Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Brandon Lucke-Wold | -- | 2387 | 2024-04-01 15:32:42 | | | |
| 2 | Peter Tang | Meta information modification | 2387 | 2024-04-02 02:50:35 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Diaz, M.J.; Mark, I.; Rodriguez, D.; Gelman, B.; Tran, J.T.; Kleinberg, G.; Levin, A.; Beneke, A.; Root, K.T.; Tran, A.X.V.; et al. Melanoma Brain Metastases. Encyclopedia. Available online: https://encyclopedia.pub/entry/56540 (accessed on 07 February 2026).
Diaz MJ, Mark I, Rodriguez D, Gelman B, Tran JT, Kleinberg G, et al. Melanoma Brain Metastases. Encyclopedia. Available at: https://encyclopedia.pub/entry/56540. Accessed February 07, 2026.
Diaz, Michael Joseph, Isabella Mark, Daphnee Rodriguez, Beata Gelman, Jasmine Thuy Tran, Giona Kleinberg, Anna Levin, Alice Beneke, Kevin Thomas Root, Andrew Xuan Vinh Tran, et al. "Melanoma Brain Metastases" Encyclopedia, https://encyclopedia.pub/entry/56540 (accessed February 07, 2026).
Diaz, M.J., Mark, I., Rodriguez, D., Gelman, B., Tran, J.T., Kleinberg, G., Levin, A., Beneke, A., Root, K.T., Tran, A.X.V., & Lucke-Wold, B. (2024, April 01). Melanoma Brain Metastases. In Encyclopedia. https://encyclopedia.pub/entry/56540
Diaz, Michael Joseph, et al. "Melanoma Brain Metastases." Encyclopedia. Web. 01 April, 2024.
Copy Citation
Metastasis to the central nervous system (CNS) is a common occurrence for patients with advanced-stage melanoma, where up to 60% of all melanoma patients will develop brain metastasis during the progression of their disease .
melanoma
cerebral metastases
MBM
early detection
1. Introduction
The incidence of melanoma has exponentially increased in developed countries, accounting for 1.7% of cancer diagnoses worldwide, and remains the fifth most diagnosed cancer in the United States [1]. Incidence rates vary across geographical locations. The annual incidence rates in regions with predominately fair-skinned populations have rapidly increased between 4 and 6% in North America, Northern Europe, and New Zealand, while countries in Asia have incidence rates that remain mostly unchanged [2]. These varying incidences are likely attributed to differences in pigmentation characteristics as melanoma is distinctly more prevalent among fair-skinned individuals and is rarely seen in darker-skinned individuals [2]. Sex and gender disparities also exist in melanoma as incidence rates are higher in men than women [3]. Although melanoma represents 1% of all skin cancers, it is responsible for over 80% of skin cancer deaths, signifying the importance of screening for those with risk factors involving family or previous history of skin cancer, congenital disorders, and UV exposure from predisposing lifestyle/profession [1]. Melanoma diagnoses are classified into five stages, 0–IV, where stage 0 is known as melanoma in situ, and the last stage is defined as metastatic melanoma (MM) [4]. If melanoma is detected in its early stages, there is a 99% 5-year survival rate for primary melanoma; however, this number significantly drops to 27% for metastatic melanoma (SD ± 5–10%) [5]. Visceral metastasis sites are associated with poor prognosis and survival in melanoma; the one-year survival rate is 36%, 13%, and 1% in patients with metastasis to one, two, or three different visceral sites, respectively [6].
Metastasis to the central nervous system (CNS) is a common occurrence for patients with advanced-stage melanoma, where up to 60% of all melanoma patients will develop brain metastasis during the progression of their disease [7]. The biological propensity for melanoma to migrate to the brain is not fully understood, but epidemiologic data implicate various factors that correlate to the development of melanoma brain metastasis (MBM); some factors include male sex, primary tumor site on the trunk, and histopathologic diagnosis of primary superficial spreading [6]. The diagnosis of MBM often results in dismal outcomes associated with the rapid decline in the quality and quantity of life, with the median overall survival (OS) from diagnosis of around 4–6 months [8].
