Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yiyi Sulaeman | -- | 4620 | 2024-03-26 16:13:10 | | | |
| 2 | Jason Zhu | Meta information modification | 4620 | 2024-03-27 03:51:29 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Sulaeman, Y.; Maftuáh, E.; Noor, M.; Hairani, A.; Nurzakiah, S.; Mukhlis, M.; Anwar, K.; Fahmi, A.; Saleh, M.; Khairullah, I.; et al. Coastal Acid-Sulfate Soils of Kalimantan, Indonesia. Encyclopedia. Available online: https://encyclopedia.pub/entry/56492 (accessed on 07 February 2026).
Sulaeman Y, Maftuáh E, Noor M, Hairani A, Nurzakiah S, Mukhlis M, et al. Coastal Acid-Sulfate Soils of Kalimantan, Indonesia. Encyclopedia. Available at: https://encyclopedia.pub/entry/56492. Accessed February 07, 2026.
Sulaeman, Yiyi, Eni Maftuáh, Muhammad Noor, Anna Hairani, Siti Nurzakiah, Mukhlis Mukhlis, Khairil Anwar, Arifin Fahmi, Muhammad Saleh, Izhar Khairullah, et al. "Coastal Acid-Sulfate Soils of Kalimantan, Indonesia" Encyclopedia, https://encyclopedia.pub/entry/56492 (accessed February 07, 2026).
Sulaeman, Y., Maftuáh, E., Noor, M., Hairani, A., Nurzakiah, S., Mukhlis, M., Anwar, K., Fahmi, A., Saleh, M., Khairullah, I., Rumanti, I.A., Alwi, M., Noor, A., & Ningsih, R.D. (2024, March 26). Coastal Acid-Sulfate Soils of Kalimantan, Indonesia. In Encyclopedia. https://encyclopedia.pub/entry/56492
Sulaeman, Yiyi, et al. "Coastal Acid-Sulfate Soils of Kalimantan, Indonesia." Encyclopedia. Web. 26 March, 2024.
Copy Citation
Coastal acid-sulfate soils are crucial for producing crops and thus, for food security. However, over time, these soil resources experience degradation, leading to higher agro-input, lower yields, and environmental hazards that finally threaten food security. The optimal use of this fragile resource is only attained by implementing vigorous integrated water–soil–crop management technologies amid the climate change impact.
acid-sulfate soil
rice
soil characteristic
soil management
tidal paddy field
1. Acid-Sulfate Soil Distribution and Selected Properties
1.1. Distribution
In Kalimantan (Indonesia), the swampland is about 10.0 Mha [1], of which 2.9 Mha is tidal and about 7.1 Mha is inland. Figure 1 shows the indicative distribution of ASSs in the coastal area of Kalimantan, covering about 3.5 Mha. In the West Kalimantan province, this soil is found in the coastal area, from Sambas Regency in the north to Singkawang, Bengkayang, Mempawah, Pontianak, Kuburaya, and Ketapang Regency in the south. ASSs are mainly found in the estuary of the Kapuas River, the longest river in Indonesia (12 km long), and the Bengkayang River (2 km long).
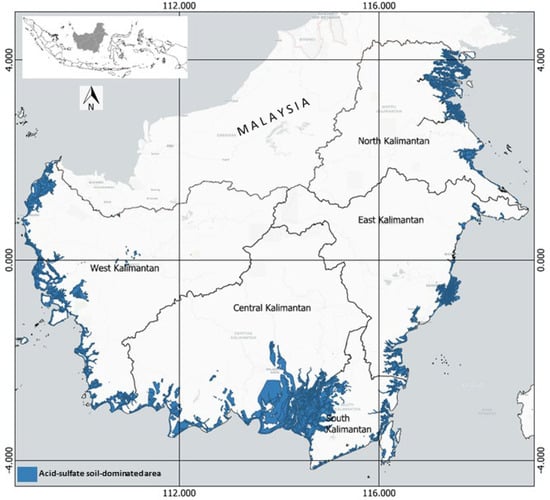
In Central Kalimantan province, ASSs extend along the coastal areas of Kotawaringin Barat Regency, Seruyan Regency, Pulangpisau Regency, and Kapuas Regency. The soils also extend to South Kalimantan province, mainly in Barito Kuala, Barito Selatan, Hulu Sungai Selatan, Tapin, Tanah Laut, Tanah Bumbu, and Kotabaru Regency.
On the eastern coast of Kalimantan, ASSs are found from the coastal area of Paser Regency in the south to Kutai Kertanegara Regency in the north of East Kalimantan province. In North Kalimantan province, these soils are found in the coastal areas of Nunukan, Tana Tidung, and Bulungan Regency.
1.2. Soil Properties
ASSs in Kalimantan are dominated by silt and clay fractions and mostly have a clay texture (the clay fraction is more than 35%). The soils are also categorized as heavy clay soils (clay fraction of more than 60%), for example in Tapin and Nunukan.
As expected, the pH of the ASSs is low (acidic), ranging from 2.6 to 5.1. The soil pH is very low in the layer containing sulfidic materials (pH 2.6–4.8). Meanwhile, sulfidic materials are found 20 cm below the surface in Kotawaringin Barat and Tana Tidung Regency, and 55 cm below the surface in Seruyan Regency. Sulfidic materials are found near the surface in several sites, such as Nunukan Regency.
Information on the depth of the sulfidic material, pyrite, is crucial in managing this soil for agriculture because pyrite (FeS2) is one of the primary sources of acidity [6][7]. Shamshuddin et al. [8] concluded that the oxidation of 1.0 moles of FeS2 produces 4.0 moles of H2SO4. Several factors affect changes in soil acidity due to pyrite oxidation, i.e., oxygen and ferric (Fe3+) availability, decomposable organic matter, the initial value of soil pH, base cation availability, pyrite content, and the hydrological condition of the land. However, soil moisture and the hydrological condition of the land are the main factors that determine soil acidity [9]. Variations in groundwater levels control pH and Eh. Decreased groundwater level or soil moisture during the dry season or due to drainage of the land leads to the oxidation of pyrite and other ferrous (Fe2+) species [9][10]. Conversely, flooded soil leads to reduced soil conditions, increasing soil pH [9][11].
Soil organic carbon (SOC) content also varies, ranging from 2.14 to 8.40%. In some areas, the soils are covered with peat soils, called peaty ASSs, such as in Kotawaringin Barat, Tapin, and Nunukan Regency. This organic matter is less than 50 cm thick, hence excluded from organic soils (Histosols). The peat material contains very high soil organic carbon ranging from 11.84 to 42.17%.
The cation exchange capacity (CEC) of soil is between 15.27 and 83.47 cmol kg−1, while base saturation (BS) varies between locations, ranging from 7% to 67%. High organic carbon and clay are responsible for this relatively high CEC. Variations in BS are closely associated with the variations in exchangeable Ca and exchangeable Mg. Soils from Sambas and Kotawaringin Barat Regency are low in exchangeable Ca and Mg, leading to lower BS. Meanwhile, the base cation content is alleviated and fertilizer application is required to support crop growth.
