduced cell viabilities to under 10% and 13%, respectively [44][45]. Benzimidazole-LNPs induced cancer cell apoptosis, generated reactive oxygen species, and inhibited Bcl-2 expression in cancer cells while sparing toxicity among healthy HEK293T cells [46]. In vivo murine studies were additionally conducted with plumbagin-loaded lipid–polymer hybrid NPs (LPNP) and TMZ-LNPs. Intravenous administration of plumbagin-LPNPs resulted in the disappearance of 40% and regression of 10% of B16-F10 melanoma tumors [47]. Similarly, TMZ-LNPs inhibited B16-F10 melanoma growth and vascularization without apparent toxicity [48]. Selective targeting capability of aptamer-associated LNPs has also been evaluated. Compared to free SA and SA-LPNPs lacking CD20 aptamers, CD20+ melanoma cells demonstrated significantly greater uptake of CD20-SA-LPNPs, resulting in enhanced cytotoxicity in vitro and in vivo with murine models [49]. Aluminum-phthalocyanine-LNPs also demonstrated strong intro photodynamic activity among melanoma cells, suggesting utility in photodynamic therapy [15].
In addition to primary cytotoxicity, LNPs have been evaluated as a therapeutic strategy to combat drug resistance, specifically, among BRAF-mutant melanoma cell lines [50][51].
4.2. Polymeric Nanoparticles
Polymeric NPs are composed of biocompatible polymers, which may be synthetic or natural in origin. In addition to the previously described studies conducted by Zeng et al. [49] and Sakpakdeejaroen et al. [47], employing lipid–polymer hybrid NPs, three studies evaluated the therapeutic potential of polymeric nanoparticle (PNP) in the management of melanoma. A 2021 study developed and evaluated an α-mangostin-loaded PNP topical gel formulation [52]. Measurable outcomes included drug release, skin permeation, cytotoxic effects against B16-F10 melanoma cells, and in vitro radical scavenging activity [52]. The PNPs demonstrated biphasic drug release, which is characterized by immediate release followed by sustained release. Confocal microscopy on rat skin demonstrated α-mangostin-PNP penetration up to 230.02 µm compared to dye solution penetration of only 15.21 µm [52]. Compared to free α-mangostin gel, the α-mangostin-PNPs depicted a significantly greater cytotoxic and antioxidant effect (p < 0.05) [52]. After 48 h, melanoma cell viability was 18.50% for α-mangostin-PNPs compared to 80.87% for free α-mangostin gel [52]. These findings suggest that α-mangostin-PNPs may be a promising approach for the treatment of skin cancer due to their biphasic drug release, enhanced skin permeation, direct cytotoxicity, and radical scavenging activity.
References
- Melanoma Skin Cancer Statistics. Available online: https://www.cancer.org/cancer/melanoma-skin-cancer/about/key-statistics.html (accessed on 14 April 2023).
- Guy, G.P.; Machlin, S.R.; Ekwueme, D.U.; Yabroff, K.R. Prevalence and costs of skin cancer treatment in the U.S., 2002–2006 and 2007–2011. Am. J. Prev. Med. 2015, 48, 183–187.
- Kao, S.Y.Z.; Ekwueme, D.U.; Holman, D.M.; Rim, S.H.; Thomas, C.C.; Saraiya, M. Economic burden of skin cancer treatment in the USA: An analysis of the Medical Expenditure Panel Survey Data, 2012–2018. Cancer Causes Control 2023, 34, 205–212.
- Mofidi, A.; Tompa, E.; Spencer, J.; Kalcevich, C.; Peters, C.E.; Kim, J.; Song, C.; Mortazavi, S.B.; Demers, P.A. The economic burden of occupational non-melanoma skin cancer due to solar radiation. J. Occup. Environ. Hyg. 2018, 15, 481–491.
- Sun, H.; Li, Y.; Zeng, F.; Meng, Y.; Du, S.; Deng, G. Melanoma survivors are at increased risk for second primary keratinocyte carcinoma. Int. J. Dermatol. 2022, 61, 1397–1404.