2. Detection and Diagnosis of Melanoma Brain Metastases
Metastatic melanoma that travels to the brain is a severe outcome of melanoma and is often given a poor prognosis [9]. Patients with metastatic brain melanomas have very high mortality rates, and the median survival rate after diagnosis is six months [10]. Additionally, in many cases, brain metastases are only detected in the later stages of cancer and are usually made up of multiple lesions [11]. The location of the metastasis often makes surgical resection difficult, which is compounded when the number of lesions is greater and when the size of the lesions is smaller. Moreover, even the effectiveness of treatment for a single or solitary brain metastasis is unclear and varies by patient [11]. Presymptomatic contrast CTs or MRIs are the primary method to identify CNS lesions. This identification is followed by characterization, conventionally achieved by the use of the Breslow thickness index and Clark level staging system as well as the notation of its subtype, mutation status, and anatomical location. The Breslow thickness index measures how vertically deep the tumor penetrates the skin layers to foretell the general likeliness of it spreading. The Clark scale is a 5-level scale that similarly specifies the number of skin layers a tumor invades. Though not fully comprehensive or infallible, these techniques are reliable for melanoma diagnoses/prognoses. As for determining the most effective treatment, however, there is still no reliable biomarker for MBM [10]. Studies have also not shown how these biomarkers may change throughout treatment and how they may signify improvement [11][12].
A promising biomarker is cell-free circulating tumor DNA (ctDNA) in determining the prognosis. A study revealed through quantitative PCR that the elevated pretreatment BRAFV600-mutant ctDNA concentration was associated with worse survival rates; conversely, low ctDNA and high lactate dehydrogenase (LDH) are associated with better survival rates when measured at week four, as demonstrated by univariate analysis (p < 0.0001) [12]. Evidence is increasingly demonstrating that pretreatment ctDNA, which is undetectable, is associated with significantly longer progression-free survival and overall survival [12]. Syeda et al. found that detectable ctDNA is associated with LDH concentration, the number of metastatic sites, the median sum of lesion diameters, and increased tumor burdens [12]. These results suggest that BRAFV600-mutant ctDNA could function as a predictive biomarker for overall and progression free survival prognosis. Furthermore, patients treated with dabrafenib plus trametinib, which maintained normal LDH levels and fewer than three organ sites with metastases, had the longest progression-free survival and overall survival [13]. Conversely, patients with more than two times the normal LDH concentrations had the shortest progression-free survival and overall survival rates [13]. Moreover, the patients with disease progression and new CNS lesions reported worse outcomes with a median survival time after progression of 4 months (n = 171) [13]. Due to the severe prognoses associated with metastatic brain melanoma, many studies often exclude this patient population from their studies. However, one study specifically examined melanoma patients with brain metastases and assessed the risk factors associated with the progression of their disease. Eastern Cooperative Oncology Group (ECOG) scores are frequently used by oncology health care professionals to assess the performance status of a patient in terms of functional status and ability for self-care for directing treatment decision and prognosis, where the scores are between 1 and 5, indicating increasing levels of disability [14]. Progression-free survival (PFS) is the duration of time between the initiation of a treatment to the progression of a disease or worsening, and this measure is used to determine how well a treatment works [15]. The results indicated that the following factors were associated with lower progression-free survival: ECOG ≳1, elevated serum LDH ≳three metastatic sites, and non-naive status [16]. Conversely, patients with ECOG 0, less than three metastatic sites, and LDH under the normal limits, typically had the highest progression-free survival (PFS) after six months [16].
Additional risk factors have been assessed for the development of brain metastasis. Despite the previous proposal, a recent study found that an ulcerated primary was not associated with an increased risk of CNS progression [10]. When comparing patients with macroscopic lymph node involvement (or in-transit metastases) and patients with lymph node micrometastases, there was no significant difference in the CNS relapse rates across both groups [10]. Patients in stage IIIC were also more likely to have CNS progression than stage IIIA or IIIB [10]. In a comparable study, 10% of patients with stage III disease were at risk for, and up to 46% of stage IV patients will develop brain metastasis [10].