Nitrogen content varies from low to very high [6][12][13], with total nitrogen ranging from 0.17 to 1.04%. Soil pH is essential when determining nutrient availability and toxicity in these soils; low soil pH causes aluminum and iron solubility to increase, and the capacity for fixing phosphorus is large [14]; phosphorus adsorption capacity may reach 800 mg kg−1 [15]. However, flooding in these soils decreases Eh and solubilizes Fe oxides, increasing P availability [16].
During ASS formation, the natural oxidation of sulfide-bearing minerals and sulfuric acid attack clay minerals, resulting in changes to the clay mineral structure. The sulfuric acid lowers pH, which makes nutrients less available; low soil pH causes aluminum and iron solubility to increase, displacing K, Ca, and Mg from the exchange complex. Furthermore, the exchange complex contains aluminum and iron. Therefore, ASS is likely deficient in Ca and K. Soil pH was positively and significantly correlated with exchangeable K, Ca, and Mg content in the soil [17]. However, flooding ASSs increases the availability of K, Ca, and Mg due to the increase in soil pH and the precipitation of aluminum and iron [18][19].
Iron (Fe) is abundant in ASSs. Fe concentrations in flooded ASSs can reach 4700 mg kg−1 [15]. Fe solubility depends on environmental conditions, such as Eh, pH, organic matter, soil moisture, microorganisms, anion presence [8][9][12], and land management systems. Fe solubility is also influenced by its characteristics, such as specific surface area and solubility [20].
Aluminum toxicity is the most critical limiting factor for plant growth in ASSs. A substantial amount of H+ ions are supplied to the soil solution as a result of pyrite oxidation, and the acid reacts with soil minerals, dissolving Al in the soil solution. Al solubility is relatively higher at low pH [21]. Exchangeable Al in ASS ranges from 1.8 to 4.3 cmol kg−1. These levels are toxic to plants and limit the availability of essential nutrient elements such as P, Ca, and Mg [8].
Salinity occurs in soils inundated by seawater daily. These areas are generally covered with mangrove forests and are not used for rice cultivation. During prolonged droughts (such as El Nino), salt concentrations in water increase [22]. The level of seawater increases from the tide to the upper stream, leading to an increase in the coverage of salt-affected soil obstructing crop growth [23]. During the rainy season, salt concentrations return to normal and rice can be planted on land in that area. Haloculture is another system for the sustainable use of saline water for crop production [24].
For rice cultivation, ASS has several constraints because pyrite oxidation increases acidity. In addition, this land has a low content of macro and micronutrients [25] and iron toxicity that can decrease rice yield from 30 to 100% depending on variety tolerance levels, toxicity intensity, and soil fertility status [26]. Managing water conditions is an option for controlling pyrite oxidation. In fact, insights into hydrological characteristics for water management technology are the key to successful crop production in ASS-dominated agricultural land.
2. Hydrological Characteristics and Water Management
Sea tide activities control the hydrological characteristics of ASSs. Pushing spring tide weakens with distance from the estuary (river or primary canal estuary), leading to lower water potency that inundates the ASS. Such a condition is caused by increasing topography upstream and water is pushed by the tide to balance the conditions in the water. The wave decreases moving energy, impacting the irrigation and drainage potency.
In areas that are routinely inundated by big tides and small tides (Type A), water floods ASSs every day. The potency of pyrite oxidation is very low and hence rarely found in acidic water (water pH < 4.0). In areas inundated only during big tides (Type B), irrigation water is always available; however, some big tides cannot reach the ASSs during small tides in the dry season (DS). Such conditions trigger pyrite oxidation in the soil layer, forming acids from leached soil that accumulate in the quarter/tertiary canal during the early wet season (WS) and move to secondary and primary canals. Some areas are not inundated by spring tides but only seepage below the soil surface (Type C or D); the only water source is rainfall. The potency of pyrite oxidation is high in these areas, primarily during the DS, resulting in acidic soils. Leached acidic compounds (organic acids, Fe, Al) lead to much lower water pH in the canals than in the Type B areas. The influence of the spring tide on water pH is presented in Figure 2, where water acidity increases with distance from the estuary.
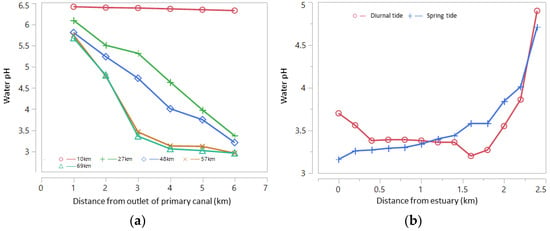
Figure 2. (a) Water pH at the peak of the spring tide based on distance from river/sea estuary in the primary canal along Barito River, South Kalimantan, measured at distances of 1, 2, 3, 4, 5 and 6 km from the canal estuary, and (b) water pH during the spring tide and the diurnal tide in the secondary canal of the Betaguh wetland irrigation region of Pulang Pisau Regency, Central Kalimantan, as measured on 10 June 2021. Source: primary data from Author/Khairil Anwar.
The depth of the pyrite layer also influences water quality in the ASS area. In the secondary canal (SC) edges with deep pyrite layers and more acidic irrigation water, the water pH will increase upstream of the SC. Mixing tidewater and drainage water in the SC leads to variations in water quality (Figure 3). In the SC, a regular pattern of water pH is more common during the diurnal tide than during the spring tide. The pH of the water in the primary canal (PC) is lower than that of the water in the SC during the diurnal tide; however, the pH of the water in the PC is higher than that of the water in the SC during the spring tide.
The water’s acidity level is an indicator that pyrite has been oxidized. In Type B ASS areas with poor drainage, water acidity negatively correlates with the water’s electrical conductivity (EC), Al3+, Fe2+, Mn2+, SO24−, Ca2+, Mg2+, Na+, and SiO2. Poor drainage in the PC leads to the accumulation of leached ions in the PC water body. Multazam et al. [27] confirmed that water pH is negatively correlated with the EC value. This correlation pattern differs from that in areas with better water circulation, as in the Type A area, where ions easily leach to the edge of the PC.
The sea surface is higher during the WS than during the DS [28]; therefore, the potency of tide overflow during the WS is higher than during the DS. Moreover, the volume of water in rivers, tributaries, and other water bodies in the upper region increases the tide overflow potency. Figure 3 shows the monthly rainfall patterns in South Kalimantan province, Indonesia, where the overflow potency is low from July to September. Overflow potency is associated with pyrite oxidation, the leaching of acids from the soil, and acidic compounds in the water canal. Therefore, pyrite oxidation occurs during the DS, and acids leach at the beginning of the WS. As a result, water pH is very low in areas with poor drainage at the start of the rainy season (October–December). Rainfall leaches acidic compounds and toxic ions from the ASSs and increases the acidic content and toxic ions in water bodies downstream [29]. Water pH increased in January along with the increasing rainfall intensity, leading to the dilution of acidic compounds. The fluctuation in standing water levels in the primary canal is influenced by rainfall [27].
