Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Xu, X.; Zuo, Y.; Chen, S.; Hatami, A.; Gu, H. In Vivo/In Vitro Electrochemical Detection of Neurochemicals. Encyclopedia. Available online: https://encyclopedia.pub/entry/56490 (accessed on 07 February 2026).
Xu X, Zuo Y, Chen S, Hatami A, Gu H. In Vivo/In Vitro Electrochemical Detection of Neurochemicals. Encyclopedia. Available at: https://encyclopedia.pub/entry/56490. Accessed February 07, 2026.
Xu, Xiaoxuan, Yimei Zuo, Shu Chen, Amir Hatami, Hui Gu. "In Vivo/In Vitro Electrochemical Detection of Neurochemicals" Encyclopedia, https://encyclopedia.pub/entry/56490 (accessed February 07, 2026).
Xu, X., Zuo, Y., Chen, S., Hatami, A., & Gu, H. (2024, March 26). In Vivo/In Vitro Electrochemical Detection of Neurochemicals. In Encyclopedia. https://encyclopedia.pub/entry/56490
Xu, Xiaoxuan, et al. "In Vivo/In Vitro Electrochemical Detection of Neurochemicals." Encyclopedia. Web. 26 March, 2024.
Copy Citation
Neurochemicals, crucial for nervous system function, influence vital bodily processes and their fluctuations are linked to neurodegenerative diseases and mental health conditions. Monitoring these compounds is pivotal, yet the intricate nature of the central nervous system poses challenges. Researchers have devised methods, notably electrochemical sensing with micro-nanoscale electrodes, offering high-resolution monitoring despite low concentrations and rapid changes. Implantable sensors enable precise detection in brain tissues with minimal damage, while microdialysis-coupled platforms allow in vivo sampling and subsequent in vitro analysis, addressing the selectivity issues seen in other methods.
neurochemicals
bioanalysis
microelectrode
1. Introduction
Brain neurochemicals constitute a range of crucial chemicals for the central nervous system’s function, collectively encoding brain activities in physiological and pathological processes [1][2]. These neurochemicals intricately regulate the nervous system, ensuring its proper function and significantly contributing to signaling, learning, motor control, and even treating neurological diseases. The evolution of advanced in vitro neurochemical detection technologies has revolutionized comprehension of neurochemical action mechanisms. However, these assays pose challenges, like potential neurochemical degradation and limitations in understanding real-time dynamic bioprocesses. Consequently, quantitatively monitoring brain neurochemicals in vivo holds profound significance in understanding human cognitive brain function.
The complexity of the brain’s environment and the fluctuating nature of neurochemicals during different physiological and pathological processes set stringent requirements for analytical methods in in vivo cerebral neurochemical monitoring. Within this domain, two major categories—implanted microsensors and microdialysis sampling—have demonstrated robust application prospects [3]. These technologies have rapidly advanced in recent years, fortifying in vivo neurochemical analysis. The popularity of microelectrodes in neuroscience surged after Gilbert Ning Ling successfully developed glass microelectrodes with apertures smaller than 1 micron. These microelectrodes, due to their minute size, allow brain implantation with reduced damage while providing excellent temporal and spatial resolutions [4][5]. In the late 1960s, Ralph Adams [6] and colleagues studied the electrochemical behavior of various biogenic amines and implanted a carbon paste electrode in an anesthetized rat’s brain, marking the initial attempt to monitor neurochemicals using conventional voltammetric techniques.
Moreover, various electrochemical techniques, including differential pulse voltammetry, amperometry, among others, have been employed for the in vivo monitoring of chemical neuro-substances [7][8][9][10][11][12], laying a solid foundation for microelectrode applications in neurochemistry. Initially focused on electroactive molecules, like ascorbic acid and 5-hydroxytryptamine, microelectrode-based research encountered challenges with substances like catecholamines due to an overlapping electrochemical redox potential. However, recent advancements in microelectrodes designed to recognize molecules via enzymes, aptamers, or electrochemical probes have significantly overcome these hurdles, and these electrodes have been used for monitoring non-electroactive molecules. For example, Gerhard’s team, in 2001, successfully monitored non-electrochemically active glutamate molecules in the rat prefrontal cortex using glutamate oxidase through a self-referencing microelectrode array (MEA) platform [13].
Despite many advantages, microelectrodes are susceptible to the biological contamination of the tissue microenvironment and struggle with quantitatively determining neurochemicals at basal levels [14][15]. Nonetheless, these limitations have not notably diminished the prominence of microsensors compared to other analytical techniques [16][17][18][19][20][21].
In parallel, microdialysis, introduced in the late 1950s, transformed understanding of in vivo neurochemicals by measuring endogenous compound concentrations in animal brains [16][17]. This technology, often combined with instruments like high-performance liquid chromatography (HPLC) or capillary electrophoresis (CE), enhances selectivity and time resolution. Continuous efforts in coupling analytical methods with microdialysis have substantially improved its time resolution to seconds, challenging the initial notion of a poor time resolution associated with microdialysis [21][22][23][24]. Recent advancements, especially coupling microdialysis with biosensors [25][26][27], have revolutionized its capabilities. This combination leads to a sensitive analysis, low detection limit, and prevents analyte degradation. For example, in 2001, M. M. Rhemrev-Boom’s group utilized mobile biosensors for direct-coupled continuous low-flow microdialysis, showcasing enhanced analyte selectivity for glucose and lactic acid [28].
