
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | MARINA VENTURINI COPETTI | -- | 1711 | 2024-03-28 15:09:53 | | | |
| 2 | Wendy Huang | Meta information modification | 1711 | 2024-03-29 01:53:28 | | |
Video Upload Options
Contamination caused by fungi stands out as a significant microbiological issue in the food industry, particularly leading to premature spoilage across various food segments, including the dry-fermented meat industry. The emergence of undesired fungi on product surfaces results in substantial economic losses. Once microorganisms infiltrate the food, contamination ensues, and their subsequent proliferation can adversely impact the product’s appearance, odor, flavor, and texture. This, in turn, leads to consumer rejection and negatively affects the commercial brand. Additionally, concerns persist regarding the potential presence of mycotoxins in these products. Given the detrimental effects of spoilage fungi in the food industry, practices such as thorough cleaning and sanitization become crucial to prevent contamination and subsequent premature deterioration. These measures play a pivotal role in ensuring the quality and safety of food, while also extending the shelf life of products.
1. Introduction
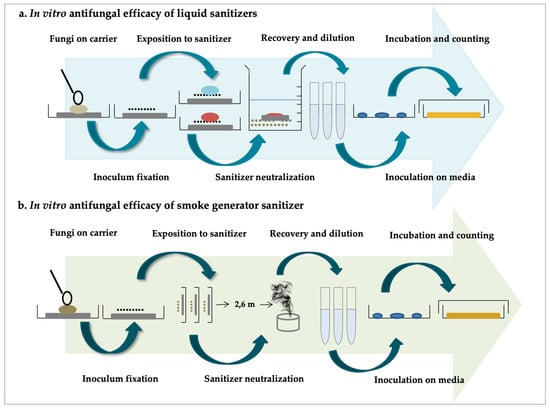
2. Sodium Hypochlorite
3. Peracetic Acid
4. Benzalkonium Chloride
References
- Perrone, G.; Rodriguez, A.; Magista, D.; Magan, N. Insights into existing and future fungal and mycotoxin contamination of cured meats. Curr. Opin. Food Sci. 2019, 29, 20–27.
- Asefa, D.T.; Kure, C.F.; Gjerde, R.O.; Omer, M.K.; Langsrud, S.; Nesbakken, T.; Skaar, I. Fungal growth patern, sources and factors of mould contamination in a dry-cured meat production facility. Int. J. Food Microbiol. 2010, 140, 131–135.
- Davies, C.R.; Wohlgemuth, F.; Young, T.; Violet, J.; Dickinson, M.; Sanders, J.W.; Vallieres, C.; Avery, S.V. Challenges and evolving strategies for mold control in the food supply chain. Fungal Biol. Rev. 2021, 36, 15–26.
- Krisch, J.; Tserennadmid, R.; VagvOlgyi, C. Essential oils against yeasts and molds that cause food spoilage. In Science against Microbial Pathogens: Communicating Current Research and Technological Advances; Mendez-Vilas, A., Ed.; FORMATEX: Badajoz, Spain, 2011; pp. 1135–1142.
- Visconti, V.; Coton, E.; Rigalma, K.; Dantigny, P. Effects of disinfectants on inactivation of mold spores relevant to the food industry: A review. Fungal Biol. Rev. 2021, 38, 44–66.
- Parussolo, G.; Oliveira, M.S.; Garcia, M.V.; Bernardi, A.O.; Lemos, J.G.; Stefanello, A.; Mallmann, C.A.; Copetti, M.V. Ochratoxin A production by Aspergillus westerdijkiae in Italian-type salami. Food Microbiol. 2019, 83, 134–140.
- Pitt, J.I.; Hocking, A.D. Fungi and Food Spoilage; Blackie Academic and Professional: London, UK, 2009; 593p.
- Battilani, P.; Pietri, A.; Giorni, P.; Formenti, S.; Bertuzzi, T.; Toscani, T.; Virgili, R.; Kozakiewicz, Z. Penicillium populations in dry-cured ham manufacturing plants. J. Food Prot. 2007, 70, 975–980.