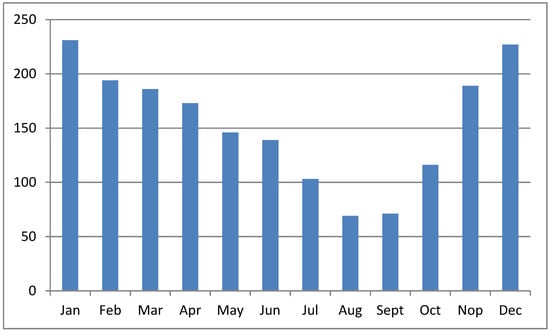
Figure 3. Monthly average rainfall (in millimeters) in South Kalimantan province, Indonesia. Source: https://dataonline.bmkg.go.id, accessed on 21 January 2024.
Standing water in the tidal region follows the sea tide dynamically, and changes by hour, day, and month depending on the moon’s position relative to the earth and the sun. Such dynamics lead to different potencies in the overflow and drainage over time. Standing water can supply irrigated sources in paddy fields, adjust application time during rice cultivation, leach toxic ions, determine planting time, and select water management technologies [27]. In general, tide overflow supplies good quality water and is used to leach toxic ions into the land by the receding water.
Water management is critical to the sustainable management of ASSs because water management can prevent pyrite oxidation, the leaching of acidic and toxic elements and compounds from the soil, and the supply of water for crops. Water management is based on tidal overflow type, potency, and water table depth (Types A, B, C, and D) [30][31]. Water management can be tailored to the soil, hydrological characteristics, and rice needs and adjusted to the problems in each tidal overflow type.
Water management in ASSs fails because soil and water become more acidic, and crop productivity decreases [32]. This conclusion is supported by the results of research on water acidity [27]. Hence, water management must (i) be tailored to the hydrological characteristics, climate, and soil in each location, (ii) be supported by the government (central and local) and the farmer using the water, and (iii) conducted at macro scales (wetland irrigation regions) and micro-scales (paddy fields).
Type A tidal areas show high inundation during the rainy season, and the areas experience saltwater intrusion during the dry season. Therefore, water infrastructure, including periphery dikes and flapped water gates, is required to prevent overflow and saltwater intrusion (Figure 4a). Type B tidal areas with impeded drainage and poor water quality are a problem because leached acids in waterways cannot go out to river estuaries due to the push from the tide. This condition requires implementing a one-way direction of fork-like canals (macro water management) supported by implementation in paddy fields (micro-scale) for better water circulation. In addition, the primary, secondary, and tertiary canals are shorter than the existing ones and are tailored to each area’s irrigated/drainage potency in such a way that acids move out from the site with the subsided water. A one-way directional system requires a swaying water gate and stop log (overflow) in the paddy fields. A swaying water gate is used to ascertain water circulation during the WS. During the DS, a stop log conserves water and prevents pyrite oxidation (Figure 5b).

Figure 4. Types of water gates: (a) flapped water gate, (b) swaying water gate, (c) stoplog water gate. Photo by Author/Khairil Anwar.
In Type C tidal areas, acids formed in soils during the DS are leached during the WS; paddy fields are inundated 5–10 cm to prevent pyrite oxidation by using overflow cascades starting from the paddy fields to tertiary and quarter canals (Figure 4c). Lowering inundation and the water table leads to pyrite oxidation, resulting in very acidic soils and water; hence, the water table requires better management.
The ASSs need leaching so that acids and toxic ions can leave the paddy field, but the water needs maintenance to prevent pyrite oxidation [33]. Precise water management, low prices, and economics are key factors in sustainable ASS management [34]. After implementing correct water management, ASSs need amelioration and fertilizers to support crop production [35].
3. Soil Amelioration
Soil amelioration improves soil properties so that the soil is favorable for crop growth and production.
Application of lime on ASSs raises soil pH, reduces Al and Fe content, and improves rice growth. Applying magnesium limestone plus biofertilizers increases soil nutrient content, i.e., total N, available P, exchangeable Ca, and exchangeable Mg [36]. Liming and application of organic fertilizer increased rice yields in acid-sulfate paddy soils [37].
Rice-husk biochar was better than ash at improving the chemical properties of ASSs (namely pH, SOC, available P, exchangeable K, exchangeable Na, exchangeable Mg, and CEC) and decreasing Al and Fe content [38]. Applying rice-husk biochar increased soil pH to 5.0 or more and rice yields by 20% [39]. Combining rice-husk biochar (5 Mg ha−1) and chicken manure (0.5 Mg ha−1) increased soil pH and P availability, decreased Fe and iron toxicity, and enhanced rice growth and yield [40]. Combining biochar (from empty fruit bunches of oil palm) increased rice yield from 141 to 472% and decreased Al toxicity [41].
Compost increases available P, whereas biochar is more effective at mitigating GHG by suppressing CO2 emissions [42]. Compost increases soil pH and improves rice growth in ASSs [42]. Applying organic material (compost) to soil improves the pH of ASSs and its effect depends on the Eh and sulfate contents. Organic matter can temporarily replace liming in land management [43]. Adding organic matter can increase P availability in ASSs and P release from the soil [44]. Compost is potent in the arrangement of nematode communities by increasing biodiversity, trophic structure, and metabolic tract in ASS-based paddy fields [45].
Applying biofertilizers (microbes) improves the quality of ASSs. Phosphate-solvent bacteria secrete organic acids, which deactivate Al and Fe through chelation. It also increases soil pH, precipitating Al or Fe as inert hydroxide Al or Fe, decreasing Al and Fe availability [46]. Sulfate-reduction bacteria (Desulfovibrio sp.) are essential in reducing acid-sulfate soils, increasing soil pH and rice yield [40].
Soil amelioration is an essential treatment for ASSs. Ameliorants (lime, biochar, organic fertilizer, compost, ash, and fly ash) and their rates and effects on soil properties have been discussed. Nevertheless, crops still need nutrient input due to the low nutrient content of these soils; thus, fertilizer application is required.
4. Fertilizer Application
The nutrient (N, P, and K) content of ASSs in Kalimantan is generally low to medium, while that of exchangeable Ca is very low to low and the soil pH is 4.5 or lower. Thus, fertilizer application is a priority in soil management and the rate and application depend on the crop, tidal type, and land typology.
Levels of N input and N loss in N cycles determine soil nitrogen content variations. Low N content occurs because N is taken up by crops, leached, and volatilized [47]. On average, nutrient loss for every ton of superior rice variety at harvest is about 17.5 kg ha−1 of N, 3.0 kg ha−1 of P, and 17.0 kg ha−1 of K [48]. ASSs with low N status need N fertilizer application. Applying 90 kg ha−1 of N to ASSs containing a total N of 0.25% yielded 4.1 Mg ha−1 of rice while adding 135 kg ha−1 of N showed no increase in yield [49]. Farmers commonly apply urea at more than the recommended rate.