2. Types of Neurochemicals and Associated Diseases
The brain, composed of billions of neurons and neuroglia cells, hosts a diverse array of neurochemicals that continuously interact, forming a dynamic neural network responsible for regulating consciousness and behavior. These neurochemicals fall into two main classifications: neurotransmitters and neuromodulators. Neurotransmitters act as messengers, facilitating information transfer between synapses through direct electrical contact and converting action potentials into chemical signals. In contrast, neuromodulators often regulate neurotransmission and some bioprocesses. Neurochemicals encompass small ions, gases, reactive oxygen species (ROS), energy suppliers, peptides, and bioactive macromolecules, collectively governing vesicles, neurons, and circuits. They play specific roles in regulating daily physiological behavior, and imbalances in neurochemicals can lead to diseases, disrupting organism functions (Figure 1).
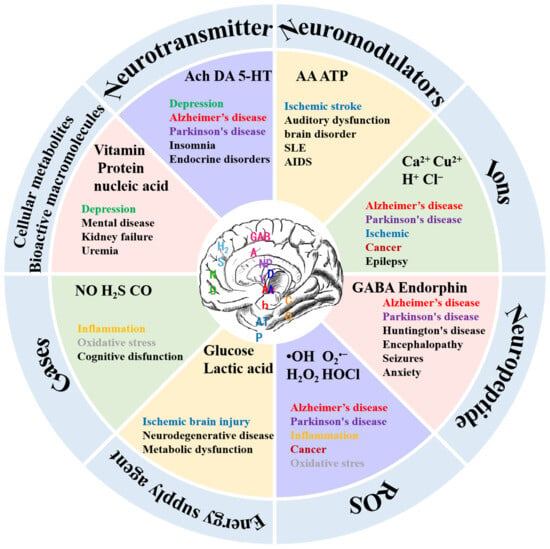
Figure 1. Classification of neurochemicals and associated diseases. The figure contains only some typical neurochemicals, not all of them (Ach: acetylcholine; DA: dopamin; 5-HT: 5-hydroxytryptamine; AA: ascorbic acid; ATP: adenosine triphosphate; SLE: systemic lupus erythematosus; AIDS: acquired immune deficiency syndrome; GABA: γ-aminobutyric acid; •OH: hydroxyl radical; H2O2: hydrogen peroxide; O2•− superoxide radical; HOCl: hypochlorous acid; NO: nitric oxide; H2S: hydrogen sulfide; CO: carbon monoxide.).
Neurotransmitters, like dopamine (DA), serotonin (5-HT), epinephrine (E), norepinephrine (NE), glutamate, and acetylcholine, amplify, transmit, and convert signals within cells, influencing mood regulation, cognition, memory formation, learning, and motor control. Catecholamine neurotransmitters, including DA and E, are extensively investigated, with alterations in their concentrations linked to neurodegenerative and psychiatric disorders [29][30]. Oscillating NE concentrations correlate with disorders like Parkinson’s disease and attention deficit hyperactivity disorder [31]. Reduced levels of 5-hydroxytryptophan are associated with depression, insomnia, and endocrine disruptions [32][33]. A decline in acetylcholine is linked to age-related memory loss and Alzheimer’s disease. Glutamate (Glu) contributes to neuronal excitation, with excessive levels leading to excitotoxicity and damaging and killing nerve cells [34]. Gamma-aminobutyric acid (GABA), a primary inhibitory neurochemical, plays a pivotal role in overall neuronal function. Fluctuations in Glu and GABA levels serve as markers for various neurological and psychiatric disorders [35][36][37].
Various ions (Ca2+, Cu2+, Na+, H+, and Cl−) play vital roles as neuromodulators in cellular signaling, molecular structure formation, and (co)enzyme activation. Metal ions chelate with biomolecules, enhancing activity and stability or act as redox centers, impacting biological processes. Dysregulated ion levels correlate with neurodegenerative diseases, cancer, and diabetes. Anions like chloride and bicarbonate regulate cell volume, membrane potential, and vesicle pH, with alterations linked to diseases such as cystic fibrosis and myasthenia gravis [38][39][40][41]. Among them, H+ ion balance is crucial for monitoring the body’s acid–base balance, with deviations triggering conditions like epilepsy, ischemia, and psychiatric disorders [42][43][44].
Neurochemicals encompass soluble gases, like nitric oxide (NO), hydrogen sulfide (H2S), and carbon monoxide (CO), known as gas transmitters. These lipophilic, soluble molecules readily cross cell membranes and are synthesized only as needed. Gas transmitters impact neuropsychiatric disorders, like anxiety. For example, nitric oxide enhances long-term synaptic transmission, influencing learning and memory. Hydrogen sulfide (H2S) regulates gamma-aminobutyric acid B receptor receptors, pH balance, and calcium homeostasis and provides neuroprotection against oxidative stress [45][46][47][48][49]. Abnormal concentrations of CO are associated with inflammation, liver disease, diabetes, and cancer [50][51][52][53][54].