3. Treatment of Melanoma Brain Metastases
Although current cancer treatment paradigms may reflect any combination of surgery, chemotherapy, radiotherapy, and immunotherapy with subsequent medical management, the options for treating melanoma brain metastases remain limited. As per usual practice, neurosurgery is a viable option for patients with solitary or single brain metastasis and for those desiring symptomatic relief. These surgical resections must still be followed by radiotherapy or a drug regimen that targets clinically undetectable micrometastases that likely remain elsewhere in the brain [17]. Regardless of surgical candidacy, systemic agents such as chemotherapeutics are prescribed to metastatic patients. However, such options fall short for MBM cases because penetration through the blood–brain barrier complicates drug delivery. Of the chemotherapy drugs that can enter the brain, they still only show modest efficacy [18]. As such, a recent push to unveil therapeutic targets that do not evade MBM treatment has spurred a burst of novel immunotherapy options. Several labs have looked at two new classes of drugs, immune checkpoint inhibitors and small-molecule targeted drugs, as alternatives to conventional chemotherapy. Both have significantly improved progression-free survival rates and patient prognosis of stage IV melanoma patients with brain metastases [18]. For example, previous research has cited that the co-administration of temozolomide (TMZ) and IFN-α2b exhibits antitumor activity, increased response rates, and in some instances, complete remission [19]. Temozolomide with sorafenib also showed antiangiogenic effects [20]. In vitro studies have also reported synergistic antitumor activity from TMZ with cisplatin. Even more promising, numerous phase III trials of a TMZ-based regimen administered to melanoma patients reported a reduction in CNS metastases from developing, proposing that the drug has prophylactic potential in patients with melanoma [21]. Therefore, temozolomide exposure is an attractive and encouraging treatment option that should be considered not only for first-line brain metastasis treatment, but also for early-stage melanoma presentation (see Figure 1).
