Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Jiao, Y.; Liu, Y.; Du, J.; Xu, J.; Luo, Z.; Liu, Y.; Guo, L. Extracellular Vesicles for Bone Regeneration. Encyclopedia. Available online: https://encyclopedia.pub/entry/56534 (accessed on 07 February 2026).
Jiao Y, Liu Y, Du J, Xu J, Luo Z, Liu Y, et al. Extracellular Vesicles for Bone Regeneration. Encyclopedia. Available at: https://encyclopedia.pub/entry/56534. Accessed February 07, 2026.
Jiao, Yao, Yitong Liu, Juan Du, Junji Xu, Zhenhua Luo, Yi Liu, Lijia Guo. "Extracellular Vesicles for Bone Regeneration" Encyclopedia, https://encyclopedia.pub/entry/56534 (accessed February 07, 2026).
Jiao, Y., Liu, Y., Du, J., Xu, J., Luo, Z., Liu, Y., & Guo, L. (2024, March 31). Extracellular Vesicles for Bone Regeneration. In Encyclopedia. https://encyclopedia.pub/entry/56534
Jiao, Yao, et al. "Extracellular Vesicles for Bone Regeneration." Encyclopedia. Web. 31 March, 2024.
Copy Citation
Bone defects are intricate pathological alterations resulting from osteoporotic fractures, traumatic injuries, inflammatory responses, malignant tumors, and various other factors. Extracellular vesicles are small lipid bilayer membrane particles secreted by all cell types. The term “EVs” collectively refers to diverse vesicle types, such as exosomes, microvesicles, microparticles, shedding vesicles, and apoptotic bodies.
extracellular vesicles
bone regeneration
exosomes
bone repair
1. Introduction
Bone defects are intricate pathological alterations resulting from osteoporotic fractures, traumatic injuries, inflammatory responses, malignant tumors, and various other factors. Globally, osteoporosis-related fractures occur at a rate of one every 20 s among individuals aged 50 and above, with over 2 million bone graft procedures conducted annually [1]. The extended healing duration associated with traditional treatments contributes significantly to the substantial healthcare expenses incurred and demonstrates certain inherent limitations [2]. The primary traditional treatment for bone defects is bone grafting, which can involve autologous bone, allogeneic bone, or synthetic materials [3]. However, autologous bone graft treatment has several drawbacks, including poor bone volume, limited availability, donor site damage, and other complications [4][5]. Conversely, allogeneic transplants can increase the risk of disease transmission, angiogenesis problems, immune rejection, and other issues [6]. To address these challenges, sustainable bone regeneration therapies are emerging, such as scaffolds, bioactive substances, and cells or tissues with osteogenic potential [7]. There are also inevitable challenges associated with cell therapy, such as biological safety concerns, limited tissue sources, and ethical issues. Additionally, the ischemic microenvironment of bone injuries may lead to a reduced survival rate of transplanted cells, making it difficult to ensure efficacy. Therefore, the emergence of cell-free therapies provides a new opportunity for bone regeneration treatment. Extracellular vesicles (EVs) can induce osteogenesis, angiogenesis, and regulate immunity. They contain fewer membrane proteins, making clinical applications safer and with a higher yield. As such, EVs are expected to be an ideal component to combine with bone engineering scaffolds to guide bone regeneration [8][9].
Extracellular vesicles are small lipid bilayer membrane particles secreted by all cell types. The term “EVs” collectively refers to diverse vesicle types, such as exosomes, microvesicles, microparticles, shedding vesicles, and apoptotic bodies (Figure 1). These heterogeneous families of small vesicles are conventionally classified into the following three groups, according to their size and biogenesis: exosomes (30–100 nm), microvesicles (100–1000 nm), and apoptotic bodies (1000–5000 nm) [10]. Their contents include DNA fragments, messenger ribonucleic acids (mRNAs), proteins, and lipids [11][12]. Exosomes are formed within multivesicular bodies and are released when these bodies fuse with the plasma membrane. They contain proteins and lipids derived from the parent cells, including tetraspanin (CD9, CD63, and CD81), proteins involved in multivesicular body biosynthesis [such as Alix and tumor susceptibility gene 101, (TSG101)], heat shock proteins (HSP70 and HSP90), and membrane translocation and fusion proteins (GTPases and membrane coupling proteins) [13]. Microvesicles are produced and released by budding from the plasma membrane. Apoptotic bodies are vesicles formed during apoptosis that contain nuclear and cytoplasmic fragments surrounded by membranes when cells shrink and break apart. In recent years, researchers have discovered a new type of extracellular vesicle, called a migrasome, which is a large vesicle growing at the tip or crossing of retraction fibers in the back of migrating cells. It is about 500 nm to 3000 nm in diameter and contains numerous smaller vesicles [14]. After the cells migrate, the retraction fibers eventually break, releasing the migrasomes into the extracellular space. Compared with exosomes, migrasomes have specific proteins, such as N-Deacetylase/N-Sulfotransferase 1 (NDST1), EGF domain-specific O-linked N-acetylglucosamine transferase (EOGT), Phosphatidylinositol glycan anchor biosynthesis class K (PIGK), and Carboxypeptidase Q (CPQ) [15]. A recent study has shown that migrasomes promote angiogenesis in chick embryos [16]. However, there have been no studies on the use of migrasomes for the treatment of specific diseases.