- Sørensen, L.M.; Jacobsen, T.; Nielsen, P.V.; Frisvad, J.C.; Koch, A.G. Mycobiota in the processing areas of two different meat products. Int. J. Food Microbiol. 2008, 124, 58–64.
- Parussolo, G.; Bernardi, A.O.; Garcia, M.V.; Stefanello, A.; Silva, T.S.; Copetti, M.V. Fungi in air, raw materials and surface of dry fermented sausage produced in Brazil. LWT-Food Sci. Technol. 2019, 108, 190–198.
- de Almeida, T.S.; dos Santos, B.A.; Stefanello, A.; dos Santos, I.D.; Fracari, J.C.; Silva, M.; Giongo, C.; Wagner, R.; Nalério, E.S.; Copetti, M.V. Spontaneously growing fungi on the surface and processing areas of matured sheep ham and volatile compounds produced. Food Res. Int. 2023, 173, 113287.
- Scaramuzza, N.; Diaferia, C.; Berni, E. Monitoring the mycobiota of three plants manufacturing Culatello (a typical Italian meat product). Int. J. Food Microbiol. 2015, 203, 78–85.
- Andrade, M.J.; Peromingo, B.; Rodríguez, M.; Rodríguez, A. Effect of cured meat product ingredients on the Penicillium verrucosum growth and ochratoxin A production. Food Control 2018, 96, 310–317.
- Hayes, P.R. Microbiologia e Higiene de los Alimentos; Acribia: Zaragoza, Spain, 1993; 369p.
- Morelli, A.M.F. Escherichia coli 0157:H7: Occurrence in a Milk Production Environment in the Viçosa Microregion, Adhesion to Different Surfaces and Resistance to Sanitizers. Ph.D. Thesis, Postgraduate in Food Science and Technology, Federal University of Viçosa, UFV, Viçosa, Brazil, 2008; p. 173.
- Copetti, M.V. Sanitizers for controlling fungal spoilage in some food industries. Curr. Opin. Food Sci. 2023, 52, 101072.
- Bernardi, A.O.; Garcia, M.V.; Copetti, M.V. Food industry spoilage fungi control through facility sanitization. Curr. Opin. Food Sci. 2019, 29, 28–34.
- Bernardi, A.O.; Stefanello, A.; Garcia, M.V.; Parussolo, G.; Stefanello, R.F.; Moro, C.B.; Copetti, M.V. Efficacy of commercial sanitizers against fungi of concern in the food industry. LWT-Food Sci. Technol. 2018, 97, 25–30.
- Lee, W.-N.; Huang, C.-H. Formation of disinfection byproducts in wash water and lettuce by washing with sodium hypochlorite and peracetic acid sanitizers. Food Chem. X 2019, 1, 100003.
- Kuaye, A.Y. Limpeza e Sanitização na Indústria de Alimentos, 1st ed.; Atheneu: Rio de Janeiro, Brazil, 2017.
- European Standard No. 13697 (2001); Chemical Disinfectants and Antiseptics—Quantitative Non-Porous Surface Test for the Evaluation of Bactericidal and/or Fungicidal Activity of Chemical Disinfectants Used in Food, Industrial, Domestic, and Institutional Areas-Test Method and Requirements without Mechanical Action (Phase 2, Step 2). iTeh Standards: San Francisco, CA, USA, 2001.
- Park, K.; Mok, J.S.; Kwon, J.Y.; Ryu, A.R.; Kim, S.H.; Lee, H.J. Food-borne outbreaks, distributions, virulence, and antibiotic resistance profiles of Vibrio parahaemolyticus in Korea from 2003 to 2016: A review. Fish. Aquat. Sci. 2018, 21, 3.
- Bernardi, A.O.; Stefanello, A.; Garcia, M.V.; Copetti, M.V. The control of cheese and meat product spoilage fungi by sanitizers: In vitro testing and food industry usage. Lebensm.-Wiss. Technol. 2021, 144, 111204.