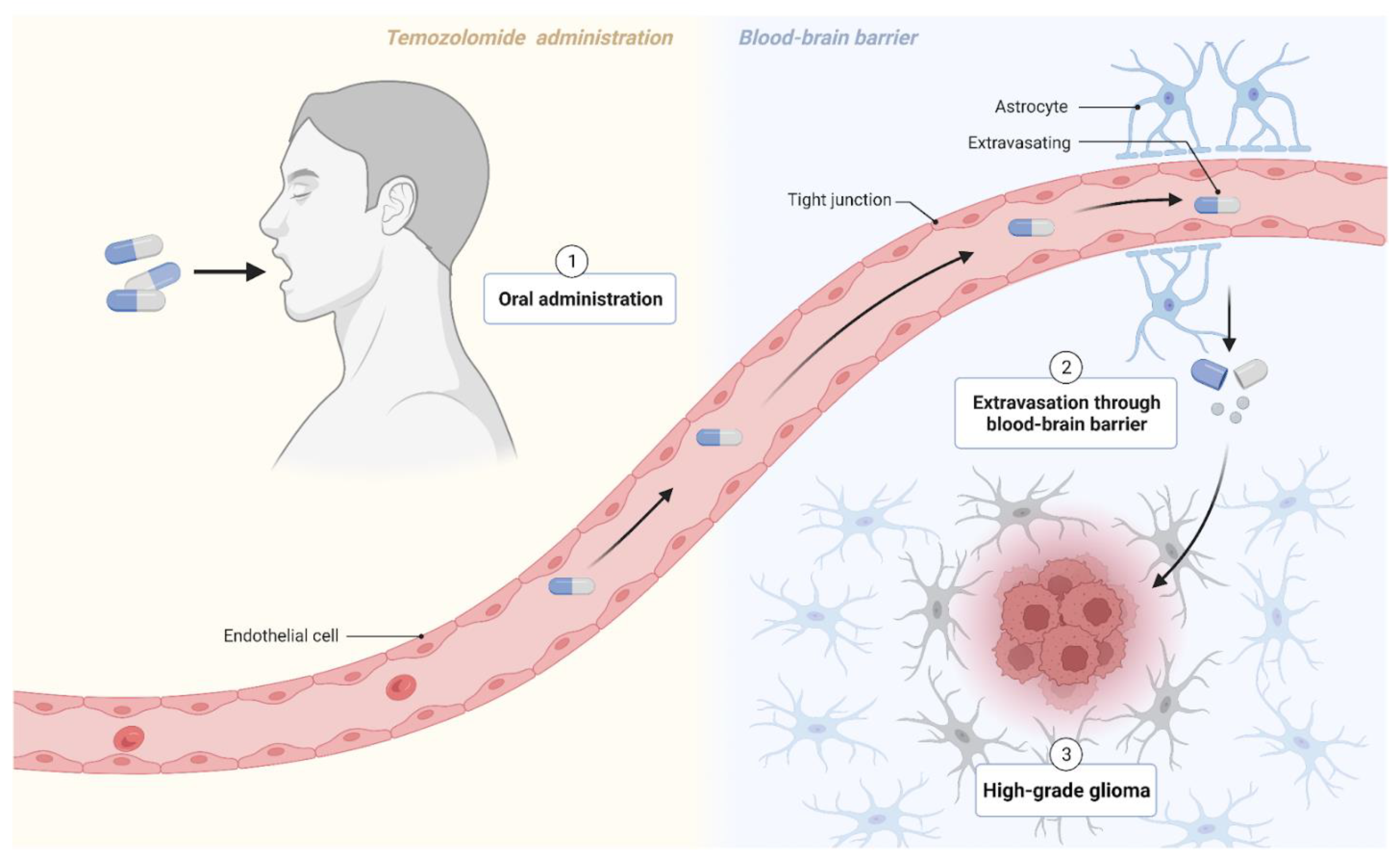
Figure 1. Temozolomide crosses the blood–brain barrier to target high-grade glioma cells. Figure created with BioRender.com (accessed on 17 December 2022).
Furthermore, evidence on the efficacy of ipilimumab and nivolumab, immune checkpoint inhibitors of PD-L1, is conflicting. Gritsch et al. reported a correlation between these agents and longer recurrence-free survival (RFS) and distant metastasis-free survival (DMFS) in MBM patients [22]. In the same year, Guo et al. claimed that PD-1 inhibitors had questionable long-term neurological and cognitive sequelae; the drugs could potentially exacerbate radiation-related necrosis [23]. High-dose injections of Allovectin-7, on the other hand, have been shown to be well-tolerated in stage III/IV MBM patients, but the results are lesion-specific [24]. Others have explored IFN-α and interleukin-2 (IL-2) both independently and jointly. Controversially, Li et al.’s randomized control trials brought them to the conclusion that subcutaneous INF-α injections are safer than IL-2 injections, but IL-2 is more effective at eradicating tumors and for relapse-free outcomes. Although IL-2 had greater brain tumor responses, it also induced more severe toxicities elsewhere in the body, ultimately diminishing the patients’ quality of life and posing a threat for their overall survivability [25]. Despite some ambiguities and the warranting of more testing, there is overwhelming evidence of immunotherapeutic (IT) approaches being key to formulating an effective management plan for complete tumor response in MBM.
For optimal success rates, IT is combined with radiation. Traditionally, two forms of radiotherapy are deferred to—whole-brain radiotherapy (WBRT) and stereotactic (SRS) radiosurgery—for treating metastatic spread to the CNS. SRS has recently gained traction, whereas WBRT has also proven to be wholly unpromising for treating MBM.