The content of available P in ASSs is low, although the total P content may be high. In the ASSs in South Kalimantan, the available P is very low to medium [50] because Al and Fe fix P [51] in very acidic soil reactions (pH of 2.5–3.9). The effect of P fertilizer application on rice yield depends on P status in the soils; in low P status, fertilizer application significantly increases rice yield. Applying 22.5 kg ha−1 of P2O5 increases rice yield from 3.23 to 4.40 Mg ha−1. Statistically, there were no differences in rice yields between using 22.5 and 45–67.5 kg ha−1 of P2O5 [52].
The availability of K in ASSs is mainly low to very low. For instance, the available K in ASSs in South Kalimantan ranges from 0.09 to 0.25 cmol kg−1, and is categorized as low to very low [50]. K is an essential macronutrient that regulates stomata movement, energy transfer, anion balance, and stress resistance [53]. K is crucial to photosynthesis, carbohydrate distribution, and starch synthesis, leading to higher rice yields [54]. Applying 25 to 37.5 kg ha−1 of K2O under low K status increases grain weight and influences seed quality. However, using a higher K fertilizer rate does not affect yield increase.
Balanced fertilizer application to ASSs has better yield than partial application of only N fertilizer, P fertilizer, or K Fertilizer. Balanced fertilizer can also be applied to local rice varieties. Adding 60 kg ha−1 of N, 60 kg ha−1 of P2O5, and 50 kg ha−1 of K2O to ASSs increased local rice yield by 42%–77% [55]. Application of NPK compound fertilizer and urea are other options for increasing crop production.
In ASSs, liming can increase the effectiveness of the fertilizer. Liming and N, P, and K fertilizer addition increased rice yield from 0.64 Mg ha−1 to 4.24 Mg ha−1. The contributions of lime, N fertilizer, P fertilizer, and K fertilizer to this yield increase were 33.9%, 33.3%, 22.7%, and 10.1%, respectively. For one hectare of this soil, the fertilizer rates for superior varieties are 67.5–135 kg N, 45–70 kg P2O5, 50–75 kg K2O, and 1–3 Mg lime [56].
Results of other studies suggest that increasing crop production in ASSs requires the application of chemical fertilizers (N, P, K), organic fertilizers, and biofertilizers. Biofertilizers contain microbes such as decomposers (Trichoderma sp.), P solvents (Bacillus sp.), and N fixers (Azospirillium sp.). Biofertilizers increase N and P availability, accelerate organic residue decomposition, and promote crop growth. Applying 25 kg ha−1 of Biotara (a biofertilizer), 400 kg ha−1 of NPK compound fertilizer, and in-situ organic matter increased rice yield by 35%–48% [52][57]. Applying 25 kg ha−1 of Biotara and 300 kg ha−1 of NPK compound fertilizer to ASSs increased total N, available P, and available K in Barito Kuala Regency, South Kalimantan province [57].
Organic fertilizer decomposition increased macro and micronutrients in the soil [58]. In tidal paddy soils, applying organic fertilizers, compost from manure, compost from rice straw, and compost from Salvinia sp. increased rice yield by 3.60 Mg ha−1, 3.73 Mg ha−1, and 3.54 Mg ha−1 compared to not applying organic fertilizers (3.15 Mg ha−1) [59]. Adding organic matter increased rice yield and reduced the use of inorganic fertilizers in tidal paddy fields [60]. Thus, for a given rice variety, fertilizer application is site-specific.
5. Adaptive Varieties and Gene Conservation
5.1. Adaptive Varieties
When selecting rice varieties for planting, farmers consider market demand and preference, plant age, high yield, plant height, tolerance to abiotic stress, and resistance to pests and diseases. The local rice variety is adaptive to environmental growth but has a low yield; thus, improving rice varieties for ASSs requires creating a rice variety that is adapted to high soil acidity, iron toxicity, and water stress (flooding and dryness). Iron toxicity limits rice growth and reduces rice yield by 30–60% [61]. The decrease in yield differs in iron-tolerant varieties [62]; the decline is up to 30% for an iron-tolerant variety but 75% for an iron-sensitive one. Iron-tolerant rice varieties absorb and translocate less iron from roots to leaves compared with iron-sensitive varieties.
Improved varieties recommended for acid-sulfate paddy soils in Indonesia include Inpara, Mekongga, and Ciherang. There are nine varieties of Inpara, from Inpara 1 to Inpara 9 [63]. The adaptation test in the tidal paddy field in Barito Kuala Regency (South Kalimantan province) showed that five of nine varieties (Inpara 3, Inpara 4, Inpara 6, Inpara 8, and Inpara 9) were adapted to local conditions. They yielded more than 3 Mg ha−1 of unmilled rice. Inpara 4, Inpara 6, Inpara 8, and Inpara 9 may be introduced to farmers as alternatives to Inpara 2 and Inpara 3. Farmers in Barito Kuala Regency (South Kalimantan province) have planted Inpara 2 and Inpara 3 since 2012, while farmers in Hulu Sungai Selatan Regency (South Kalimantan province) have planted Inpara 4. Inpara 2 and Inpara 3 gave a yield of about 4.12–6.20 Mg ha−1 [64][65].
In the tidal paddy fields of Sambas Regency (West Kalimantan province), Inpara yielded 5.43 Mg ha−1 of rice [66], higher than in South Kalimantan province, which yielded 3.09 Mg ha−1 of rice [67]. The yield difference was due to soil fertility and iron toxicity levels. In West Kalimantan, the soil pH was 5.3 and the iron content was 150 mg kg−1 (showing no symptoms of iron toxicity), whereas in South Kalimantan, the soil pH was 4.62 and the iron content was 439 mg kg−1 (showing symptoms of iron toxicity). Low soil fertility and iron toxicity are responsible for the low productivity of superior varieties, ranging from 3.0 to 4.0 Mg ha−1 [67], much lower than the potential yield of about 5.0–7.6 Mg ha−1 [63].
5.2. Conservation of Genes of Local Rice Varieties
Several local tidal rice varieties are available, and some are grown by farmers. Conservation of these local varieties is crucial for safeguarding biodiversity and as materials for improving rice varieties. The indigenous agriculturalists residing in South Kalimantan acknowledged and designated indigenous tidal rice cultivars contingent upon the visual characteristics of the lemma and palea husk coloration. From a genetic standpoint, the dissimilarity in husk coloration could signify genetic or phenotypic adaptability, precisely the capacity of individual genotypes required to generate diverse phenotypes in response to alternative environmental circumstances [68]. Conserving local rice varieties is vital to preventing genetic erosion [69][70][71].
Genebanks, exemplifying ex-situ conservation, guarantee the accessibility, thorough characterization, and documentation of stored materials, thus safeguarding them considerably from external risks [72]. It ensures germplasm preservation when plants are obliterated from their original habitats. Additionally, from the user’s perspective, it can consolidate materials from diverse and dispersed locations into a single site that is readily accessible for utilization [73].