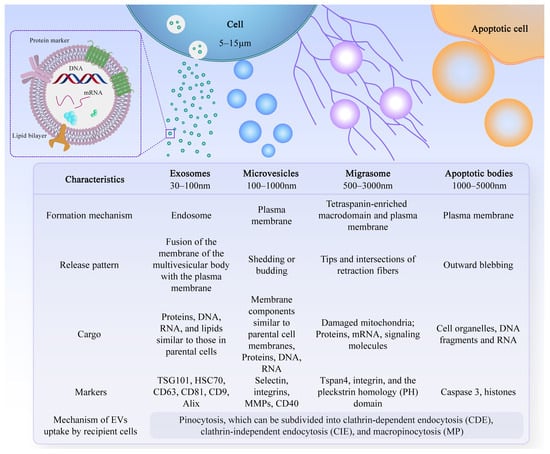
Figure 1. Characteristics of different types of EVs. There are different types of EVs, and their formation mechanisms, release patterns, cargo, and markers are not exactly the same.
After leaving the initiating cell, these vesicles can reach the target cell via markers on their membrane surface, which can interact with the receptor-ligand, and thus alter the physiological state of the target cell by transferring their contents or triggering signals on the target cell’s surface. The effective uptake of EVs by cells is crucial for their biological activity. However, the precise mechanism underlying the uptake of EVs by recipient cells remains incompletely understood. Recent research suggests that the uptake mechanism primarily involves pinocytosis, which can be categorized into clathrin-dependent endocytosis (CDE), clathrin-independent endocytosis (CIE), and macropinocytosis (MP), among which CIE and MP are the most common modalities [17][18][19][20]. Increasingly, studies have shown that EVs have multiple physiological functions, such as regulating the body’s immune response, promoting tissue regeneration and repair, and neural communication [21]. Due to their excellent biocompatibility, long-term stability, and low immunogenicity, EVs have attracted widespread exploration and application, especially in the field of bone regeneration [22].
2. Common Sources of EVs for Bone Regeneration
2.1. Immune Cells
Immune cells in the bone microenvironment release cytokines and paracrine factors that exhibit activating or inhibitory responses to bone-associated cells. Neutrophils are the most abundant white blood cells in the blood circulation, and they are also one of the first types of immune cells recruited in the microenvironment of bone injury and inflammatory response. Studies have shown that Thrombospondin-1 (TSP-1), an acellular glycoprotein associated with blood clot formation and angiogenesis, is strongly expressed in response to the stimulation of neutrophil-derived exosomes [23]. TSP-1 can trigger CD36-dependent signal that reduces the sensitivity of platelets to PGE-1 stimulated by endothelium-derived mediators, thereby impairing their ability to inhibit platelets [24]. Mast cells are widely distributed around microvessels in the skin and visceral submucosa, which promote the secretion of coagulation factors in the inflammatory process and participate in immune regulation. When activated, mast cell-derived exosomes can activate endothelial cells to secrete plasminogen activator inhibitor type 1 (PAI-1) [25]. Dendritic cells (DCs) can regulate the initiation of adaptive immunity by secreting EVs containing major histocompatibility complex (MHC) class I and II molecules to activate cognate T cells and promote humoral responses. Studies have shown that dendritic cell-derived EVs can induce osteogenesis [26]. Their exosomes contain immunomodulators, such as transforming growth factor-beta 1 (TGF-β1) and interleukin-10 (IL-10), which can be released in response to inflammation, promoting the recruitment of regulatory T cells to inhibit osteoclasts and reduce bone loss [27]. Macrophages are a ubiquitous cell type in vertebrate tissues, serving as a primary defense against pathogens by phagocytosing microorganisms, infected particles, and dead cells [28]. Their differentiation into M1 or M2 phenotypes is modulated by the local environment, with exosomes derived from macrophages reflecting their respective phenotypic characteristics [29] (Figure 2). These exosomes contain distinct biological information, resulting in unique functions; for instance, M2-Exos have been shown to contain higher levels of miR-365, whereas miR-326 is more abundant in M1-Exos [30][31]. Notably, no biomarkers have been identified to distinguish M1-Exos from M2-Exos [32]. In a study aimed at promoting osteogenesis, Chen et al. combined M2 macrophage-derived exosomes and stromal cell-derived factor-1α (SDF-1α) with hydrogels, yielding a hydrogel with good biocompatibility, hemostatic ability, and healing promotion. In vitro experiments revealed that the hydrogel could facilitate the proliferation and migration of human bone marrow mesenchymal stem cells and human umbilical vein endothelial cells, ultimately promoting osteogenesis and angiogenesis [33].