- Espinosa, P.; Pfeiffer, R.M.; García-Casado, Z.; Requena, C.; Landi, M.T.; Kumar, R.; Nagore, E. Risk factors for keratinocyte skin cancer in patients diagnosed with melanoma, a large retrospective study. Eur. J. Cancer 2016, 53, 115–124.
- Diaz, M.J.; Mark, I.; Rodriguez, D.; Gelman, B.; Tran, J.T.; Kleinberg, G.; Levin, A.; Beneke, A.; Root, K.T.; Tran, A.X.V.; et al. Melanoma Brain Metastases: A Systematic Review of Opportunities for Earlier Detection, Diagnosis, and Treatment. Life 2023, 13, 828.
- Wu, J. The Enhanced Permeability and Retention (EPR) Effect: The Significance of the Concept and Methods to Enhance Its Application. J. Pers. Med. 2021, 11, 771.
- Gavas, S.; Quazi, S.; Karpiński, T.M. Nanoparticles for Cancer Therapy: Current Progress and Challenges. Nanoscale Res. Lett. 2021, 16, 173.
- Liu, J.P.; Wang, T.T.; Wang, D.G.; Dong, A.J.; Li, Y.P.; Yu, H.J. Smart nanoparticles improve therapy for drug-resistant tumors by overcoming pathophysiological barriers. Acta Pharmacol. Sin. 2017, 38, 1–8.
- Haider, M.; Elsherbeny, A.; Pittalà, V.; Consoli, V.; Alghamdi, M.A.; Hussain, Z.; Khoder, G.; Greish, K. Nanomedicine Strategies for Management of Drug Resistance in Lung Cancer. Int. J. Mol. Sci. 2022, 23, 1853.
- Toderascu, L.I.; Sima, L.E.; Orobeti, S.; Florian, P.E.; Icriverzi, M.; Maraloiu, V.A.; Comanescu, C.; Iacob, N.; Kuncser, V.; Antohe, I.; et al. Synthesis and Anti-Melanoma Activity of L-Cysteine-Coated Iron Oxide Nanoparticles Loaded with Doxorubicin. Nanomaterials 2023, 13, 621.
- Guo, H.; Li, F.; Qiu, H.; Liu, J.; Qin, S.; Hou, Y.; Wang, C. Preparation and Characterization of Chitosan Nanoparticles for Chemotherapy of Melanoma Through Enhancing Tumor Penetration. Front. Pharmacol. 2020, 11, 317.
- Xue, J.; Zhu, Y.; Bai, S.; He, C.; Du, G.; Zhang, Y.; Zhong, Y.; Chen, W.; Wang, H.; Sun, X. Nanoparticles with rough surface improve the therapeutic effect of photothermal immunotherapy against melanoma. Acta Pharm. Sin. B 2022, 12, 2934–2949.
- Mello, V.C.; Araújo, V.H.S.; de Paiva, K.L.R.; Simões, M.M.; Marques, D.C.; da Silva Costa, N.R.; de Souza, I.F.; da Silva, P.B.; Santos, I.; Almeida, R.; et al. Development of New Natural Lipid-Based Nanoparticles Loaded with Aluminum-Phthalocyanine for Photodynamic Therapy against Melanoma. Nanomaterials 2022, 12, 3547.
- Dayan, A.; Fleminger, G.; Ashur-Fabian, O. RGD-modified dihydrolipoamide dehydrogenase conjugated to titanium dioxide nanoparticles—Switchable integrin-targeted photodynamic treatment of melanoma cells. RSC Adv. 2018, 8, 9112–9119.
- Bilkan, M.T.; Çiçek, Z.; Kurşun, A.G.C.; Özler, M.; Eşmekaya, M.A. Investigations on effects of titanium dioxide (TiO2) nanoparticle in combination with UV radiation on breast and skin cancer cells. Med. Oncol. 2022, 40, 60.
- Dam, D.H.M.; Zhao, L.; Jelsma, S.A.; Zhao, Y.; Paller, A.S. Folic acid functionalized hollow nanoparticles for selective photodynamic therapy of cutaneous squamous cell carcinoma. Mater. Chem. Front. 2019, 3, 1113–1122.
- Wang, X.; Xuan, L.; Pan, Y. Photothermal ablation of murine melanomas by Fe3O4 nanoparticle clusters. Beilstein J. Nanotechnol. 2022, 13, 255–264.