Reactive oxygen species (ROS), generated from oxygen-containing molecules, play crucial roles in physiological and pathological processes. ROS accumulation causes oxidative stress, inflammation, and cellular damage, leading to necrosis and cancer.
Glucose and lactic acid serve as primary energy sources for the brain. A dysfunctional glucose metabolism correlates with neuropathologies like ischemic brain injury and neurodegenerative disorders. Lactate contributes to brain energy metabolism, regulating microcirculation and neuronal excitability and offering neuroprotection [55][56].
Another group of neuromodulators is neuropeptides, categorized as hypothalamic and pituitary, impacting synaptic transmission, neuronal inhibition, cognitive impairment, and stress response [57][58][59]. For example, the neuropeptide γ is associated with inhibiting the transmission of excitatory amino acids and reducing neuronal excitation. Endorphins affect anxiety and pain perception.
The last group of neuromodulators comprises metabolites. Generally, cell metabolism produces metabolites, like lipids, vitamins, antibiotics, toxins, and hormones, impacting neuromodulation. An abnormal brain metabolism often coexists with psychiatric disorders, like major depressive disorder (MDD) [60].
As previously mentioned, neurochemical imbalances can cause extensive harm to organisms. Analyzing their concentrations and monitoring their interactions under pathophysiological conditions at the molecular level aids in understanding brain function, guiding diagnosis, and the treatment of neurological diseases. Among all analytical techniques, electrochemical analytical methods stand out due to their speed, sensitivity, cost effectiveness, and ability to enable online detection. These methods rely on the direct redox of electrochemically active analytes on electrode surfaces or specific recognition units for indirect detection. However, the complex in vivo environment often requires an indirect detection strategy for some electrically active molecules to prevent sensor toxicity (Figure 2).
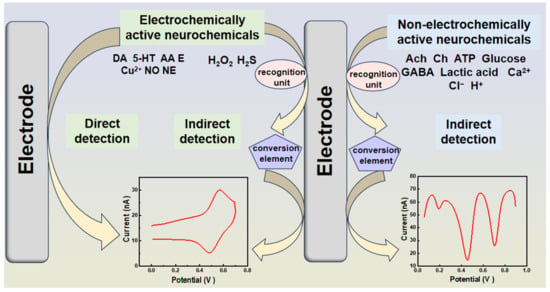
Figure 2. Electrochemical sensing strategies for the direct and indirect detection of neurochemicals.
3. In Vivo Electrochemical Measurements of Neurochemicals in the Brain Tissue
Two main types of analytical methods are commonly utilized to electrochemically detect dynamic changes in neurochemicals in vivo (Figure 3). The first method involves implantable in vivo electrochemical (bio)sensing, where miniature electrochemical sensors are directly implanted into brain regions to record real-time dynamic changes in neurochemical levels within the central nervous system (CNS). The second method involves in vivo sampling-based neurochemical analysis, typically encompassing the electroanalysis of neurochemicals sampled in vivo from brain regions in vitro. This process relies on in vivo microdialysis combined with electrochemical measurements [24][61][62], alongside sample separation and offline assays. These techniques allow for multiple neurochemical analyses to be conducted simultaneously. Alternatively, selective online assays enable the continuous monitoring of one or more neurochemicals.
3.1. Implantable Electrochemical Biosensors to Monitor Neurochemicals in the Brain Tissue
Implantable electrochemical biosensors are considered highly promising and effective technologies for real-time analyte monitoring due to their excellent spatial and temporal resolution as well as adaptable electrode interfaces. The commonly used electrode materials are divided into carbon and metal. Due to the complexity of the in vivo environment, carbon-based materials are widely used for the detection of neurochemicals in vivo because of their inertness. Common carbon electrodes include glass carbon electrodes (GCEs), carbon paste electrodes (CPEs), and screen-printed carbon electrodes. However, the most popular electrodes for neurochemical measurements are carbon fiber microelectrodes (CFMEs) because of their excellent biocompatibility, small size, and good electron transfer of neurotransmitters. In order to reduce tissue damage and inflammatory reactions during implantation, the size of the electrodes is crucial and can be regulated down to the micrometer and even nanometer levels [63][64]. Generally, electrode geometries are limited to discs, cylinders, or cones. Advances in nanolithography and 3D printing allow electrodes to have customizable geometries as well as optimized chemical and surface structures, which greatly improve the performance of the electrodes. Additionally, the advancement in microelectrode arrays facilitates the placement of multiple micrometer-sized electrodes on the same device, enabling the simultaneous detection of specific neurochemical molecules across various brain regions.
Electrochemically active substances can undergo direct redox reactions at the electrode interface, generating electrical signals for direct monitoring. However, non-electrochemically active neurochemicals require specific recognition units to capture and then convert chemical signals into electrical signals for indirect detection, because the redox potentials of some electrically active neurochemicals overlap extensively in vivo, and indirect monitoring methods are also available to improve selectivity. The in vivo neural environment is complex, featuring low analyte concentrations and thousands of potential interfering compounds, making sensitivity and selectivity crucial challenges in designing effective electrochemical sensors. In this way, advancements in various nanomaterials and recognition units have been instrumental in enhancing sensitivity and selectivity for detecting various neurochemicals.