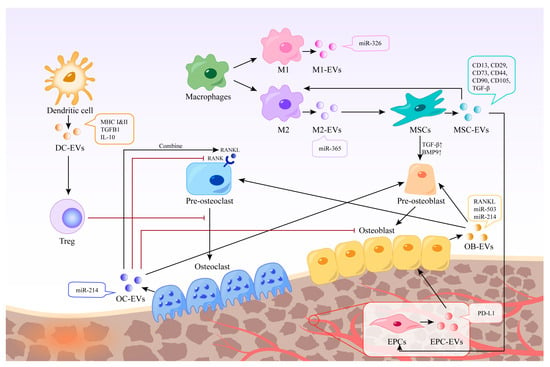
Figure 2. Different EVs in bone regeneration. EVs derived from various cells possess distinct contents and functions. Certain EVs can act directly on bone cells, whereas others can indirectly stimulate bone regeneration by regulating immune cells, inhibiting osteoclasts, and promoting endothelial cell regeneration. The black arrow represents the process of EVs production and action, and the red arrow represents the inhibitory effect of EVs or cells.
2.2. Stem Cells
Mesenchymal stem cells (MSCs) are multipotent stromal cells with various sources, such as bone marrow-derived mesenchymal stem cells (BMSCs), adipose-derived mesenchymal stem cells (ASCs), umbilical cord-derived mesenchymal stem cells (UMSCs), and others [34][35][36]. BMSCs have been widely used in bone regeneration strategies due to their osteogenic capacity [37]. EVs derived from stem cells have been shown to have stem cell-like regenerative functions. Thus, using EVs instead of stem cells to treat tissue defects can avoid the side effects of stem cell therapy, such as immune response and tumor formation [38]. EVs are also easier to store and transport. In addition to common surface markers such as CD9 and CD81, exosomes derived from MSCs also express CD73, CD44, and CD90, which are characteristic markers of MSCs [39]. Characterization of BMSC-derived exosome contents based on proteomics identified 730 functional proteins, including proteins that control the growth, proliferation, adhesion, migration, and morphogenesis capacities of MSCs [40]. These extracellular vesicles can promote the expression of osteogenic growth factors and bone-related proteins, and increase calcium deposition and matrix mineralization in vitro [41][42][43]. BMSC-derived EVs showed characteristic markers CD13, CD29, CD44, CD73, CD90, and CD105 [44], which can up-regulate the expression of TGF-β1 and bone morphogenetic protein 9 (BMP9), thereby promoting the differentiation of osteoblasts [45]. Qin et al. isolated BMSC-derived EVs and found that they positively regulate osteogenic genes and osteoblast differentiation in vitro. In vivo, experiments using rats with skull defects showed that EVs lead to more bone formation in bone defects, and miR-196a may play a crucial role [46].
2.3. Bone Cells
Bone homeostasis is regulated by interactions among osteoblasts, osteocytes, and osteoclasts and their surrounding microenvironment [47]. Exosomes from bone cells, immune cells, mesenchymal stem cells, and endothelial cells have been shown to affect bone formation and resorption, potentially influencing the development of bone-related diseases [48]. Osteoclasts are multinucleated cells derived from bone marrow monocytes and macrophages responsible for bone resorption. EVs derived from mature osteoclasts contain competitive inhibitors of receptor activator of nuclear factor kappa-Β (NF-κB), which inhibit osteoclast generation in the same environment [49]. Moreover, EVs released by mature osteoclasts can bind to receptor activator of nuclear factor-kappa B ligand (RANKL) on the surface of osteoblasts and trigger the RANKL reverse signaling pathway, thereby activating the key Runt-related transcription factor 2 (Runx2) and promoting bone formation [50]. Osteoclast-derived exosomes have been shown to promote osteogenic differentiation of stromal cells before osteogenesis [51]. However, it has also been shown to inhibit their differentiation and lead to reduced bone formation by being internalized in osteoblasts through EphrinA2/EphA2 recognition [52]. Li et al. found that miR-214-3p levels in osteoclasts were elevated in ovariectomized mice and elderly women with fractures, and that miR-214-3p in osteoclast-derived EVs was able to transfer to osteoblasts in vitro to inhibit osteoblast activity and reduce bone formation in vivo [53]. Osteoblasts are resident bone cells derived from bone marrow mesenchymal stem cells and are responsible for bone matrix synthesis and mineralization by releasing collagen and glycoproteins. Mineralized osteoblast-derived exosomes have been shown to induce osteogenic differentiation through activation of the Wnt signaling pathway, calcium signaling, and regulation of microRNA profiling [54]. Meanwhile, osteoblast-derived exosomes are also rich in RANKL protein, which can stimulate osteoclast differentiation through the RANKL-RANK signaling pathway and lead to nuclear translocation of nuclear factor of activated T cells, cytoplasmic 1 (NFATc1), a major transcriptional regulator of osteoclast differentiation [55]. In contrast, another study showed that mineralized osteoblasts were able to release EVs containing miR-503-3p, which impaired osteogenesis by inhibiting RANK expression [56]. This may be due to the heterogeneity of EVs, and the mechanisms regulating the switch between bone formation and bone resorption are not fully understood [57].
2.4. Endothelial Cells
Angiogenesis plays a crucial role in the bone regeneration microenvironment. Exosomes derived from endothelial cells can target osteocytes and stimulate bone regeneration [58]. Studies have demonstrated that exosomes derived from endothelial progenitor cells (EPCs) can promote angiogenesis through the Raf/ERK signaling pathway, thereby accelerating bone formation [59]. Moreover, EPCs were found to enhance healing and neovascularization in a mouse fracture model by recruiting osteoclast precursors. EPC-derived exosomes have also been shown to have a positive impact in animal models of osteoporosis, mainly through the high ferritin pathway in osteoblasts [60].