- Chen, W.; Qin, M.; Chen, X.; Wang, Q.; Zhang, Z.; Sun, X. Combining photothermal therapy and immunotherapy against melanoma by polydopamine-coated Al2O3 nanoparticles. Theranostics 2018, 8, 2229–2241.
- Behnam, M.A.; Emami, F.; Sobhani, Z.; Koohi-Hosseinabadi, O.; Dehghanian, A.R.; Zebarjad, S.M.; Moghim, M.H.; Oryan, A. Novel Combination of Silver Nanoparticles and Carbon Nanotubes for Plasmonic Photo Thermal Therapy in Melanoma Cancer Model. Adv. Pharm. Bull. 2018, 8, 49–55.
- Liu, X.; Xu, Y.; Yin, L.; Hou, Y.; Zhao, S. Chitosan-Poly(Acrylic Acid) Nanoparticles Loaded with R848 and MnCl2 Inhibit Melanoma via Regulating Macrophage Polarization and Dendritic Cell Maturation. Int. J. Nanomed. 2021, 16, 5675–5692.
- Mohsen, M.O.; Heath, M.; Kramer, M.F.; Velazquez, T.C.; Bullimore, A.; Skinner, M.A.; Speiser, D.E.; Bachmann, M.F. In situ delivery of nanoparticles formulated with micron-sized crystals protects from murine melanoma. J. Immunother. Cancer 2022, 10, e004643.
- Cao, M.; Yan, H.; Han, X.; Weng, L.; Wei, Q.; Sun, X.; Lu, W.; Wei, Q.; Ye, J.; Cai, X.; et al. Ginseng-derived nanoparticles alter macrophage polarization to inhibit melanoma growth. J. Immunother. Cancer 2019, 7, 326.
- Neek, M.; Tucker, J.A.; Butkovich, N.; Nelson, E.L.; Wang, S.W. An Antigen-Delivery Protein Nanoparticle Combined with Anti-PD-1 Checkpoint Inhibitor Has Curative Efficacy in an Aggressive Melanoma Model. Adv. Ther. 2020, 3, 2000122.
- Kuang, X.; Wang, Z.; Luo, Z.; He, Z.; Liang, L.; Gao, Q.; Li, Y.; Xia, K.; Xie, Z.; Chang, R.; et al. Ag nanoparticles enhance immune checkpoint blockade efficacy by promoting of immune surveillance in melanoma. J. Colloid Interface Sci. 2022, 616, 189–200.
- Mioc, M.; Pavel, I.Z.; Ghiulai, R.; Coricovac, D.E.; Farcaş, C.; Mihali, C.V.; Oprean, C.; Serafim, V.; Popovici, R.A.; Dehelean, C.A.; et al. The Cytotoxic Effects of Betulin-Conjugated Gold Nanoparticles as Stable Formulations in Normal and Melanoma Cells. Front. Pharmacol. 2018, 9, 429.
- Suarasan, S.; Campu, A.; Vulpoi, A.; Banciu, M.; Astilean, S. Assessing the Efficiency of Triangular Gold Nanoparticles as NIR Photothermal Agents In Vitro and Melanoma Tumor Model. Int. J. Mol. Sci. 2022, 23, 13724.
- Bonamy, C.; Pesnel, S.; Ben Haddada, M.; Gorgette, O.; Schmitt, C.; Morel, A.L.; Sauvonnet, N. Impact of Green Gold Nanoparticle Coating on Internalization, Trafficking, and Efficiency for Photothermal Therapy of Skin Cancer. ACS Omega 2023, 8, 4092–4105.
- Zhang, Y.; Zhan, X.; Xiong, J.; Peng, S.; Huang, W.; Joshi, R.; Cai, Y.; Liu, Y.; Li, R.; Yuan, K.; et al. Temperature-dependent cell death patterns induced by functionalized gold nanoparticle photothermal therapy in melanoma cells. Sci. Rep. 2018, 8, 8720.
- Li, X.; Zhong, S.; Zhang, C.; Li, P.; Ran, H.; Wang, Z. MAGE-Targeted Gold Nanoparticles for Ultrasound Imaging-Guided Phototherapy in Melanoma. Biomed. Res. Int. 2020, 2020, 6863231.