- Fukuzaki, S. Mechanisms of actions of sodium hypochlorite in cleaning and disinfection processes. Biocontrol Sci. 2006, 11, 147–157.
- Duarte, A.L.A.; Rosário, D.K.A.D.; Oliveira, S.B.S.; de Souza, H.L.S.; de Carvalho, R.V.; Carneiro, J.C.S.; Silva, P.I.; Bernardes, P.C. Ultrasound improves antimicrobial effect of sodium dichloroisocyanurate to reduce Salmonella Typhimurium on purple cabbage. Int. J. Food Microbiol. 2018, 269, 12–18.
- Resende, A.; Souza, P.I.M.D.; Souza, J.R.D.; Blum, L.E.B. Ação do hipoclorito de sódio no controle do Erysiphe diffusana soja. Rev. Caatinga 2009, 22, 53–59.
- Wang, D.; Fletcher, G.C.; On, S.L.; Palmer, J.S.; Gagic, D.; Flint, S.H. Biofilm formation, sodium hypochlorite susceptibility and genetic diversity of Vibrio parahaemolyticus. Int. J. Food Microbiol. 2023, 385, 110011.
- Petri, E.; Virto, R.; Mottura, M.; Parra, J. Comparison of peracetic acid and chlorine effectiveness during fresh-cut vegetable processing at industrial scale. J. Food Prot. 2021, 84, 1592–1602.
- Teng, Z.; Luo, Y.; Alborzi, S.; Zhou, B.; Chen, L.; Zhang, J.; Zhang, B.; Millner, P.; Wang, Q. Investigation on chlorine-based sanitization under stabilized conditions in the presence of organic load. Int. J. Food Microbiol. 2018, 266, 150–157.
- Pereira, S.S.P.; Oliveira, H.M.; Turrini, R.N.T.; Lacerda, R.A. Disinfection with sodium hypochlorite in hospital environmental surfaces in the reduction of contamination and infection prevention: A systematic review. Rev. Esc. Enferm. USP 2015, 49, 681–688.
- Su, Y.; Shen, X.; Chiu, T.; Green, T.; Zhu, M.-J. Efficacy of chlorine and peroxyacetic acid to control Listeria monocytogenes on apples in simulated dump tank water system. Food Microbiol. 2022, 106, 104033.
- Bernardi, A.O.; Stefanello, A.; Lemos, J.G.; Garcia, M.V.; Copetti, M.V. Antifungal activity of commercial sanitizers against strains of Penicillium roqueforti, Penicillium paneum, Hyphopichia burtonii, and Aspergillus pseudoglaucus: Bakery spoilage fungi. Food Microbiol. 2019, 83, 59–63.
- Bernardi, A.O.; da Silva, T.S.; Stefanello, A.; Garcia, M.V.; Parussolo, G.; Dornelles, R.C.P.; Copetti, M.V. Sensitivity of food spoilage fungi to a smoke generator sanitizer. Int. J. Food Microbiol. 2019, 289, 72–76.
- Stefanello, A.; Fracari, J.C.; Silva, M.; Lemos, J.G.; Garcia, M.V.; dos Santos, B.A.; Copetti, M.V. Influence of type, concentration, exposure time, temperature, and presence of organic load on the antifungal efficacy of industrial sanitizers against Aspergillus brasiliensis (ATCC 16404). Food Microbiol. 2021, 97, 103740.
- Stefanello, A.; Magrini, L.N.; Lemos, J.G.; Garcia, M.V.; Bernardi, A.O.; Cichoski, A.J.; Copetti, M.V. Comparison of electrolized water and multiple chemical sanitizer action against heat-resistant molds (HRM). Int. J. Food Microbiol. 2020, 335, 108856.
- Lemos, J.G.; Stefanello, A.; Bernardi, A.O.; Garcia, M.V.; Magrini, L.N.; Cichoski, A.J.; Wagner, R.; Copetti, M.V. Antifungal efficacy of sanitizers and electrolyzed waters against toxigenic Aspergillus. Food Res. Int. 2020, 137, 109451.