3. Functions of EVs
EVs are involved in promoting bone regeneration in various ways, including regulation of the immune environment, promotion of angiogenesis, differentiation of osteoblasts and osteoclasts, and promotion of bone mineralization (Figure 3).
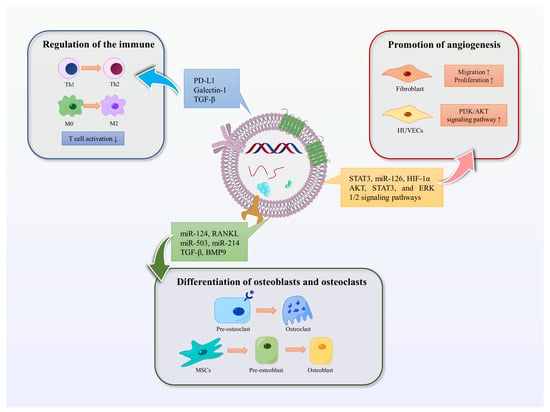
Figure 3. Functions of EVs. The functions of EVs in promoting bone regeneration include regulating immunity, promoting angiogenesis, and promoting osteoblast and osteoclast differentiation. Blue arrows represent the immunomodulatory effect of EVs in bone regeneration, red represents the promotion of angiogenesis, and green represents the effect on osteoblasts or osteoclasts.
3.1. Mediating Immune Stimulation or Immunosuppression
Moderate inflammatory response is necessary in the early stage of bone injury, while hyperactive and persistent inflammation can hinder bone regeneration and lead to inflammatory injury. EVs have the potential to act as immunomodulatory messengers by mediating immune stimulation or immunosuppression [61]. MSC-derived exosomes can influence the activity of immune cells, including T cells, B cells, NK cells, and macrophages. A clinical study has shown that MSC-derived exosomes may reduce the ability of peripheral blood mononuclear cells (PBMCs) to release proinflammatory cytokines in vivo. MSC-derived exosomes upregulate IL-10 and TGF-β1 in PBMCs, thereby promoting the proliferation and immunosuppressive capacity of Tregs to reduce inflammatory damage [62]. In addition, human umbilical vein endothelial cells (HUVECs) -derived exosomes contain a high concentration of programmed death ligand-1 (PD-L1). Exosomes overexpressing PD-L1 can specifically bind to programmed death-1 (PD-1) on T cells, inhibit the activation of T cells, and promote callus formation and fracture healing [63]. Studies have shown that mesenchymal cell-derived microvesicles (MVs) can deliver several immunomodulators such as PD-L1, galectin-1, and TGF-β, which can inhibit self-reactive cells and suppress their mediated tissue damage, induce peripheral tolerance, and modulate immune responses [64]. Furthermore, MSC-derived exosomes have been found to inhibit the concentrations of pro-inflammatory cytokines such as interleukin-1 beta (IL-1β) and tumor necrosis factor-alpha (TNF-α), while the secretion of TGF-β is increased. This induces the conversion of Th1 to Th2 cells and inhibits the pro-inflammatory response, thereby reducing inflammation and promoting anti-inflammatory response in a similar manner to MSCs [65]. In addition, M2-type macrophages have an anti-inflammatory phenotype and are mainly responsible for tissue remodeling during macrophage polarization. Studies have shown that MSC-derived microvesicles can promote the polarization of monocytes to M2-type macrophages, thereby mediating tissue repair [66].
3.2. Promotion of Angiogenesis
EVs can also play a crucial role in promoting angiogenesis. In vitro studies have shown that BMSC-Exos can promote fibroblast migration and proliferation through signaling pathways involving AKT, STAT3, and ERK 1/2 [67]. Furthermore, BMSCs are enriched in transcriptionally active STAT3, a transcription factor that is involved in angiogenesis, proliferation, migration, and growth factor production. iPS-MSC-Exos, which are secreted by induced pluripotent stem cell-derived mesenchymal stem cells, have been shown to have great potential in treating ischemic tissues. Liu et al. found that after intravenous injection into a rat model of steroid-induced osteonecrosis, iPS-MSC-Exos significantly prevented bone loss and promoted angiogenesis in the femur [68]. Hypoxic preconditioning can enhance the regenerative capacity of stem cells. Ding et al. reported that miR-126 was significantly upregulated in BMSC-Exos under hypoxic conditions compared to the normal group. MiR-126, which is involved in the process of angiogenesis, can induce the activation of the PI3K/AKT pathway in HUVECs, thereby promoting the formation of new blood vessels [69]. Moreover, research has shown that transplantation of umbilical cord MSC-derived exosomes (uMSC-Exos) combined with hydrogel into the site of injury in a rat model of femur fracture resulted in uMSC-Exos promoting bone healing through hypoxia-inducible factor 1α (HIF-1α)-mediated pro-angiogenic effects [70].