3.1.1. Non-Electroactive Neurochemicals
The detection of neurochemicals typically involves directly measuring the corresponding current generated by the target species on the electrode surface, providing a quantitative assessment of dynamic chemical changes. Electrochemically active neurochemicals can be reoxidized directly on the electrode surface. However, the detection of non-electroactive neurochemicals (e.g., choline, acetylcholine, and glutamate) necessitates modified sensors. These sensors require a recognition unit capable of interacting with the target and producing an electroactive molecule, such as H2O2, or transferring the signal to the electrochemical process, like an intermediate [65].
● Enzymes intrinsically possess catalytic activity and exhibit specific recognition toward analytes. Enzyme-based biosensors usually rely on a medium that facilitates electron transfer (MET), generating an electrical signal by oxidizing or reducing electrically active substances on the electrode surface. The resulting current is directly proportional to the analyte concentration, enabling the detection of the target analyte. Examples include choline oxidase and glutamate oxidase, known for their simplicity, stability, and high sensitivity. O. Frey’s group [66] used a semipermeable m-phenylenediamine layer to significantly enhance sensor immunity to interference. They developed a micro-biosensor for the simultaneous detection of glutamate and choline in the rat brain using in silico process technology, opening new directions for detecting these substances within physiologically relevant concentration ranges. For reliable in vivo detection, Gerhard’s group [13] created a self-referencing microelectrode array (MEA) platform. By introducing a self-reference electrode and subtracting its current signal as background noise, interference from other species was eliminated. MEAs have been extensively used to measure rapid changes in glutamate levels in anesthetized and awake animals [13][67][68][69][70][71][72][73][74][75], lactate and glucose levels, and for the real-time monitoring of choline, acetylcholine (ACh) [76], and oxygen [77] in vivo.
The ideal enzyme-based sensor capable of direct electron transfer (DET) from the redox-active center to the electrode surface belongs to the DET enzyme sensors category. DET enzyme sensors remain unaffected by changes in ambient oxygen concentration or additional mediators, making them adaptable to complex in vivo environments, ideal for neurochemical detection. Yu’s group [78] contributed significantly to ROS detection using functionalized ionic liquid polymers (PILs) coated on Prussian blue nanoparticles and carbon nanotubes (CNTs), enhancing sensor sites for SOD to improve sensitivity and stability to low O2•− concentrations. Hence, the sensor effectively tracked changes in O2•− levels under normal and pathological conditions in the living brain system. Overall, enzymes play a crucial role in in vivo neurochemical detection owing to their unique selectivity and rapid kinetics. The development of various enzymes has expanded the scope of detectable analytes, and the emergence of synthetic nano-enzymes [79] holds promise for the future of biosensors.
● Aptamers, single-stranded DNA or RNA molecules from randomly sequenced nucleic acid pools, have gained attention for their high affinity and specificity to the target. Compared to enzymes, they offer convenient synthesis, design flexibility, and chemical stability. Aptamer-based electrochemical sensors undergo conformational changes when the aptamer on the electrode surface combines with the ligand, triggering electron transfer between the electrode and the modified REDOX group on the aptamer [80]. Currently, aptamers are used to detect neurochemicals, like DA [81], 5-HT [82], and ATP [83]. Aptamers possess a good biological compatibility. Due to a complex screening process, only specific neurochemicals have aptamer recognition units. In 2005, Yi Xiao et al. [84] pioneered an electrochemical aptamer sensor, sparking research interest. Subsequently, various aptamer electrochemical sensors have been developed, mostly for blood analyte detection [85][86]. However, few electrochemical aptamers have been applied successfully to brain tissue detection. Cui’s group [87] developed an electrochemical aptamer-based in vivo cocaine sensor on a silica-based neurorecording probe platform capable of measuring cocaine directly from discrete brain locations using square wave voltammetry, capturing real-time cocaine transient events in multiple brain regions over the entire pharmacokinetic time course.
Despite the many advantages of aptamers, several challenges persist, including limited measurement duration and obtaining high-performance aptamers for new targets. New aptamers need adaptation to sensing platforms, potentially bottlenecking sensor development for novel targets. Specificity remains a challenge; aptamers often bind chemical groups present in multiple targets, leading to cross-reactivity during detection [88][89]. This presents challenges for in vivo monitoring. The potential duration of in vivo aptamer-based measurements remains uncertain; prolonged in vivo measurements risk signal loss and electrode contamination, mitigated by surface coating or increasing single-layer packaging density to reduce protein contamination [90][91][92]. Despite being in its infancy, aptamer-based in vivo detection offers excellent time resolution, miniaturization, and other advantages, holding potential for in situ detection platforms.
● Recognition Elements for Ions play a crucial role in maintaining the central nervous system’s normal functioning. For instance, calcium ions act as second messengers in neurotransmitter regulation, while dysregulated Cl− levels and brain pH disorders are associated with various neurological disorders. Efforts have been made to detect ions accurately in vivo. To enhance in vivo detection accuracy, a dual-channel recognition strategy using ratiometric microelectrodes was proposed for real-time monitoring. Fan Zhao et al. [93] achieved dual-channel recognition by modifying recognition elements on different microelectrodes, enabling the real-time in situ monitoring of pH in the rat brain, minimizing brain damage and inaccuracies between the electrodes. Gu’s group [94] constructed a ratiometric microsensor for pH monitoring, utilizing electrochemically oxidized graphene oxide (EOGO) to generate a built-in correction signal and a poly(melamine) (PMel) film as a pH-selective recognition membrane. PMel, a highly pH-sensitive conductive polymer, facilitated the successful real-time monitoring of rat brain pH post-whole-brain ischemia/reperfusion events, confirming the robustness of the proposed ratiometric electrochemical microsensor platform.