- Silva, S.; Stefanello, A.; Santos, B.; Fracari, J.; Leães, G.; Copetti, M. Factors that interfere in the action of sanitizers against ochratoxigenic fungi deteriorating dry-cured meat products. Fermentation 2023, 9, 83.
- Parish, M.; Beuchat, L.; Suslow, T.; Harris, L.; Garrett, E.; Farber, J.; Busta, F. Methods to reduce/eliminate pathogens from fresh and fresh-cut produce. Compr. Rev. Food Sci. Food Saf. 2003, 2, 161–173.
- Elhalwagy, M.; Biabani, R.; Bertanza, G.; Wisdom, B.; Goldman-Torres, J.; McQuarrie, J.; Straatman, A.; Santoro, D. Mechanistic modeling of peracetic acid wastewater disinfection using computational fluid dynamics: Integrating solids settling with microbial inactivation kinetics. Water Res. 2021, 201, 117355.
- Ao, X.-W.; Eloranta, J.; Huang, C.-H.; Santoro, D.; Sun, W.-J.; Lu, Z.-D.; Li, C. Peracetic acid-based advanced oxidation processes for decontamination and disinfection of water: A review. Water Res. 2021, 188, 116479.
- Fallik, E. Microbial quality and safety of fresh produce. In Postharvest Handling; Elsevier: Amsterdam, The Netherlands, 2014; pp. 313–339.
- Osaili, T.M.; Alaboudi, A.R.; Al-Quran, H.N.; Al-Nabulsi, A.A. Decontamination and survival of Enterobacteriaceae on shredded iceberg lettuce during storage. Food Microbiol. 2018, 73, 129–136.
- Singh, P.; Hung, Y.; Qi, H. Efficacy of Peracetic Acid in Inactivating Foodborne Pathogens on Fresh Produce Surface: Use of PAA to ensure produce safety. J. Food Sci. 2018, 83, 432–439.
- Srey, S.; Jahid, I.K.; Ha, S.-D. Biofilm formation in food industries: A food safety concern. Food Control 2013, 31, 572–585.
- Kim, J.; Huang, C.-H. Reactivity of peracetic acid with organic compounds: A critical review. ACS ES&T Water 2021, 1, 15–33.
- Lazado, C.C.; Sveen, L.R.; Soleng, M.; Pedersen, L.-F.; Timmerhaus, G. Crowding reshapes the mucosal but not the sys-temic response repertoires of Atlantic salmon to peracetic acid. Aquaculture 2021, 531, 735830.
- Du, P.; Liu, W.; Cao, H.; Zhao, H.; Huang, C.-H. Oxidation of amino acids by peracetic acid: Reaction kinetics, pathways and theoretical calculations. Water Res. X 2018, 1, 100002.
- Lieke, T.; Meinelt, T.; Hoseinifar, S.H.; Pan, B.; Straus, D.L.; Steinberg, C.E.W. Sustainable aquaculture requires environmental-friendly treatment strategies for fish diseases. Rev. Aquac. 2019, 12, 943–965.
- Izumi, H. Process Hygiene: Overall Approach to Hygienic Processing; Academic Press: Cambridge, MA, USA, 2014.
- Lazado, C.C.; Voldvik, V. Temporal control of responses to chemically induced oxidative stress in the gill mucosa of Atlantic salmon (Salmosalar). J. Photochem. Photobiol. B Biol. 2020, 205, 111851.
- Acosta, F.; Montero, D.; Izquierdo, M.; Galindo-Villegas, J. High-level biocidal products effectively eradicate pathogenic γ-proteobacteria biofilms from aquaculture facilities. Aquaculture 2021, 532, 736004.
- Banach, J.L.; Sampers, I.; Van Haute, S.; Van Der Fels-Klerx, H. Effect of disinfectants on preventing the cross-contamination of pathogens in fresh produce washing water. Int. J. Environ. Res. Public Health 2015, 12, 8658–8677.