3.3. Differentiation of Osteoblasts and Osteoclasts
EVs play a role in promoting the differentiation of bone marrow mesenchymal stem cells into osteoblasts and osteoclasts, thus maintaining the balance of bone metabolism [71]. Paracrine signaling mediated by EVs regulates bone homeostasis by affecting osteoblasts and osteoclasts [72]. Furthermore, miR-214-3p in osteoblast-derived exosomes can be transferred to osteoblasts, inhibiting osteoblast activity in vitro and reducing bone formation in vivo [53]. Annexins and sodium-dependent inorganic phosphate transporters transport calcium and phosphate for the initial formation and accumulation of hydroxyapatite crystals in matrix vesicles. These vesicles later release these crystals into the extracellular fluid inducing calcification following collagen calcification [73][74].
References
- Abbasi, N.; Hamlet, S.; Love, R.M.; Nguyen, N. Porous scaffolds for bone regeneration. J. Sci. Adv. Mater. Devices 2020, 5, 1–9.
- Zhao, J.; Zhou, C.; Xiao, Y.; Zhang, K.; Zhang, Q.; Xia, L.; Jiang, B.; Jiang, C.; Ming, W.; Zhang, H.; et al. Oxygen generating biomaterials at the forefront of regenerative medicine: Advances in bone regeneration. Front. Bioeng. Biotechnol. 2024, 12, 1292171.
- Martin, V.; Bettencourt, A. Bone regeneration: Biomaterials as local delivery systems with improved osteoinductive properties. Mater. Sci. Eng. C 2018, 82, 363–371.
- Zhang, Y.; Ma, W.; Zhan, Y.; Mao, C.; Shao, X.; Xie, X.; Wei, X.; Lin, Y. Nucleic acids and analogs for bone regeneration. Bone Res. 2018, 6, 37.
- Deng, C.; Zhu, H.; Li, J.; Feng, C.; Yao, Q.; Wang, L.; Chang, J.; Wu, C. Bioactive Scaffolds for Regeneration of Cartilage and Subchondral Bone Interface. Theranostics 2018, 8, 1940–1955.
- Einhorn, T.A.; Gerstenfeld, L.C. Fracture healing: Mechanisms and interventions. Nat. Rev. Rheumatol. 2015, 11, 45–54.
- Wong, R.C.W.; Tideman, H.; Kin, L.; Merkx, M.A.W. Biomechanics of mandibular reconstruction: A review. Int. J. Oral Maxillofac. Surg. 2010, 39, 313–319.
- Li, Y.; Chen, M.; Zhao, Y.; Li, M.; Qin, Y.; Cheng, S.; Yang, Y.; Yin, P.; Zhang, L.; Tang, P. Advance in Drug Delivery for Ageing Skeletal Muscle. Front. Pharmacol. 2020, 11, 1016.
- Huber, J.; Griffin, M.F.; Longaker, M.T.; Quarto, N. Exosomes: A Tool for Bone Tissue Engineering. Tissue Eng. Part B Rev. 2022, 28, 101–113.
- Gyorgy, B.; Szabo, T.G.; Pasztoi, M.; Pal, Z.; Misjak, P.; Aradi, B.; Laszlo, V.; Pallinger, E.; Pap, E.; Kittel, A.; et al. Membrane vesicles, current state-of-the-art: Emerging role of extracellular vesicles. Cell Mol. Life Sci. 2011, 68, 2667–2688.
- Malda, J.; Boere, J.; van de Lest, C.H.; van Weeren, P.; Wauben, M.H. Extracellular vesicles—New tool for joint repair and regeneration. Nat. Rev. Rheumatol. 2016, 12, 243–249.
- Geeurickx, E.; Tulkens, J.; Dhondt, B.; Van Deun, J.; Lippens, L.; Vergauwen, G.; Heyrman, E.; De Sutter, D.; Gevaert, K.; Impens, F.; et al. The generation and use of recombinant extracellular vesicles as biological reference material. Nat. Commun. 2019, 10, 3288.
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 19.
- Ma, L.; Li, Y.; Peng, J.; Wu, D.; Zhao, X.; Cui, Y.; Chen, L.; Yan, X.; Du, Y.; Yu, L. Discovery of the migrasome, an organelle mediating release of cytoplasmic contents during cell migration. Cell Res. 2015, 25, 24–38.
- Zhao, X.; Lei, Y.; Zheng, J.; Peng, J.; Li, Y.; Yu, L.; Chen, Y. Identification of markers for migrasome detection. Cell Discov. 2019, 5, 27.
- Strzyz, P. Migrasomes promote angiogenesis. Nat. Rev. Mol. Cell Biol. 2023, 24, 84.
- Feng, D.; Zhao, W.; Ye, Y.; Bai, X.; Liu, R.; Chang, L.; Zhou, Q.; Sui, S. Cellular Internalization of Exosomes Occurs through Phagocytosis. Traffic 2010, 11, 675–687.
- Doherty, G.J.; McMahon, H.T. Mechanisms of Endocytosis. Annu. Rev. Biochem. 2009, 78, 857–902.
- Jadli, A.S.; Ballasy, N.; Edalat, P.; Patel, V.B. Inside(sight) of tiny communicator: Exosome biogenesis, secretion, and uptake. Mol. Cell. Biochem. 2020, 467, 77–94.