● Molecularly Imprinted Polymers (MIPs), formed by a template molecule’s size, shape, and functional groups, draw inspiration from the specific binding of antigens and antibodies—the ‘lock and key’ mechanism. These polymers, like a template, can absorb specific targets that fit the fabricated template in terms of shape, size, and chemical function. Due to their distinct structure and specific recognition ability or high selectivity, MIPs find extensive use, particularly in molecularly imprinted electrochemical sensors (MIPESs) [95]. Mosbach and Haupt [96] pioneered the integration of electrochemical sensors with MIPs, introducing MIPESs in 1999. Since then, MIPESs have detected organic compounds, heavy metal ions, emerging pollutants, and in vitro biomolecules. In 2012 [97], the first in vivo microsensor based on a MIP detected dopamine in the rat brain. Subsequently, focusing on neurochemical detection in the body, it has been employed for selective DA detection [98], as well as norepinephrine (NE) [99] and epinephrine (EP) [100].
● Electrochemical Probes: While in vitro environments can be corrected, the complexity of in vivo environments poses a risk to the selectivity of in vivo assays. To address this issue, electrochemical probes based on a dual recognition strategy were proposed for in vivo measurements, enhancing assay selectivity through the synergistic chemical recognition of specific ligands and the redox activity of chemicals. Recent studies suggest that biological activities initially attributed to hydrogen sulfide may actually be mediated by H2Sn, which converts endogenous hydrogen sulfide to hydrogen polysulfide in the presence of ROS. H2Sn is hypothesized to have a stronger oxidative capacity and reactivity than hydrogen sulfide, potentially being the true regulator in cellular signaling [101][102]. The further development of sensors that selectively detect multiple neurochemicals simultaneously is required for an in-depth understanding of in vivo molecular mechanisms. Despite the direct oxidation of H2S on the electrode surface, the slow oxidation of other electroactive biomolecules and the organism’s internal complexity cause significant interference. Tian’s group [8] designed two electrochemical probes, 3,4-bis((2-fluoro-5-nitrobenzoyl)oxy)-benzoic acid and N-(4-(2,5-dinitrophenoxy) phenyl)-5-(1, 2-dithiolan-3-yl)pentanamide, specifically recognizing H2Sn and hydrogen sulfide, respectively. Co-assembling these probes on a mesoporous gold membrane yielded a microsensor that responded well to both hydrogen sulfide (0.2–50 µM) and H2Sn (0.2 to 40 µM).
● Electrochemical Microarray Detection, an electrochemical microarray detection platform, offers a promising approach to simultaneously sense multiple ions in the body under specific physiological or pathological conditions. Tian’s group [103] developed an electrochemical physiological microarray (ECPM) to quantify K+, Ca2+, and Na+ concentrations and pH using an open-circuit potentiostatic method. Different ion-recognizing elements designed for simultaneous electrical signal recording without cross-talk were employed. An internal reference electrode coated with a polyvinyl chloride membrane avoided the complex brain environment interference, ensuring the accuracy and selectivity of the developed ECPM. It provides new paths for the real-time monitoring of K+, Ca2+, and Na+ ions’ dynamics and quantitative concentrations, establishing the correlation between electrical and chemical signals.
3.1.2. Electroactive Neurochemicals
Electrochemical sensing for detecting neurochemicals typically involves the direct oxidation or reduction of the target species on the electrode surface, generating corresponding electric currents that quantify dynamic chemical changes. Electrochemically active neurochemicals undergo direct oxidation or reduction on the electrode surface, producing detectable electrical signals. However, challenges arise due to the presence of multiple redox substances and the overlapping peak potentials of their reactions, hindering selective monitoring.
Nevertheless, functional brain regulatory networks enable electrochemical monitoring. Techniques, such as cyclic voltammetry (CV), chronoamperometry (CA), and potentiometric pulse (DPV), are commonly employed. CV is the recording of current–potential curves by controlling the electrode potential at different rates over time with one or more repeated scans in a triangular waveform. The basic principle is that a pulsed voltage with a triangular waveform is applied to the closed loop formed by the working electrode and the counter electrode to change the potential at the working electrode/electrolyte interface at a certain rate, forcing the active material at the working electrode to undergo an oxidation/reduction reaction. CA monitors the gain or loss of electrons in the presence of a fixed potential. In addition to a three-electrode setup consisting of a working electrode, a reference electrode, and a counter electrode, it can also be performed using a two-electrode setup with the reference and counter short-circuited together. The differential current of DPV is a symmetrical volt–ampere peak whose intensity is proportional to the concentration of the analyte. Due to the high Faraday to charging current ratio of the DPV, the direct detection of the base analyte at low concentrations can be achieved. Fast scan cyclic voltammetry (FSCV) stands out due to its scanning potentials and high scan rate, enabling the detection of small changes in neurochemical concentrations and differentiation based on unique redox potentials. In their study, Butcher et al. [104] utilized carbon fiber microelectrodes to monitor electroactive neurochemicals, such as dopamine, epinephrine, norepinephrine, and 5-hydroxytryptophan, enhancing selectivity and temporal resolution.