- Chi, Y.F.; Qin, J.J.; Li, Z.; Ge, Q.; Zeng, W.H. Enhanced anti-tumor efficacy of 5-aminolevulinic acid-gold nanoparticles-mediated photodynamic therapy in cutaneous squamous cell carcinoma cells. Braz. J. Med. Biol. Res. Rev. Bras. Pesqui Med. E Biol. 2020, 53, e8457.
- Zhao, L.; Xie, H.; Li, J. Red Blood Cell Membrane-Camouflaged Gold Nanoparticles for Treatment of Melanoma. J. Oncol. 2022, 2022, 3514984.
- Jeon, H.J.; Choi, B.B.R.; Park, K.H.; Hwang, D.S.; Kim, U.K.; Kim, G.C. Induction of Melanoma Cell-Selective Apoptosis Using Anti-HER2 Antibody-Conjugated Gold Nanoparticles. Yonsei Med. J. 2019, 60, 509–516.
- Malindi, Z.; Barth, S.; Abrahamse, H. The Potential of Antibody Technology and Silver Nanoparticles for Enhancing Photodynamic Therapy for Melanoma. Biomedicines 2022, 10, 2158.
- Himalini, S.; Uma Maheshwari Nallal, V.; Razia, M.; Chinnapan, S.; Chandrasekaran, M.; Ranganathan, V.; Gatasheh, M.K.; Hatamleh, A.A.; Al-Khattaf, F.S.; Kanimozhi, S. Antimicrobial, anti-melanogenesis and anti-tyrosinase potential of myco-synthesized silver nanoparticles on human skin melanoma SK-MEL-3 cells. J. King Saud Univ.—Sci. 2022, 34, 101882.
- Kim, D.; Amatya, R.; Hwang, S.; Lee, S.; Min, K.A.; Shin, M.C. BSA-Silver Nanoparticles: A Potential Multimodal Therapeutics for Conventional and Photothermal Treatment of Skin Cancer. Pharmaceutics 2021, 13, 575.
- Valenzuela-Salas, L.M.; Girón-Vázquez, N.G.; García-Ramos, J.C.; Torres-Bugarín, O.; Gómez, C.; Pestryakov, A.; Villarreal-Gómez, L.J.; Toledano-Magaña, Y.; Bogdanchikova, N. Antiproliferative and Antitumour Effect of Nongenotoxic Silver Nanoparticles on Melanoma Models. Oxid. Med. Cell Longev. 2019, 2019, 4528241.
- Zhang, X.; Chen, F.; Turker, M.Z.; Ma, K.; Zanzonico, P.; Gallazzi, F.; Shah, M.A.; Prater, A.R.; Wiesner, U.; Bradbury, M.S.; et al. Targeted melanoma radiotherapy using ultrasmall 177Lu-labeled α-melanocyte stimulating hormone-functionalized core-shell silica nanoparticles. Biomaterials 2020, 241, 119858.
- Chen, F.; Zhang, X.; Ma, K.; Madajewski, B.; Benezra, M.; Zhang, L.; Phillips, E.; Turker, M.Z.; Gallazzi, F.; Penate-Medina, O.; et al. Melanocortin-1 Receptor-Targeting Ultrasmall Silica Nanoparticles for Dual-Modality Human Melanoma Imaging. ACS Appl. Mater. Interfaces 2018, 10, 4379–4393.
- Clemente, N.; Miletto, I.; Gianotti, E.; Sabbatini, M.; Invernizzi, M.; Marchese, L.; Dianzani, U.; Renò, F. Verteporfin-Loaded Mesoporous Silica Nanoparticles’ Topical Applications Inhibit Mouse Melanoma Lymphangiogenesis and Micrometastasis In Vivo. Int. J. Mol. Sci. 2021, 22, 13443.
- Marinheiro, D.; Ferreira, B.J.M.L.; Oskoei, P.; Oliveira, H.; Daniel-da-Silva, A.L. Encapsulation and Enhanced Release of Resveratrol from Mesoporous Silica Nanoparticles for Melanoma Therapy. Materials 2021, 14, 1382.