Indonesia is an archipelago distinguished by various climatic conditions, ecological geography, and agricultural practices that sustain extensive rice diversity. With Indonesia’s vast biodiversity, the abundance of genetic resource variability is considerable, encompassing diverse geographical areas. Each specific area in Indonesia possesses numerous distinct genetic resources, often dissimilar to those found in other regions [74]; these are primarily local varieties, and thus immensely different cross islands. Local rice varieties in South Kalimantan exhibit distinctive characteristics. These varieties range in plant height from 105 to 180 cm, with 10–24 tillers. The panicle is prominently exposed and grain threshing is moderate (6%–25%). The leaf angle is horizontal and the flag leaf angle is intermediate and flat, lacking the upright angle in high-yielding varieties. Similarly, the stem angle is generally moderate, falling between upright and open. More than 3300 rice accession numbers are stored in the Indonesian Gene Bank [75].
In the history of rice breeding, numerous studies have shown that rice landraces are the progenitor lines of promising new varieties. The development of IR8 [76], the identification of genes for submergence tolerance [77], and the improvement of rice yield [78] are noteworthy among these studies. IR8 is a hybrid of two landraces, Peta, an active and tall rice variety from Indonesia, and Dee-geo-woo-gen, a Chinese semi-dwarf rice type [76]. In Indonesia, the development of superior rice varieties through cross-breeding began in the 1900s using germplasm originating from various sources. Until 1965, rice breeding was directed at establishing varieties suitable for multiple land conditions, including land with medium and low fertility levels [79]. The most significant increase in production occurred from the 1970s to the 1980s with the introduction of new high-yielding varieties that were more responsive to fertilizers and matured early, for example, IR36, Cisadane, IR64, and IR66, with a growth rate of 3.3% each year [80].
Genetic diversity is also valuable in the gene conservation of natural resources [81]. Several essential genes were discovered and significantly contributed to the rice breeding process. Regarding submergence tolerance, submergence 1 quantitative trait locus (SUB1 QTL) is the origin of the rice landrace FR13A [77]. The narrow leaf 1 (NAL1) allele in the Tropical Japonica rice landrace Daringan is responsible for the substantial enhancement of the yield of contemporary rice varieties [78]. Yustisia et al. [82] reported that the levels of iron and zinc in brown rice varied across five high-yielding varieties (Ciherang, Widas, IR64, Cisokan, and Cimelati) that were cultivated in Inseptisols, with Fe ranging from 10.84 to 19.80 mg kg−1, and Zn ranging from 19.64 to 24.55 mg kg−1. The Widas variety possessed the highest Fe concentration whereas the Cisokan variety had the lowest.
The above information significantly contributed to the development of rice varieties and increased rice production in Indonesia over the years. During the pre-green revolution period, there was a notable enhancement in rice productivity in Indonesia. Notably, the mean yield per hectare, as recorded by FAOStat, went up from 1.76 Mg ha−1 in 1961 to 2.25 Mg ha−1 in 1969, subsequently increasing to 2.38 Mg ha−1 in 1970. This increase can be primarily attributed to the extensive adoption of high-yielding varieties [83]. During the Green Revolution decade, rice varieties with excellent yields and tolerance to numerous pests and plant diseases were released. One of these was IR-64, which has dominated rice fields in Indonesia since its introduction in 1986 due to its high yield and resistance to brown planthoppers. Superior cultivars boosted national rice productivity from 3.96 Mg ha−1 year−1 on average during the 1980–1990 period to 4.35 Mg ha−1 year−1 during the 1990–2000 period, an average gain of 0.23% yearly [83].
In addition to water conditions, soil management, adaptive rice varieties, farming systems, and technology adoption are other essential factors required for successful crop production in coastal acid-sulfate soils. Human resource characteristics and conditions (farmers and other stakeholders) also play crucial roles.
References
- BBSDLP. Sumber Daya Lahan Pertanian Indonesia: Luas, Penyebaran dan Potensi Ketersediaan, 2015th ed.; Balai Besar Penelitian dan Pengembangan Sumberdaya Lahan Pertanian: Bogor, Indonesia, 2015; ISBN 978-602-344-083-2.
- Hidayat, A.; Hikmatullah; Suparto. Peta Sumberdaya Tanah Tingkat Tinjau Provinsi Kalimantan Barat, Skala 1:250.000; Balai Besar Penelitian dan Pengembangan Sumberdaya Lahan Pertanian: Bogor, Indonesia, 2010; ISBN 978-602-8977-02-9.
- Suparto; Hidayat, H.; Prasodjo, N.; Ponidi. Peta Sumberdaya Tanah Tingkat Tinjau Provinsi Kalimantan Tengah, Skala 1:250.000; Balai Besar Penelitian dan Pengembangan Sumberdaya Lahan Pertanian: Bogor, Indonesia, 2013; ISBN 978-602-8977-55-4.
- Hidayat, A.; Suparto; Hikmatullah. Peta Sumberdaya Tanah Tingkat Tinjau Provinsi Kalimantan Selatan, Skala 1:250.000; Balai Besar Penelitian dan Pengembangan Sumberdaya Lahan Pertanian: Bogor, Indonesia, 2011; ISBN 978-602-8977-11-1.
- Suparto; Hikmatullah; Prasodjo, N.; Ropik; Ponidi; Kuncoro, R.D.; Amalia, L.; Widiastuti, F. Peta Sumberdaya Tanah Tingkat Tinjau Provinsi Kalimantan Timur, Skala 1:250.000; Balai Besar Penelitian dan Pengembangan Sumberdaya Lahan Pertanian: Bogor, Indonesia, 2014; ISBN 978-602-8977-55-5.
- Jayalath, N.; Fitzpatrick, R.W.; Mosley, L.; Marschner, P. Type of Organic Carbon Amendment Influences pH Changes in Acid Sulfate Soils in Flooded and Dry Conditions. J. Soils Sediments 2016, 16, 518–526.
- Jayalath, N.; Mosley, L.; Fitzpatrick, R.; Marschner, P. Addition of Organic Matter Influences pH Changes in Reduced and Oxidised Acid Sulfate Soils. Geoderma 2016, 262, 125–132.
- Shamshuddin, J.; Azura, A.E.; Shazana, M.; Fauziah, C.; Panhwar, Q.; Naher, U. Properties and Management of Acid Sulfate Soils in Southeast Asia for Sustainable Cultivation of Rice, Oil Palm, and Cocoa. Adv. Agron. 2014, 124, 91–142.
- Karimian, N.; Johnston, S.G.; Burton, E.D. Acidity Generation Accompanying Iron and Sulfur Transformations during Drought Simulation of Freshwater Re-Flooded Acid Sulfate Soils. Geoderma 2017, 285, 117–131.
- Johnston, S.G.; Burton, E.D.; Hagan, R.; Aaso, T.; Tuckerman, G. A Revised Method for Determining Existing Acidity in Re-Flooded Acid Sulfate Soils. Appl. Geochem. 2015, 52, 16–22.