The second form of radiotherapy, stereotactic radiosurgery (SRS), has experienced a rapid uptake among patients with brain metastases. It has become the preferred radiotherapeutic option for numerous reasons. In SRS, external ionizing radiation beams are precisely focused on metastatic tumors, minimizing toxicity exposure. SRS has been demonstrated to be well-tolerated and to extend survivability when paired with immunotherapy [26][27][28][29]. Alone, SRS does provide a durable early response, but the effects are minimal when not administered concomitantly with IT [22].
Among the described treatment methods, the combined treatment of SRS and immunotherapy for MBM yields the best treatment pattern outcomes, and presently, it is the most scientifically supported therapeutic approach for targeting MBM. The most optimal results for OS and PFS are derived from conjunctive treatments via immunotherapies and radiotherapies in patients with MBM. Future work should pursue the optimal combination ratio and timing of the two therapies.
4. Emerging Clinical Evidence and Focus Areas
There is a variety of emerging clinical evidence relevant to melanoma brain metastases. A large quantity of new research seeks to identify and improve novel and established treatment options. Emerging trials showcase how newly tested combinations of medications can increase the clinical efficacy for metastatic melanoma. For example, the characterization of nivolumab and ipilimumab therapy is ongoing as the effects still need to be fully understood, especially in active brain metastases. A phase II trial led by Long achieved significant intracranial response in patients with active melanoma brain metastases [30]. With no treatment-related deaths, intracranial response was achieved across three patient cohorts, with 46% (95% CI, 29–63) in the first cohort showing intracranial response, followed by 20% (95% CI, 7–41) in the second cohort, and 6% (95% CI, 0–30) in the third cohort [30]. Complete intracranial response was stimulated across the three patient cohorts at rates of 17%, 12%, and 0%; however, grade 3 or 4 treatment-related adverse events occurred in all three cohorts in 54%, 16%, and 13% of patients, respectively [30]. Despite treatment-related adverse effects, joint nivolumab and ipilimumab therapy showed a high intracranial response, indicating its potential as a primary therapy option for untreated and asymptomatic melanoma brain metastases [30].
In addition to improving drug therapy, emerging research addressing other clinical course features for patients with melanoma brain metastases is also abundant. Radiotherapy approaches, in particular, can often improve the accuracy and accessibility. A study conducted by Tran et al. assessed the pre-trial cost-effectiveness of whole-brain radiotherapy, hippocampal-avoidant radiotherapy, and observational methods such as stereotactic radiosurgery or surgery alone (Figure 2) [31]. Hippocampal avoidant radiotherapy was shown using probabilistic sensitivity analysis as the preferred method in 77% of simulations. Other studies have confirmed the data quality from observational methods such as whole brain radiotherapy and the preferred hippocampal avoidant radiotherapy [26][32]. Aside from the cost considerations, additional research addressing improvable features of utilized observational methods could lead to better patient outcomes and conserve clinical resources. Despite the magnitude of emerging research, further studies are needed to identify prospective front-line therapy options that promote greater clinical efficacy.

Figure 2. Illustration of two observational methods: radiosurgery and surgery alone. Figure created with BioRender.com (accessed on 18 December 2022).
The BRAF gene plays a crucial role in determining patient treatment. Inhibitors of BRAF downstream MAPK and/or MEK are more effective than chemotherapy in the treatment of BRAFV600E-mutated metastatic melanoma [33]. However, the issue of drug resistance remains a significant challenge. A novel drug, E6201, acts as an ATP-competitive MEK1 inhibitor that has shown success in patients with brain metastasis with BRAF V600E and CTNNB1 mutations [33]. While the effect of CTNNB1 on the MEK pathway is unknown, E6201 provides insights into the mechanism of metastatic melanoma progression into brain metastasis [33]. A different study using patient derived xenografts highlighted RAS and MAP2K1/2 mutations as conferring resistance to BRAF inhibition [34]. Additionally, the established melanoma cell lines showed a significant bias toward BRAF, TP53 mutations, and CDKN2A loss [34]. Pre-clinical data by several groups have suggested that combining BRAF/MEK inhibitors with PI3K/mTOR inhibitors may overcome resistance in BRAF mutant melanomas [34]. A case study focused on BRAF G466E and BRAF K601E mutations with decreased and increased kinase activity, respectively. The results indicated that mutations resulting in increased MAP kinase activity may respond better to MEK inhibitors, whereas patients with mutations resulting in low MAP kinase activities may benefit from treatment with inhibitors of various immune checkpoints [35].