- Costa Verdera, H.; Gitz-Francois, J.J.; Schiffelers, R.M.; Vader, P. Cellular uptake of extracellular vesicles is mediated by clathrin-independent endocytosis and macropinocytosis. J. Control. Release 2017, 266, 100–108.
- Yáñez-Mó, M.; Siljander, P.R.M.; Andreu, Z.; Bedina Zavec, A.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066.
- Liu, M.; Sun, Y.; Zhang, Q. Emerging Role of Extracellular Vesicles in Bone Remodeling. J. Dent. Res. 2018, 97, 859–868.
- Xiao, Y.; Ding, Y.; Zhuang, J.; Sun, R.; Sun, H.; Bai, L. Osteoimmunomodulation role of exosomes derived from immune cells on osseointegration. Front. Bioeng. Biotechnol. 2022, 10, 989537.
- Roberts, W.; Magwenzi, S.; Aburima, A.; Naseem, K.M. Thrombospondin-1 induces platelet activation through CD36-dependent inhibition of the cAMP/protein kinase A signaling cascade. Blood 2010, 116, 4297–4306.
- Al-Nedawi, K.; Szemraj, J.; Cierniewski, C.S. Mast Cell–Derived Exosomes Activate Endothelial Cells to Secrete Plasminogen Activator Inhibitor Type 1. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1744–1749.
- Wang, Z.; Ding, L.; Zheng, X.L.; Wang, H.X.; Yan, H.M. DC-derived exosomes induce osteogenic differentiation of mesenchymal stem cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2014, 22, 600–604.
- Elashiry, M.; Elashiry, M.M.; Elsayed, R.; Rajendran, M.; Auersvald, C.; Zeitoun, R.; Rashid, M.H.; Ara, R.; Meghil, M.M.; Liu, Y.; et al. Dendritic cell derived exosomes loaded with immunoregulatory cargo reprogram local immune responses and inhibit degenerative bone disease in vivo. J. Extracell. Vesicles 2020, 9, 1795362.
- Verdeguer, F.; Aouadi, M. Macrophage heterogeneity and energy metabolism. Exp. Cell Res. 2017, 360, 35–40.
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage Activation and Polarization: Nomenclature and Experimental Guidelines. Immunity 2014, 41, 14–20.
- Binenbaum, Y.; Fridman, E.; Yaari, Z.; Milman, N.; Schroeder, A.; Ben David, G.; Shlomi, T.; Gil, Z. Transfer of miRNA in Macrophage-Derived Exosomes Induces Drug Resistance in Pancreatic Adenocarcinoma. Cancer Res. 2018, 78, 5287–5299.
- Bai, Z.; Li, H.; Li, C.; Sheng, C.; Zhao, X. M1 Macrophage-Derived Exosomal MicroRNA-326 Suppresses Hepatocellular Carcinoma Cell Progression via Mediating NF-κB Signaling Pathway. Nanoscale Res. Lett. 2020, 15, 221.
- Shan, X.; Zhang, C.; Mai, C.; Hu, X.; Cheng, N.; Chen, W.; Peng, D.; Wang, L.; Ji, Z.; Xie, Y. The Biogenesis, Biological Functions, and Applications of Macrophage-Derived Exosomes. Front. Mol. Biosci. 2021, 8, 715461.
- Chen, L.; Yu, C.; Xiong, Y.; Chen, K.; Liu, P.; Panayi, A.C.; Xiao, X.; Feng, Q.; Mi, B.; Liu, G. Multifunctional hydrogel enhances bone regeneration through sustained release of Stromal Cell-Derived Factor-1α and exosomes. Bioact. Mater. 2022, 25, 460–471.
- Zhang, Y.; Xie, Y.; Hao, Z.; Zhou, P.; Wang, P.; Fang, S.; Li, L.; Xu, S.; Xia, Y. Umbilical Mesenchymal Stem Cell-Derived Exosome-Encapsulated Hydrogels Accelerate Bone Repair by Enhancing Angiogenesis. ACS Appl. Mater. Interfaces 2021, 13, 18472–18487.
- Liu, J.; Gao, J.; Liang, Z.; Gao, C.; Niu, Q.; Wu, F.; Zhang, L. Mesenchymal stem cells and their microenvironment. Stem Cell Res. Ther. 2022, 13, 429.
- Qi, X.; Zhang, J.; Yuan, H.; Xu, Z.; Li, Q.; Niu, X.; Hu, B.; Wang, Y.; Li, X. Exosomes Secreted by Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Repair Critical-Sized Bone Defects through Enhanced Angiogenesis and Osteogenesis in Osteoporotic Rats. Int. J. Biol. Sci. 2016, 12, 836–849.
- Liao, H. Osteogenic potential: Comparison between bone marrow and adipose-derived mesenchymal stem cells. World J. Stem Cells 2014, 6, 288.
- Girón, J.; Maurmann, N.; Pranke, P. The role of stem cell-derived exosomes in the repair of cutaneous and bone tissue. J. Cell. Biochem. 2022, 123, 183–201.
- Rezabakhsh, A.; Sokullu, E.; Rahbarghazi, R. Applications, challenges and prospects of mesenchymal stem cell exosomes in regenerative medicine. Stem Cell Res. Ther. 2021, 12, 521.