- Johnston, S.G.; Burton, E.D.; Aaso, T.; Tuckerman, G. Sulfur, Iron and Carbon Cycling Following Hydrological Restoration of Acidic Freshwater Wetlands. Chem. Geol. 2014, 371, 9–26.
- Anda, M.; Subardja, D. Assessing Soil Properties and Tidal Behaviors as a Strategy to Avoid Environmental Degradation in Developing New Paddy Fields in Tidal Areas. Agric. Ecosyst. Environ. 2013, 181, 90–100.
- Yli-Halla, M.; Virtanen, S.; Regina, K.; Österholm, P.; Ehnvall, B.; Uusi-Kämppä, J. Nitrogen Stocks and Flows in an Acid Sulfate Soil. Environ. Monit. Assess. 2020, 192, 751.
- Zin, K.P.; Lim, L.H.; Mallikarjunaiah, T.H.; Bandara, J.M.R.S. Chemical Properties and Phosphorus Fractions in Profiles of Acid Sulfate Soils of Major Rice Growing Areas in Brunei Darussalam. Geoderma Reg. 2015, 6, 22–30.
- Prade, K.; Ottow, J.C.G.; Jacq, V. Excessive Iron Uptake (Iron Toxicity) by Wetland Rica (Oryza sativa L.) on Acid Sulphate Soil in the Casamance/Senegal. In Selected Papers of the Dakkar Symposium on Acid Sulphate Soils, Dakkar, Senegal, January, 1986; Dost, H., Ed.; ILRI Publication No. 44; International Land Reclamation Institute: Wageningen, The Netherlands, 1986; pp. 150–162.
- Wisawapipat, W.; Charoensri, K.; Runglerttrakoolchai, J. Solid-Phase Speciation and Solubility of Phosphorus in an Acid Sulfate Paddy Soil during Soil Reduction and Reoxidation as Affected by Oil Palm Ash and Biochar. J. Agric. Food Chem. 2017, 65, 704–710.
- Dhanya, K.; Gladis, R. Acid Sulfate Soils-Its Characteristics and Nutrient Dynamics. Asian J. Soil Sci. 2017, 12, 221–227.
- Sahrawat, K.L. Soil Fertility in Flooded and Non-Flooded Irrigated Rice Systems. Arch. Agron. Soil Sci. 2012, 58, 423–436.
- Bhaduri, D.; Mandal, A.; Chakraborty, K.; Chatterjee, D.; Dey, R. Interlinked Chemical-Biological Processes in Anoxic Waterlogged Soil—A Review. Indian J. Agric. Sci. 2017, 87, 1587–1599.
- Poggenburg, C.; Mikutta, R.; Schippers, A.; Dohrmann, R.; Guggenberger, G. Impact of Natural Organic Matter Coatings on the Microbial Reduction of Iron Oxides. Geochim. Cosmochim. Acta 2018, 224, 223–248.
- Mosley, L.M.; Fitzpatrick, R.W.; Palmer, D.; Leyden, E.; Shand, P. Changes in Acidity and Metal Geochemistry in Soils, Groundwater, Drain and River Water in the Lower Murray River after a Severe Drought. Sci. Total Environ. 2014, 485, 281–291.
- Kusmiyati, F.; Sumarsono, K.; Karno, K. Pengaruh Perbaikan Tanah Salin Terhadap Karakter Fisiologi Calopogonium Mucunoides. Pastura 2014, 4, 1–6.
- Yaduvanshi, N.P.S.; Lal, K.; Swarup, A. Effect of Sodic Water Irrigation with Sulphur through Single Super Phosphate on Yield, Mineral Composition and Soil Properties in Rice-Wheat System. J. Soil Salin. Water Qual. 2015, 7, 35–39.
- Pirasteh-Anosheh, H.; Parnian, A.; Spasiano, D.; Race, M.; Ashraf, M. Haloculture: A System to Mitigate the Negative Impacts of Pandemics on the Environment, Society and Economy, Emphasizing COVID-19. Environ. Res. 2021, 198, 111228.
- Panhwar, Q.A.; Naher, U.A.; Shamshuddin, J.; Radziah, O.; Hakeem, K.R. Management of Acid Sulfate Soils for Sustainable Rice Cultivation in Malaysia. In Soil Science: Agricultural and Environmental Prospectives; Hakeem, K., Akhtar, J., Sabir, M., Eds.; Spirnger: Cham, Switzerland, 2016; pp. 91–104.
- Mahender, A.; Swamy, B.; Anandan, A.; Ali, J. Tolerance of Iron-Deficient and -Toxic Soil Conditions in Rice. Plants 2019, 8, 31.
- Multazam, Z.; Utami, S.N.H.; Maas, A.; Anwar, K. The Impact of Seasonal Changes on Tidal Water Quality in Acid Sulfate Soils for Rice Cultivation and Water Management Strategies in South Kalimantan, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2022, 1005, 012023.
- Auliyani, D.; Basuki, T.M.; Nugrahanto, E.B.; Widjaja, W.W. Penentuan Nisbah Hantar Sedimen Sub Daerah Aliran Sungai Watujali Dan Silengkong Kabupaten Kebumen. In Proceedings of the Seminar Nasional ke 4 Pengelolaan Pesisir Dan Daerah Aliran Sungai, Yogyakarta, Indonesia, 24 October 2018; Universitas Gajah Mada: Yogyakarta, Indonesia, 2018; pp. 1–8.
- Toivonen, J.; Hudd, R.; Nystrand, M.; Osterholm, P. Climatic Effects on Water Quality in Areas with Acid Sulfate Soils with Commensurable Consequences on the Reproduction of Burbot (Lota lota L.). Environ. Geochem. Health 2020, 4, 3141–3156.
- Suriadikarta, D.A.; Sutriadi, M.T. Jenis Jenis Lahan Berpotensi Untuk Pengembangan Pertanian Lahan Rawa. J. Penelit. Dan Pengemb. Pertan. 2007, 26, 115–122.
- Ar-Riza, I. Alkasuma Pertanian Lahan Rawa Pasang Surut Dan Strategi Pengembangannya Dalam Era Otonomi Daerah. J. Sumberd. Lahan 2008, 2, 95–104.
- Bronswijk, J.J.B.; Groenenberg, J.E.; Ritsema, C.J.; van Wijk, A.L.M.; Nugroho, K. Evaluation of Water Management Strategies for Acid Sulphate Soils Using a Simulation Model: A Case Study in Indonesia. J. Agric. Water Manag. 1995, 27, 125–142.
- Anda, M.; Siswanto, A.B. Properties of Organic and Acid Sulfate Soils and Water of a ‘Reclaimed’ Tidal Backswamp in Central Kalimantan, Indonesia. Geoderma 2009, 149, 54–65.
- Österholm, P.; Virtanen, S.; Rosendahl, R.; Uusi-Kämppä, J.; Ylivainio, K.; Yli-Halla, M. Groundwater Management of Acid Sulfate Soils Using Controlled Drainage, by-Pass Flow Prevention, and Subsurface Irrigation on a Boreal Farmland. Soil Plant Sci. 2015, 65, 110–120.