References
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Barsouk, A. Epidemiology of Melanoma. Med. Sci. 2021, 9, 63.
- Erdei, E.; Torres, S.M. A new understanding in the epidemiology of melanoma. Expert Rev. Anticancer Ther. 2010, 10, 1811–1823.
- Bellenghi, M.; Puglisi, R.; Pontecorvi, G.; De Feo, A.; Carè, A.; Mattia, G. Sex and Gender Disparities in Melanoma. Cancers 2020, 12, 1819.
- Eddy, K.; Shah, R.; Chen, S. Decoding Melanoma Development and Progression: Identification of Therapeutic Vulnerabilities. Front. Oncol. 2021, 10, 626129.
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33.
- Damsky, J.W.E.; Rosenbaum, L.E.; Bosenberg, M. Decoding Melanoma Metastasis. Cancers 2010, 3, 126–163.
- Ajithkumar, T.; Parkinson, C.; Fife, K.; Corrie, P.; Jefferies, S. Evolving treatment options for melanoma brain metastases. Lancet Oncol. 2015, 16, e486–e497.
- Oliva, I.G.; Tawbi, H.; Davies, M.A. Melanoma Brain Metastases: Current Areas of Investigation and Future Directions. Cancer J. 2017, 23, 68–74.
- Hauswald, H.; Habl, G.; Krug, D.; Kehle, D.; Combs, S.; Bermejo, J.L.; Debus, J.; Sterzing, F. Whole brain helical Tomotherapy with integrated boost for brain metastases in patients with malignant melanoma–a randomized trial. Radiat. Oncol. 2013, 8, 234.
- Samlowski, W.E.; Moon, J.; Witter, M.; Atkins, M.B.; Kirkwood, J.M.; Othus, M.; Ribas, A.; Sondak, V.K.; Flaherty, L.E. High frequency of brain metastases after adjuvant therapy for high-risk melanoma. Cancer Med. 2017, 6, 2576–2585.
- Fuentes, R.; Osorio, D.; Hernandez, J.E.; Simancas-Racines, D.; Martinez-Zapata, M.J.; Cosp, X.B. Surgery versus stereotactic radiotherapy for people with single or solitary brain metastasis. Cochrane Database Syst. Rev. 2018, 2018, CD012086.
- Syeda, M.M.; Wiggins, J.M.; Corless, B.C.; Long, G.V.; Flaherty, K.T.; Schadendorf, D.; Nathan, P.D.; Robert, C.; Ribas, A.; Davies, M.A.; et al. Circulating tumour DNA in patients with advanced melanoma treated with dabrafenib or dabrafenib plus trametinib: A clinical validation study. Lancet Oncol. 2021, 22, 370–380.
- Long, G.V.; Grob, J.-J.; Nathan, P.; Ribas, A.; Robert, C.; Schadendorf, D.; Lane, S.R.; Mak, C.; Legenne, P.; Flaherty, K.T.; et al. Factors predictive of response, disease progression, and overall survival after dabrafenib and trametinib combination treatment: A pooled analysis of individual patient data from randomised trials. Lancet Oncol. 2016, 17, 1743–1754.
- Azam, F.; Latif, M.F.; Farooq, A.; Tirmazy, S.H.; AlShahrani, S.; Bashir, S.; Bukhari, N. Performance Status Assessment by Using ECOG (Eastern Cooperative Oncology Group) Score for Cancer Patients by Oncology Healthcare Professionals. Case Rep. Oncol. 2019, 12, 728–736.