Despite advancements like waveform alterations enhancing sensitivity in rapid CV [105][106][107], varying chemical basal concentrations pose detection challenges. To achieve sensitivity and selectivity in in vivo electrochemical sensors, the modulation of specific electrochemical processes at the electrode/brain interface is essential. Nanomaterial advancements, encompassing carbon nanotubes, graphene, MOFs (metal–organic frameworks), metals, and metal oxides, bolster electron transfer kinetics. This enhancement elevates sensitivity, diminishes activation overpotential, and minimizes interference from unwanted substances. Additionally, recognition units, such as enzymes, aptamers, and electrochemical probes, play crucial roles in the construction of electroactive neurochemical sensors.
Among these electrode modifiers, metallic nanomaterials exhibit exceptional properties in amplifying electrical signals. Guoyue Shi et al. [108] pioneered the development of a glass-sealed Au nanoelectrode with cluster-like gold nanostructures modified with Nafion, marking the first instance of improving sensitivity and selectivity for the in vivo detection of dopamine (DA). This electrochemical biosensor offers several advantages, including easy insertion, low tissue interference, good biocompatibility, and strong analytical performance. It provides an effective means to monitor brain DA.
In addition to metallic nanomaterials, certain carbon-based nanomaterials possess remarkable electrochemical catalytic properties. Graphene, renowned for its high conductivity, mechanical strength, expansive surface area, and catalytic characteristics [109][110], is extensively used as an electrode in efficient electrochemical sensors. Its wide potential window and rapid electron transfer rate lead to a minimal charge transfer resistance and heightened electrochemical activity. The hydrophobic and p-p interactions between dopamine and graphene enable the distinct separation of the oxidation potentials of dopamine and uric acid. Chen’s group [111] significantly increased the electrode surface area and enhanced the electrocatalytic performance of platinum wires by employing gold nanoparticles (AuNPs) and reduced graphene oxide (rGO)-modified platinum wire microelectrodes on AuNPs/rGO composites, achieving the successful detection of dopamine in the rat brain striatum. Single- and multi-walled carbon nanotubes (SWCNTs and MWCNTs, respectively) were utilized to augment the electroactive/adsorption sites, ensuring a higher sensitivity, and electrocatalytic/defect-enriched sites, ensuring a higher selectivity in monitoring neurochemicals.
In 2019 [112], Taylor et al. enhanced the sensitivity to dopamine by modifying the coating on carbon fiber surfaces, introducing a highly sensitive and selective carbon fiber electrode (CFE). PEDOT finds wide utility in neutral physiological environment analysis for detecting electrophysiological signals and neurotransmission due to its strong adhesion and porous structure. PEDOT/CNT-functionalized sensors have been extensively applied in in vivo DA detection. Cai’s group [113] utilized SWCNTs/PEDOT: PSS-modified microelectrode arrays, creating a four-stalk implantable microelectrode array (MEA) for simultaneously and in real-time detecting bimodal signals—electrophysiological signals and dopamine (DA) concentration—in the rat striatum. This setup effectively monitored the dynamic changes in striatal bimodal signals during isoflurane anesthesia.
To improve the selectivity, some recognition units, such as enzymes, aptamers, and electrochemical probes, have been developed for the detection of electroactive neurochemicals. Mao’s group [114] introduced a novel interface functionalization strategy involving the assembly of aptamer–cholesterol amphiphiles (aptCAs) on alkyl chain-functionalized carbon fiber electrodes (CFEs). This approach proved more effective than pre-treating the electrode surface with a positively charged coating, enabling the efficient immobilization of aptamers on the CFE surface via electrostatic interaction. The resulting modified electrode exhibited increased stability in physiological fluids and demonstrated versatility across various oligonucleotide sequences. Upon implantation into the nucleus ambiguus (NAc) and medial forebrain bundle (MFB) regions of rats, this modified electrode successfully monitored dopamine (DA) dynamics in the living rat brain using amperometry to track changes in DA levels during electrical stimulation. Mao’s group also proposed an electrochemical coupling strategy, covalently placing a specific catechol on the carbon fiber surface, initiating an initial electrochemical coupling on the CFE. This process resulted in a thin layer of quinone intermediates that rapidly bound to thiol-containing oligonucleotides at controlled potentials. This innovative strategy not only simplified and enhanced the efficiency of carbon surface modification but also significantly improved sensitivity and stability in dopamine sensing. It established a robust system for continuously detecting dopamine dynamics in the living animal brain [115].