- Shamshuddin, J.; Muhrizal, S.; Fauziah, I.; Husni, M.H.A. Effects of Adding Organic Materials to an Acid Sulfate Soil on Growth of Cocoa (Theobroma cacao L.). Sci. Total Environ. 2004, 323, 33–45.
- Panhwar, Q.A.; Naher, U.A.; Shamshuddin, J.; Ismail, M.R. Effects of Biochar and Ground Magnesium Limestone Application, with or without Bio-Fertilizer Addition, on Biochemical Properties of an Acid Sulfate Soil and Rice Yield. Agronomy 2020, 10, 1100.
- Maftu’ah, E.; Lestari, Y.; Pangaribuan, E.B.; Mayasari, V. Amelioration of Actual Acid Sulfate Soils to Improve Soil Chemical Properties and Rice Yields. IOP Conf. Ser. Earth Environ. Sci. 2021, 648, 012167.
- Masulili, A.; Utomo, W.H.; Syechfani, M.S. Rice Husk Biochar for Rice Based Cropping System in Acid Soil: 1. The Characteristics of Rice Husk Biochar and Its Influence on the Properties of Acid Sulfate Soils and Rice Growth in West Kalimantan, Indonesia. J. Agric. Sci. 2010, 2, 39–47.
- Annisa, W.; Mukhlis, M.; Hairani, A. Biochar-Materials for Remediation on Swamplands: Mechanisms and Effectiveness. J. Sumberd. Lahan 2021, 15, 13–22.
- Mukhlis; Khairullah, I. Effectivity of bioAmeliorant to Increase Rice Productivity in Swamplands. In Strategies and Technologies for the Utilization and Improvement of Rice; IAARD Press: Jakarta, Indonesia, 2020; pp. 277–288.
- Bakar, R.A.; Razak, Z.A.; Ahmad, S.H.; Seh-Bardan, B.J.; Tsong, L.C.; Meng, C.P. Influence of Oil Palm Empty Fruit Bunch Biochar on Floodwater pH and Yield Components of Rice Cultivated on Acid Sulphate Soil under Rice Intensification Practices. Plant Prod. Sci. 2015, 18, 491–500.
- Phuong, N.T.K.; Khoi, C.M.; Ritz, K.; Van Sinh, N.; Tarao, M.; Toyota, K. Potential Use of Rice Husk Biochar and Compost to Improve P Availability and Reduce GHG Emissions in Acid Sulfate Soil. Agronomy 2020, 10, 685.
- Michael, P.S.; Fitzpatrick, R.W.; Reid, R. The Role of Organic Matter in Ameliorating Acid Sulfate Soils with Sulfuric Horizons. Geoderma 2015, 225, 42–49.
- Mayakaduwage, S.; Mosley, L.M.; Marschner, P. Phosphorus Pools in Acid Sulfate Soil Are Influenced by pH, Water Content, and Addition of Organic Matter. J. Soil Sci. Plant Nutr. 2021, 21, 1066–1075.
- Sinh, V.N.; Khoi, C.M.; Phuong, N.T.K.; Linh, T.B.; Minh, D.D.; Perry, R.N.; Toyota, K. Impacts of Fallow Conditions, Compost and Silicate Fertilizer on Soil Nematode Community in Salt–Affected Paddy Rice Fields in Acid Sulfate and Alluvial Soils in the Mekong Delta, Vietnam. Agronomy 2021, 11, 425.
- Shamshuddin, J.; Panhwar, Q.A.; Alia, F.J.; Shazana, M.A.R.S.; Radziah, O.; Fauziah, C.I. Formation and Utilisation of Acid Sulfate Soils in Southeast Asia for Sustainable Rice Cultivation. Pertanika J. Trop. Agric. Sci. 2017, 40, 225–246.
- Kurnain, A.; Ifansyah, H. Dinamika Ion Surjan Di Lahan Rawa Pasang Surut. In Proceedings of the Seminar Nasional FKPTPI; Fakultas Pertanian Univaersitas Lambung Mangkurat: Banjarbaru, Indonesia, 2015; pp. 161–163.
- Dobermann, A.; Fairhurst, T. Rice: Nutrient Disorders and Nutrient Management, 1st ed.; IRRI: Manila, Philippines, 2000; ISBN 981-04-2742-5.
- Gribaldi; Nurlaili; Danial, E. Peningkatan Produktivitas Padi Hibrida Melalui Pemberian Pupuk N Dengan System Ratun Di Lahan Rawa Pasang Surut. J. Agrotek Trop. 2020, 8, 185–192.
- Noor, A.; Ningsih, R.D.; Napisah, K.; Yuliani, N. Tanah Rawa Kalimantan Selatan. In Kesuburan Tanah Rawa; IPB Press: Bogor, Indonesia, 2022; pp. 142–157.
- Bahri, S.; Basri, T.H.; Rahmatsyah; Faisal, T.M. Kajian Kecukupan Hara Fosfor Pada Lahan Sulfat Masam Potensial Terhadap Pertumbuhan Dan Produksi Beberapa Varietas Kedelai. J. Agroqua 2021, 19, 1–4.
- Suriadikarta, D.A.; Simanugkalit, R.D.M. Pendahuluan. In Pupuk Organik dan Pupuk Hayati; Badan Penelitian dan Pengembangan Pertanian: Bogor, Indonesia, 2012; pp. 1–10.
- Wang, M.Q.; Zheng, Q.; Guo, S. The Critical Role of Potassium in Plant Stress Response. Int. J. Mol. Sci. 2013, 14, 7370–7390.
- Philip, J.W.; Broadley, M.R.; Gregory, P.J. Managing the Nutrition of Plants and People. Appl. Environ. Soil Sci. 2012, 2012, 1155–11167.
- Nursyamsi, D.; Alwi, M. Ameliorasi Dan Pemupukan Di Lahan Rawa. In Proceedings of the Prosiding Semnas Teknologi Pemupukan dan Pemulihan Lahan Terdegradasi, Bogor, Indonesia, 29–30 July 2012; Balitbangtan-Kementan: Bogor, Indonesia, 2012; pp. 687–700.
- Khairullah, I.; Noor, A. Upaya Peningkatan Produktivitas Padi Melalui Pemupukan Di Lahan Pasang Surut Sulfat Masam. J. Pertan. Agros 2018, 20, 123–133.
- Mukhlis. Uji Keefektivan Pupuk Hayati Biotara Terhadap Tanaman Padi Di Lahan Rawa Sulfat Masam; Laporan Hasil Penelitian; Balai Penelitian Pertanian Lahan Rawa: Banjarbaru, Indonesia, 2011.
- Juarsah, I. Pemanfaatan Pupuk Organik Untuk Pertanian Organik Dan Lingkungan Berkelanjutan. In Proceedings of the Seminar Nasional Pertanian Organik, Bogor, Indonesia, 18–19 June 2014; Balai Besar Penelitian dan Pengembangan Sumberdaya Lahan Pertanian: Bogor, Indonesia, 2014; pp. 127–136.