- Hess, L.M.; Brnabic, A.; Mason, O.; Lee, P.; Barker, S. Relationship between Progression-free Survival and Overall Survival in Randomized Clinical Trials of Targeted and Biologic Agents in Oncology. J. Cancer 2019, 10, 3717–3727.
- Dutriaux, C.; Robert, C.; Grob, J.-J.; Mortier, L.; Dereure, O.; Lebbe, C.; Mansard, S.; Grange, F.; Neidhardt, E.-M.; Lesimple, T.; et al. An open label, non-randomised, phase IIIb study of trametinib in combination with dabrafenib in patients with unresectable (stage III) or distant metastatic (stage IV) BRAF V600-mutant melanoma: A subgroup analysis of patients with brain metastases. Eur. J. Cancer 2022, 175, 254–262.
- Fogarty, G.; Morton, R.L.; Vardy, J.; Nowak, A.K.; Mandel, C.; Forder, P.M.; Hong, A.; Hruby, G.; Burmeister, B.; Shivalingam, B.; et al. Whole brain radiotherapy after local treatment of brain metastases in melanoma patients—A randomised phase III trial. BMC Cancer 2011, 11, 142.
- Pasquali, S.; Hadjinicolaou, A.V.; Sileni, V.C.; Rossi, C.R.; Mocellin, S. Systemic treatments for metastatic cutaneous melanoma. Cochrane Database Syst. Rev. 2018, 2, CD011123.
- Richtig, E.; Hofmann-Wellenhof, R.; Pehamberger, H.; Forstinger, C.; Wolff, K.; Mischer, P.; Raml, J.; Fritsch, P.; Zelger, B.; Ratzinger, G.; et al. Temozolomide and interferon alpha2b in metastatic melanoma stage IV. Br. J. Dermatol. 2004, 151, 91–98.
- Amaravadi, R.K.; Schuchter, L.M.; McDermott, D.F.; Kramer, A.; Giles, L.; Gramlich, K.; Carberry, M.; Troxel, A.B.; Letrero, R.; Nathanson, K.L.; et al. Phase II Trial of Temozolomide and Sorafenib in Advanced Melanoma Patients with or without Brain Metastases. Clin. Cancer Res. 2009, 15, 7711–7718.
- Chiarion-Sileni, V.; Guida, M.; Ridolfi, L.; Romanini, A.; Del Bianco, P.; Pigozzo, J.; Brugnara, S.; Colucci, G.; Ridolfi, R.; De Salvo, G.L. Central nervous system failure in melanoma patients: Results of a randomised, multicentre phase 3 study of temozolomide- and dacarbazine- based regimens. Br. J. Cancer 2011, 104, 1816–1821.
- Gritsch, D.M.; Mrugala, M.M.M.; Marks, L.A.M.; Wingerchuk, D.M.M.; O’Carroll, C.B.M. In Patients With Melanoma Brain Metastases, Is Combination Immune Checkpoint Inhibition a Safe and Effective First-Line Treatment? A Critically Appraised Topic. Neurologist 2022, 27, 290–297.
- Guo, T.; Chu, L.; Chu, X.; Yang, X.; Li, Y.; Zhou, Y.; Xu, D.; Zhang, J.; Wang, S.; Hu, J.; et al. Brain metastases, patterns of intracranial progression, and the clinical value of upfront cranial radiotherapy in patients with metastatic non-small cell lung cancer treated with PD-1/PD-L1 inhibitors. Transl. Lung Cancer Res. 2022, 11, 173–187.
- Bedikian, A.Y.; Richards, J.; Kharkevitch, D.; Atkins, M.B.; Whitman, E.; Gonzalez, R. A phase 2 study of high-dose Allovectin-7 in patients with advanced metastatic melanoma. Melanoma Res. 2010, 20, 218–226.
- Li, S.; Wu, X.; Chen, P.; Pei, Y.; Zheng, K.; Wang, W.; Qiu, E.; Zhang, X. Interferon-α versus interleukin-2 in Chinese patients with malignant melanoma: A randomized, controlled, trial. Anti-Cancer Drugs 2019, 30, 402–409.