- Kim, H.; Choi, D.; Yun, S.J.; Choi, S.; Kang, J.W.; Jung, J.W.; Hwang, D.; Kim, K.P.; Kim, D. Proteomic Analysis of Microvesicles Derived from Human Mesenchymal Stem Cells. J. Proteome Res. 2012, 11, 839–849.
- Takeuchi, R.; Katagiri, W.; Endo, S.; Kobayashi, T. Exosomes from conditioned media of bone marrow-derived mesenchymal stem cells promote bone regeneration by enhancing angiogenesis. PLoS ONE 2019, 14, e225472.
- Xu, T.; Luo, Y.; Wang, J.; Zhang, N.; Gu, C.; Li, L.; Qian, D.; Cai, W.; Fan, J.; Yin, G. Exosomal miRNA-128-3p from mesenchymal stem cells of aged rats regulates osteogenesis and bone fracture healing by targeting Smad5. J. Nanobiotechnol. 2020, 18, 47.
- Li, X.; Zheng, Y.; Hou, L.; Zhou, Z.; Huang, Y.; Zhang, Y.; Jia, L.; Li, W. Exosomes derived from maxillary BMSCs enhanced the osteogenesis in iliac BMSCs. Oral Dis. 2020, 26, 131–144.
- Ramos, T.L.; Sánchez-Abarca, L.I.; Muntión, S.; Preciado, S.; Puig, N.; López-Ruano, G.; Hernández-Hernández, Á.; Redondo, A.; Ortega, R.; Rodríguez, C.; et al. MSC surface markers (CD44, CD73, and CD90) can identify human MSC-derived extracellular vesicles by conventional flow cytometry. Cell Commun. Signal. 2016, 14, 2.
- Narayanan, R.; Huang, C.; Ravindran, S. Hijacking the Cellular Mail: Exosome Mediated Differentiation of Mesenchymal Stem Cells. Stem Cells Int. 2016, 2016, 3808674.
- Qin, Y.; Wang, L.; Gao, Z.; Chen, G.; Zhang, C. Bone marrow stromal/stem cell-derived extracellular vesicles regulate osteoblast activity and differentiation in vitro and promote bone regeneration in vivo. Sci. Rep. 2016, 6, 21961.
- Al-Bari, A.A.; Al, M.A. Current advances in regulation of bone homeostasis. FASEB Bioadv. 2020, 2, 668–679.
- Hu, Y.; Wang, Y.; Chen, T.; Hao, Z.; Cai, L.; Li, J. Exosome: Function and Application in Inflammatory Bone Diseases. Oxidative Med. Cell. Longev. 2021, 2021, 6324912.
- Huynh, N.; VonMoss, L.; Smith, D.; Rahman, I.; Felemban, M.F.; Zuo, J.; Rody, W.J.; McHugh, K.P.; Holliday, L.S. Characterization of Regulatory Extracellular Vesicles from Osteoclasts. J. Dent. Res. 2016, 95, 673–679.
- Ikebuchi, Y.; Aoki, S.; Honma, M.; Hayashi, M.; Sugamori, Y.; Khan, M.; Kariya, Y.; Kato, G.; Tabata, Y.; Penninger, J.M.; et al. Coupling of bone resorption and formation by RANKL reverse signalling. Nature 2018, 561, 195–200.
- Chen, C.; Zheng, R.Q.; Cao, X.C.; Zhang, G.C. Biological characteristics of osteoclast exosomes and their role in the osteogenic differentiation of somatic cells prior to osteogenesis. J. Biol. Regul. Homeost. Agents 2018, 32, 815.
- Sun, W.; Zhao, C.; Li, Y.; Wang, L.; Nie, G.; Peng, J.; Wang, A.; Zhang, P.; Tian, W.; Li, Q.; et al. Osteoclast-derived microRNA-containing exosomes selectively inhibit osteoblast activity. Cell Discov. 2016, 2, 16015.
- Li, D.; Liu, J.; Guo, B.; Liang, C.; Dang, L.; Lu, C.; He, X.; Cheung, H.Y.; Xu, L.; Lu, C.; et al. Osteoclast-derived exosomal miR-214-3p inhibits osteoblastic bone formation. Nat. Commun. 2016, 7, 10872.
- Cui, Y.; Luan, J.; Li, H.; Zhou, X.; Han, J. Exosomes derived from mineralizing osteoblasts promote ST2 cell osteogenic differentiation by alteration of microRNA expression. FEBS Lett. 2016, 590, 185–192.
- Deng, L.; Wang, Y.; Peng, Y.; Wu, Y.; Ding, Y.; Jiang, Y.; Shen, Z.; Fu, Q. Osteoblast-derived microvesicles: A novel mechanism for communication between osteoblasts and osteoclasts. Bone 2015, 79, 37–42.
- Chen, C.; Cheng, P.; Xie, H.; Zhou, H.; Wu, X.; Liao, E.; Luo, X. MiR-503 Regulates Osteoclastogenesis via Targeting RANK. J. Bone Miner. Res. 2014, 29, 338–347.