- Noor, A.; Lubis, I.; Ghulamahdi, M.; Ningsih, R.; Anwar, K.; Chozin, M.; Wirnas, D. The Response by Selected Rice Genotypes to Organic Ameliorants in Tidal Swampland Which Is Affected by Fe Toxicity. Agron. Res. 2022, 20, 1044–1059.
- Ningsih, R.D.; Napisah, K.; Noor, A. Menghemat Pupuk Kimia Hingga 50% Dengan Menggunakan Pupuk Organik Pada Lahan Pasang Surut. In Proceedings of the Prosiding Balai Besar Penelitian Tanaman Padi; Balai Besar Penelitian Tanaman Padi: Subang, Indonesia, 2017; pp. 321–327.
- Majerus, V.; Bertin, P.; Lutts, S. Effects of Iron Toxicity on Osmotic Potential, Osmolytes and Polyamines Concentrations in the African Rice (Oryza glaberrima Steud.). Plant Sci. 2007, 173, 96–105.
- Virmani, S.S. Varietal Tolerance of Rice to Iron Toxicity in Liberia. Int. Rice Res. News 1977, 2, 4–5.
- Suprihatno, B.; Daradjat, A.A.; Satoto; Baehaki. Deskripsi Varietas Padi; Besar Penelitian Tanaman Padi: Sukamandi, Indonesia, 2010; ISBN 978-979-540-047-9.
- Adri, A.; Yardha, Y. Peningkatan Produktivitas Padi Melalui Varietas Unggul Baru Mendukung Swasembada Berkelanjutan Di Provinsi Jambi. J. Agroekotek 2014, 6, 1–11.
- Helmi, H. Peningkatan Produktivitas Padi Lahan Rawa Lebak Melalui Penggunaan Varietas Unggul Padi Rawa. J. Pertan. Trop. 2015, 2, 78–92.
- Koesrini, K.; Alwi, M.; Saleh, M. Adaptasi Dan Keragaan Hasil Varietas Unggul Padi Di Lahan Rawa Wilayah Perbatasan Kalimantan Barat. J. Penelit. Pertan. Tanam. Pangan 2019, 3, 53–59.
- Saleh, M.; Nurzakiah, S. Adaptabilitas Varietas Inpara Di Lahan Rawa Pasang Surut Tipe Luapan Air B Pada Musim Kemarau. J. Agron. Indones. 2017, 45, 117–123.
- Fusco, G.; Minelli, A. Phenotypic Plasticity in Development and Evolution: Facts and Concepts. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 547–556.
- Hawkes, J.G.; Maxted, N.; Ford-Lloyd, B.V. The Ex Situ Conservation of Plant Genetic Resources, 1st ed.; Springer: Dordrecht, The Netherlands, 2000; ISBN 978-0-7923-6442-9.
- Hairmansis, A.; Aswidinnoor, H.; Trikoesmanitya; Suwarno. Evaluasi Daya Pemulih Kesuburan Padi Lokal Dari Kelompok Tropical Japonica. Bul. Agron. 2005, 33, 1–6.
- Sitaresmi, T.; Wening, R.H.; Rakhmi, A.T.; Yunani, N.; Susanto, U. Pemanfaatan Plasma Nutfah Padi Varietas Lokal Dalam Perakitan Varietas Unggul. J. Iptek Tanam. Pangan 2013, 8, 22–30.
- Ishaq, M.; Falusi, A. Germplasm Conservation and Its Impact on Crop Improvement in Nigeria. Crop Res. 2008, 36, 285–896.
- Ferrer, M.C.; Duldulao, M.D.; Caguiat, X.G.I.; Mananghaya, T.E.; Newingham, M.; Nombrere, J.; Castro, J.; Alfonso, D.O.; Regalario, J.B.; Alvarino, J.B.M.; et al. PhilRice Genebank: Recent Developments in Managing and Sharing the Philippine Rice Germplasm. IOP Conf. Ser. Earth Environ. Sci. 2020, 482, 012010.
- Mulsanti, I.W.; Yunani, N.; Sitaresmi, T. Morphology-Based Genetic Diversity of Early Maturity Rice Germplasm, the Relation to Yield Component. Ecol. Environ. Conserv. 2020, 26, S193–S198.
- Prasetiyono, J.; Hidayatun, N.; Tasliah, T. Genetic Diversity Analysis of 53 Indonesian Rice Genotypes Using 6K Single Nucleotide Polymorphism Markers. J. AgroBiogen 2018, 14, 1–10.
- Rabara, R.C.; Ferrer, M.C.; Calayugan, M.I.C.; Duldulao, M.D.; Rabara, J.J. Conservation of Rice Genetic Resources for Food Security. Adv. Food Technol. Nutr. Sci.-Open J. 2015, SE, S51–S56.
- Bailey-Serres, J.; Fukao, T.; Ronald, P.; Ismail, A.; Heuer, S.; Mackill, D. Submergence Tolerant Rice: SUB1′s Journey from Landrace to Modern Cultivar. Rice 2010, 3, 138–147.
- Fujita, D.; Trijatmiko, K.R.; Tagle, A.G.; Sapasap, M.V.; Koide, Y.; Sasaki, K.; Tsakirpaloglou, N.; Gannaban, R.B.; Nishimura, T.; Yanagihara, S.; et al. NAL1 Allele from a Rice Landrace Greatly Increases Yield in Modern Indica Cultivars. Proc. Natl. Acad. Sci. USA 2013, 110, 20431–20436.
- Swastika, D.K.S.; Agustian, A.; Suryana, A.; Muslim, C.; Sunarsih; Perdana, R.P. Tinjauan Historis Teknologi Varietas Unggul Dan Program Intensifikasi Dalam Peningkatan Produktivitas Padi Berkelanjutan. Forum Penelit. Agro Ekon. 2021, 39, 103–114.
- Nugraha, Y.; Sitaresmi, T. Upaya Peningkatan Produktivitas Padi Dari Sisi Pendekatan Genetik. J. Iptek Tanam. Pangan 2018, 13, 1–10.
- Zhang, P.; Li, J.; Li, X.; Liu, X.; Zhao, X.; Lu, Y. Population Structure and Genetic Diversity in a Rice Core Collection (Oryza sativa L.) Investigated with SSR Markers. PLoS ONE 2011, 6, e27565.
- Yustisia; Tohari; Shiddieq, D.; Subowo, G. Pengkayaan Besi (Fe) Dan Seng (Zn) Dalam Beras Dan Karakter Penentu Varietas Padi Sawah Efisien Pada Tanah Vertisol Dan Inseptisol. Agrotop J. Agric. Sci. 2012, 2, 67–75.
- Frankham, R.; Ballou, J.D.; Briscoe, D.A. A Primer of Conservation Genetics; Cambridge University Press: Cambridge, UK, 2004; ISBN 978-0-511-81735-9.
More
Information
Subjects:
Soil Science
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Revisions:
2 times
(View History)
Update Date:
27 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