- Fogarty, G.B.; Hong, A.; Dolven-Jacobsen, K.; Reisse, C.H.; Burmeister, B.; Haydu, L.H.; Dhillon, H.; Steel, V.; Shivalingam, B.; Drummond, K.; et al. First interim analysis of a randomised trial of whole brain radiotherapy in melanoma brain metastases confirms high data quality. BMC Res. Notes 2015, 8, 192.
- Janavicius, M.; Lachej, N.; Anglickiene, G.; Vincerzevskiene, I.; Brasiuniene, B. Outcomes of Treatment for Melanoma Brain Metastases. J. Ski. Cancer 2020, 2020, 7520924.
- Kirkpatrick, J.P.; Wang, Z.; Sampson, J.H.; McSherry, F.; Herndon, J.E.; Allen, K.J.; Duffy, E.; Hoang, J.K.; Chang, Z.; Yoo, D.S.; et al. Defining the optimal planning target volume in image-guided stereotactic radiosurgery of brain metastases: Results of a randomized trial. Int. J. Radiat. Oncol. 2015, 91, 100–108.
- Tétu, P.; Allayous, C.; Oriano, B.; Dalle, S.; Mortier, L.; Leccia, M.; Guillot, B.; Dalac, S.; Dutriaux, C.; Lacour, J.-P.; et al. Impact of radiotherapy administered simultaneously with systemic treatment in patients with melanoma brain metastases within MelBase, a French multicentric prospective cohort. Eur. J. Cancer 2019, 112, 38–46.
- Long, G.V.; Atkinson, V.; Lo, S.; Sandhu, S.; Guminski, A.D.; Brown, M.P.; Wilmott, J.S.; Edwards, J.; Gonzalez, M.; Scolyer, R.A.; et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: A multicentre randomised phase 2 study. Lancet Oncol. 2018, 19, 672–681.
- Tran, A.D.; Fogarty, G.; Nowak, A.K.; Diaby, V.; Hong, A.; Watts, C.; Morton, R.L. Cost-Effectiveness of Subsequent Whole-Brain Radiotherapy or Hippocampal-Avoidant Whole-Brain Radiotherapy Versus Stereotactic Radiosurgery or Surgery Alone for Treatment of Melanoma Brain Metastases. Appl. Health Econ. Health Policy 2020, 18, 679–687.
- Martinage, G.; Hong, A.M.; Fay, M.; Thachil, T.; Roos, D.; Williams, N.; Lo, S.; Fogarty, G. Quality assurance analysis of hippocampal avoidance in a melanoma whole brain radiotherapy randomized trial shows good compliance. Radiat. Oncol. 2018, 13, 132.
- Larkin, J.R.; Dickens, A.M.; Claridge, T.D.W.; Bristow, C.; Andreou, K.; Anthony, D.C.; Sibson, N.R. Early Diagnosis of Brain Metastases Using a Biofluids-Metabolomics Approach in Mice. Theranostics 2016, 6, 2161–2169.
- Krepler, C.; Sproesser, K.; Brafford, P.; Beqiri, M.; Garman, B.; Xiao, M.; Shannan, B.; Watters, A.; Perego, M.; Zhang, G.; et al. A Comprehensive Patient-Derived Xenograft Collection Representing the Heterogeneity of Melanoma. Cell Rep. 2017, 21, 1953–1967.
- Babiker, H.M.; Byron, S.A.; Hendricks, W.P.D.; Elmquist, W.F.; Gampa, G.; Vondrak, J.; Aldrich, J.; Cuyugan, L.; Adkins, J.; De Luca, V.; et al. E6201, an intravenous MEK1 inhibitor, achieves an exceptional response in BRAF V600E-mutated metastatic malignant melanoma with brain metastases. Investig. New Drugs 2019, 37, 636–645.
More
Information
Subjects:
Neurosciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.7K
Revisions:
2 times
(View History)
Update Date:
02 Apr 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