- Tamura, T.; Yoshioka, Y.; Sakamoto, S.; Ichikawa, T.; Ochiya, T. Extracellular vesicles in bone homeostasis: Key roles of physiological and pathological conditions. J. Bone Miner. Metab. 2022, 41, 345–357.
- Vig, S.; Fernandes, M.H. Bone Cell Exosomes and Emerging Strategies in Bone Engineering. Biomedicines 2022, 10, 767.
- Jia, Y.; Zhu, Y.; Qiu, S.; Xu, J.; Chai, Y. Exosomes secreted by endothelial progenitor cells accelerate bone regeneration during distraction osteogenesis by stimulating angiogenesis. Stem Cell Res. Ther. 2019, 10, 12.
- Song, H.; Li, X.; Zhao, Z.; Qian, J.; Wang, Y.; Cui, J.; Weng, W.; Cao, L.; Chen, X.; Hu, Y.; et al. Reversal of Osteoporotic Activity by Endothelial Cell-Secreted Bone Targeting and Biocompatible Exosomes. Nano Lett. 2019, 19, 3040–3048.
- Silva, A.M.; Teixeira, J.H.; Almeida, M.I.; Gonçalves, R.M.; Barbosa, M.A.; Santos, S.G. Extracellular Vesicles: Immunomodulatory messengers in the context of tissue repair/regeneration. Eur. J. Pharm. Sci. 2017, 98, 86–95.
- Du, Y.; Zhuansun, Y.; Chen, R.; Lin, L.; Lin, Y.; Li, J. Mesenchymal stem cell exosomes promote immunosuppression of regulatory T cells in asthma. Exp. Cell Res. 2018, 363, 114–120.
- Lin, Z.; Xiong, Y.; Meng, W.; Hu, Y.; Chen, L.; Chen, L.; Xue, H.; Panayi, A.C.; Zhou, W.; Sun, Y.; et al. Exosomal PD-L1 induces osteogenic differentiation and promotes fracture healing by acting as an immunosuppressant. Bioact. Mater. 2022, 13, 300–311.
- Mokarizadeh, A.; Delirezh, N.; Morshedi, A.; Mosayebi, G.; Farshid, A.; Mardani, K. Microvesicles derived from mesenchymal stem cells: Potent organelles for induction of tolerogenic signaling. Immunol. Lett. 2012, 147, 47–54.
- Chen, W.; Huang, Y.; Han, J.; Yu, L.; Li, Y.; Lu, Z.; Li, H.; Liu, Z.; Shi, C.; Duan, F.; et al. Immunomodulatory effects of mesenchymal stromal cells-derived exosome. Immunol. Res. 2016, 64, 831–840.
- Chu, C.; Deng, J.; Sun, X.; Qu, Y.; Man, Y. Collagen Membrane and Immune Response in Guided Bone Regeneration: Recent Progress and Perspectives. Tissue Eng. Part B Rev. 2017, 23, 421–435.
- Shabbir, A.; Cox, A.; Rodriguez-Menocal, L.; Salgado, M.; Badiavas, E.V. Mesenchymal Stem Cell Exosomes Induce Proliferation and Migration of Normal and Chronic Wound Fibroblasts, and Enhance Angiogenesis In Vitro. Stem Cells Dev. 2015, 24, 1635–1647.
- Liu, X.; Li, Q.; Niu, X.; Hu, B.; Chen, S.; Song, W.; Ding, J.; Zhang, C.; Wang, Y. Exosomes Secreted from Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Prevent Osteonecrosis of the Femoral Head by Promoting Angiogenesis. Int. J. Biol. Sci. 2017, 13, 232–244.
- Ding, J.; Wang, X.; Chen, B.; Zhang, J.; Xu, J. Exosomes Derived from Human Bone Marrow Mesenchymal Stem Cells Stimulated by Deferoxamine Accelerate Cutaneous Wound Healing by Promoting Angiogenesis. BioMed Res. Int. 2019, 2019, 9742765.
- Zhang, Y.; Hao, Z.; Wang, P.; Xia, Y.; Wu, J.; Xia, D.; Fang, S.; Xu, S. Exosomes from human umbilical cord mesenchymal stem cells enhance fracture healing through HIF-1α-mediated promotion of angiogenesis in a rat model of stabilized fracture. Cell Prolif. 2018, 52, e12570.
- Liu, J.; Li, D.; Wu, X.; Dang, L.; Lu, A.; Zhang, G. Bone-derived exosomes. Curr. Opin. Pharmacol. 2017, 34, 64–69.
- Li, Q.; Huang, Q.; Wang, Y.; Huang, Q. Extracellular vesicle-mediated bone metabolism in the bone microenvironment. J. Bone Miner. Metab. 2018, 36, 1–11.
- Golub, E.E. Biomineralization and matrix vesicles in biology and pathology. Semin. Immunopathol. 2011, 33, 409–417.
- Chu, C.; Wei, S.; Wang, Y.; Wang, Y.; Man, Y.; Qu, Y. Extracellular vesicle and mesenchymal stem cells in bone regeneration: Recent progress and perspectives. J. Biomed. Mater. Res. Part A 2019, 107, 243–250.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
415
Revisions:
2 times
(View History)
Update Date:
01 Apr 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