- Drača, D.; Edeler, D.; Saoud, M.; Dojčinović, B.; Dunđerović, D.; Đmura, G.; Maksimović-Ivanić, D.; Mijatović, S.; Kaluđerović, G.N. Antitumor potential of cisplatin loaded into SBA-15 mesoporous silica nanoparticles against B16F1 melanoma cells: In vitro and in vivo studies. J. Inorg. Biochem. 2021, 217, 111383.
- Kelidari, H.R.; Alipanah, H.; Roozitalab, G.; Ebrahimi, M.; Osanloo, M. Anticancer Effect of Solid-Lipid Nanoparticles Containing Mentha longifolia and Mentha pulegium Essential Oils: In Vitro Study on Human Melanoma and Breast Cancer Cell Lines. Biointerface Res. Appl. Chem. 2021, 12, 2128–2137.
- Valizadeh, A.; Khaleghi, A.A.; Roozitalab, G.; Osanloo, M. High anticancer efficacy of solid lipid nanoparticles containing Zataria multiflora essential oil against breast cancer and melanoma cell lines. BMC Pharmacol. Toxicol. 2021, 22, 52.
- Movahedi, F.; Gu, W.; Soares, C.P.; Xu, Z.P. Encapsulating Anti-Parasite Benzimidazole Drugs into Lipid-Coated Calcium Phosphate Nanoparticles to Efficiently Induce Skin Cancer Cell Apoptosis. Front. Nanotechnol. 2021, 3, 693837.
- Sakpakdeejaroen, I.; Somani, S.; Laskar, P.; Mullin, M.; Dufès, C. Regression of Melanoma Following Intravenous Injection of Plumbagin Entrapped in Transferrin-Conjugated, Lipid-Polymer Hybrid Nanoparticles. Int. J. Nanomed. 2021, 16, 2615–2631.
- Clemente, N.; Ferrara, B.; Gigliotti, C.L.; Boggio, E.; Capucchio, M.T.; Biasibetti, E.; Schiffer, D.; Mellai, M.; Annovazzi, L.; Cangemi, L.; et al. Solid Lipid Nanoparticles Carrying Temozolomide for Melanoma Treatment. Preliminary In Vitro and In Vivo Studies. Int. J. Mol. Sci. 2018, 19, 255.
- Zeng, Y.B.; Yu, Z.C.; He, Y.N.; Zhang, T.; Du, L.B.; Dong, Y.M.; Chen, H.W.; Zhang, Y.Y.; Wang, W.Q. Salinomycin-loaded lipid-polymer nanoparticles with anti-CD20 aptamers selectively suppress human CD20+ melanoma stem cells. Acta Pharmacol. Sin. 2018, 39, 261–274.
- Fattore, L.; Cafaro, G.; Di Martile, M.; Campani, V.; Sacconi, A.; Liguoro, D.; Marra, E.; Bruschini, S.; Stoppoloni, D.; Cirombella, R.; et al. Oncosuppressive miRNAs loaded in lipid nanoparticles potentiate targeted therapies in BRAF-mutant melanoma by inhibiting core escape pathways of resistance. Oncogene 2023, 42, 293–307.
- Fattore, L.; Campani, V.; Ruggiero, C.F.; Salvati, V.; Liguoro, D.; Scotti, L.; Botti, G.; Ascierto, P.A.; Mancini, R.; De Rosa, G.; et al. In Vitro Biophysical and Biological Characterization of Lipid Nanoparticles Co-Encapsulating Oncosuppressors miR-199b-5p and miR-204-5p as Potentiators of Target Therapy in Metastatic Melanoma. Int. J. Mol. Sci. 2020, 21, 1930.
- Md, S.; Alhakamy, N.A.; Neamatallah, T.; Alshehri, S.; Mujtaba, M.A.; Riadi, Y.; Radhakrishnan, A.K.; Khalilullah, H.; Gupta, M.; Akhter, M.H. Development, Characterization, and Evaluation of α-Mangostin-Loaded Polymeric Nanoparticle Gel for Topical Therapy in Skin Cancer. Gels 2021, 7, 230.
More
Information
Subjects:
Neurosciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
02 Apr 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