- Kim, J.-M.; Zhang, B.-Z.; Park, J.-M. Comparison of sanitization efficacy of sodium hypochlorite and peroxyacetic acid used as disinfectants in poultry food processing plants. Food Control 2023, 152, 109865.
- da Silva Fernandes, M.; Kabuki, D.Y.; Kuaye, A.Y. Behavior of Listeria monocytogenes in a multi-species biofilm with Enterococcus faecalis and Enterococcus faecium and control through sanitation procedures. Int. J. Food Microbiol. 2015, 200, 5–12.
- Hrudey, S.E. Chlorination disinfection by-products, public health risk tradeoffs and me. Water Res. 2009, 43, 2057–2092.
- Wang, R.Y.; Shen, X.; Su, Y.; Critzer, F.; Zhu, M.-J. Chlorine and peroxyacetic acid inactivation of Listeria monocytogenes in simulated apple dump tank water. Food Control 2023, 144, 109314.
- Ahmad, R.; Cho, E.; Rakhmat, S.; Hyun, M.; Park, C.-B.; Kim, S. Characterization of structure isomers of ethylbenzalkyl dimethyl ammonium chlorides and quantification in commercial household disinfectant products. Environ. Technol. Innov. 2023, 29, 102979.
- Kuca, K.; Marek, J.; Stodulka, P.; Musilek, K.; Hanusova, P.; Hrabinova, M.; Jun, D. Preparation of benzalkonium salts differing in the length of a side alkyl chain. Molecules 2007, 12, 2341–2347.
- Gerba, C.P. Quaternary ammonium biocides: Efficacy in application. Appl. Environ. Microbiol. 2015, 81, 464–469.
- Prieto-Blanco, M.C.; Planas-Franco, A.; Muniategui-Lorenzo, S.; González-Castro, M.J. Mixed-mode chromatography of mixed functionalized analytes as the homologues of benzalkonium chloride. Application to pharmaceutical formulations. Talanta 2023, 255, 124228.
- Núñez, O.; Moyano, E.; Galceran, M.T. Determination of quaternary ammonium biocides by liquid chromatography–mass spectrometry. J. Chromatogr. A 2004, 1058, 89–95.
- Barber, O.W.; Hartmann, E.M. Benzalkonium chloride: A systematic review of its environmental entry through wastewater treatment, potential impact, and mitigation strategies. Crit. Rev. Environ. Sci. Technol. 2021, 52, 2691–2719.
- Makvandi, P.; Jamaledin, R.; Jabbari, M.; Nikfarjam, N.; Borzacchiello, A. Antibacterial quaternary ammonium compounds in dental materials: A systematic review. Dent. Mater. 2018, 34, 851–867.
- Zhang, C.; Cui, F.; Zeng, G.-M.; Jiang, M.; Yang, Z.-Z.; Yu, Z.-G.; Zhu, M.-Y.; Shen, L.-Q. Quaternary ammonium compounds (QACs): A review on occurrence, fate and toxicity in the environment. Sci. Total Environ. 2015, 518–519, 352–362.
- Lavorgna, M.; Russo, C.; D’Abrosca, B.; Parrella, A.; Isidori, M. Toxicity and genotoxicity of the quaternary ammonium compound benzalkonium chloride (BAC) using Daphnia magna and Ceriodaphnia dubia as model systems. Environ. Pollut. 2016, 210, 34–39.
- Wessels, S.; Ingmer, H. Modes of action of three disinfectant active substances: A review. Regul. Toxicol. Pharmacol. 2013, 67, 456–467.
- Schmidt, R.H. Basic Elements of Equipment Cleaning and Sanitizing in Food Processing and Handling Operations; University of Florida Cooperative Extension Service Institute of Food and Agriculture Sciences EDIS, 1997; Available online: http://purl.fcla.edu/UF/lib/FS077 (accessed on 19 January 2024).