3.2. Microdialysis Coupled with Electrochemical Detection
Microdialysis serves as a pivotal analytical technique for sampling in neurochemical analyses. Especially beneficial for in vivo applications, microdialysis minimizes damage to the brain tissue and exhibits extensive applicability in various extracellular fluid analyses, encompassing heart, fat, liver, and brain tissues [116]. Originating in 1966, the technique relies on analyte diffusion across a porous membrane. Bito’s group [17][117] accessed a dog’s cortical layer using a sterile dialysis capsule, allowing the collection of analytes into a brine stream, subsequently detectable in the flowing salt species. Notably, microdialysis often integrates with chromatography or electrophoresis systems for sample separation. When combined with electrochemical detection systems, it forms a separation-based sensor capable of achieving the highly selective, near real-time monitoring of multiple analytes. However, microdialysis monitoring relying on separation systems requires sample pretreatment, risking sample degradation, causing delays, and limiting spatial resolution due to separation properties. The coupling of microdialysis with biosensors, driven by advancements in electrochemical biosensors, enables the direct analysis of biological samples from living bodies. While less selective than separation-based microdialysis monitoring, this approach’s simplicity negates the need for additional pretreatment, avoids potential analyte degradation, and allows the monitoring of biological events over extended periods, such as 2 h [118].
3.2.1. Detection Techniques with Separation Means
Microdialysis can be paired with separation devices, like high-performance liquid chromatography (HPLC) and capillary electrophoresis (CE), along with associated detectors, to detect samples in dialysate. Continuous efforts have substantially improved microdialysis coupled with separation systems, achieving temporal resolutions in seconds, overturning the prior notion of a poor temporal resolution. As a result, it has also been favored by researchers, with a variety of microdialysis-coupled separation systems emerging as detection strategies that can be applied to the detection of different neurochemicals.
● Microdialysis–capillary electrophoresis/microchip electrophoresis (MD–CE/ME): MD–CE/ME encounters a challenge due to the high-volume injection requirements (1–10 µL) of conventional separation techniques, leading to a compromised time resolution—a consistent limitation in microdialysis systems. However, the emergence of capillary electrophoresis has significantly alleviated this issue with its high separation efficiency and shorter separation times [119][120][121][122][123]. As early as the 1990s [124][125], the coupling of MD with CE enabled the efficient analysis of polar and charged compounds in biological samples. Initially, the integration of MD and CE was often paired with laser-induced fluorescence (LIF) detection, facilitating the in vivo detection of various neurochemicals, like norepinephrine, glutamate, and aspartate [123][126].
Despite CE-LIF detection enhancing the potential for the high temporal resolution monitoring of neurochemicals in microdialysis fluids, it necessitates derivatization to introduce a fluorophore into compounds, limiting its applicability to all metabolites and reducing selectivity for biomolecules. However, capillary electrophoresis (CE) combined with electrochemical detection has emerged as a robust analytical tool [127][128][129]. Thomas J. O’Shea et al. [130] demonstrated the continuous monitoring of amino acids in the brain by integrating CE with electrochemical detection using microdialysis sampling. This setup was showcased by observing “K+-induced stimulation of excitatory amino acid release.” Qian’s group [20] developed an integrated end column decoupler with conductive discs in a fused silica capillary wall, providing a low detection limit. This CE-EC system effectively determined dopamine in 1 min brain microdialysis fluid samples from anesthetized rats.
On the other hand, microchip electrophoresis (ME) not only facilitates rapid MD-LC separation and demands minimal sample volumes but also enables the continuous, simultaneous monitoring of multiple analytes using online separation sensors. When combined with continuous microdialysis sampling, microchip electrophoretic separation-based sensors provide near real-time dynamic information on sample chemical composition. EC detection in ME was first described in 1998 [131] and offers unique advantages [132] over the initially used laser-induced fluorescence techniques [133][134][135]. It allows the direct detection of small, naturally electrically active biomolecules using an amperometric method. Additionally, the integration of microchip electrophoresis separation with the electrochemical system eliminates the need for bulky optical detection instruments, enhancing convenience.
Fabricating MD-ME-EC systems faces challenges in integrating working electrodes into the device. Rachel A. Saylor et al. [136] employed a PDMS/glass hybrid device fabrication process, enabling the successful integration of a high ionic-strength, pressure-driven MD stream with ME and CE on carbon electrodes. This integration allowed the near real-time in vivo monitoring of catecholamines in the rat brain. To enhance electrode alignment reproducibility with the separation channel, Shamal M. Gunawardhana et al. [137] utilized a thermally cracked photoresist film working electrode and a poly (Dimethylsiloxane) microchip with a flow-gated sample injection interface. This setup coupled MD for the online monitoring of levodopa conversion to dopamine and monitoring dopamine release in anesthetized rats after high K+ stimulation.
Flow-gated capillary electrophoresis combines conventional CE and microchip CE, using a quartz capillary as a separation channel and adopting a fast flow-gated injection technique primarily from microchip CE. This method utilizes silica capillaries as separation tubes while employing a single-cross microchip configuration for rapid flow-gated injection [19]. As early as 1996 [138], Kennedy’s group investigated capillary electrophoresis coupled with microdialysis using a flow-gated interface for the in vivo quantitative monitoring of compounds like aspartic acid and glutamate in the rat caudate nucleus. This strategy led to an enhanced separation efficiency and rapid sampling despite trace sample amounts. Subsequently, to further enhance flow-gated CE in vivo monitoring, Bowser and Kennedy [139] reported an improved separation efficiency using smaller diameter capillaries (10 µm), higher electric field strength (2000 V/cm), and sheath-flow cuvettes. Additionally, Kennedy and Bowser [140] utilized derivatization with NDA and NBD-F to enhance sensitivity in detecting primary amines for rat brain neurotransmitter detection.
● Microdialysis–liquid chromatography (MD–LC): MD–LC is a prevalent method for analyzing microdialysis samples, often employing liquid chromatography (LC) coupled with electrochemical, fluorescence, mass spectrometry (MS), or absorbance detection. While capillary electrophoresis boasts a high temporal resolution, HPLC has emerged as the preferred method for studying low concentrations of neurochemicals, like dopamine and 5-HT, due to its reliability and reproducibility. HPLC can provide both base concentrations and dynamic changes. However, conventional liquid chromatography necessitates sufficient dialysate, leading to longer processing times, inadequate temporal resolution, and significant delays between the sample collection and clinical response. Conversely, nano (capillary or microporous)-based LC methods coupled with fluorescence or electrochemical detection offer the selective analysis of neurochemicals in microvolume samples. Previous efforts to enhance the speed of HPLC techniques include using high pressure to increase the linear velocity of the mobile phase, elevating column temperature, and utilizing shorter columns filled with shorter particles [141][142][143]. The temporal resolution of measurements at HPLC not only depends on the sample stream flow rate but also on the sample volume. Capillary columns possess significantly smaller peak volumes compared to the standard or microbore (1 mm diameter) HPLC columns, minimizing the sample volume dilution and greatly improving the temporal resolution. Weber’s group integrated these modifications to determine serotonin (5-HT) levels in the central nervous system of awake animals using capillary LC and electrochemical detection [144]. To further expedite 5-HT chromatographic determinations and enhance the temporal resolution, Weber’s group explored on-column pre-concentration as part of optimization, highlighting the column diameter’s importance in achieving high speed and optimal sensitivity conditions. Smaller particle diameters and higher temperatures were found to yield faster optimal separations and a greater sensitivity. Under ideal conditions, the separation time for 5-HT was reduced to approximately 22.7 s. Online analysis feasibility was demonstrated by simulating repeated injections to achieve a time resolution of 36 s [21].
3.2.2. Detection Techniques with Biosensors
The use of biosensors offers a viable alternative to separation devices, which often necessitate bulky hardware and intricate operational mechanisms. Coupling biosensors with microdialysis introduces near real-time capabilities and shorter analysis times. This method involves fewer technical requirements as it avoids the need for sample collection and separation. While the use of biosensors has significantly improved the drawback of low temporal resolution, other challenges have emerged, including issues related to stability, reproducibility, etc. [145]
An ideal biosensor should have the capability to continuously and reliably monitor analytes in complex bodily fluids from living organisms over an extended period. Typically, a biosensor comprises one or more recognition elements designed to identify target molecules [146][147]. This process generates a chemical signal captured by the sensor component and translated into an optical or electrochemical signal.
The utilization of enzymes stands as a common strategy in biosensors. In 2005, Mao’s group [24] summarized the principles, development, and notable applications of early enzyme-based continuous online monitoring combined with microdialysis sampling and biosensors. Notably, their work involved the development of enzyme-based biosensors integrated with microdialysis, employing thionine and xanthine oxidase (XOD) as low-potential mediators and oxidases [22]. This demonstrated that the use of low-potential mediators for electron transfer in oxidases offers a novel approach for developing oxidase-based biosensors, both theoretically and technologically simple.
The combination of microdialysis enables the highly selective detection of endogenous species within the brain system. Their research showcased an online electrochemical system (OECS) for the selective and continuous measurement of acetylcholine (ACh) [23], achieved by efficiently integrating in vivo microdialysis, a multienzymatic microreactor, and an electrochemical detector.
In a separate study, Yanyan Yu et al. [148] synthesized a new room-temperature ionic liquid, [C3(OH)2mim][BF4], with two hydroxyl functionalities in the imidazolium core. This introduction of functional groups significantly enhanced the stabilization of Au/Pt alloys and facilitated the formation of well-dispersed small metal nanoparticles, exhibiting a good catalytic activity against hydrogen peroxide. Using glutamate oxidase as a biorecognition element, the continuous detection of glutamate was achieved in the rat striatum, observing normal levels and changes in concentration following various stimuli.
To further enhance in vivo sensing accuracy and selectivity, a dual recognition unit strategy (DRUS) was proposed. Yanyan Yu et al. [149] constructed a DRUS ATP biosensor with high selectivity and sensitivity. Utilizing aptamer-to-base and polyimidazole-to-phosphate recognition abilities, the biosensor exhibited an ultra-high sensitivity to subinosinazole levels of ATP-LOD and a remarkable selectivity against interfering ADP and AMP sensing in the extracellular ATP microdialysate sampled from the brain system.
Ratiometric electrochemical sensors (RECSs) have shown improved reproducibility, stability, and reliability in correcting errors during in vivo sensing, making them ideally suited for repetitive in vivo analyses [8][150][151]. Building upon this concept, Gu’s group constructed RECSs by modifying graphene oxide on electrodes via electrodeposition. Subsequently, methylene blue was adsorbed onto the graphene oxide using electrostatic attraction, serving as an effective internal reference ratio. The introduction of graphene sp3 C-C defects via subsequent electroreduction facilitated the electrochemical oxidation of AA at low potentials, ensuring a high selectivity in the brain system against potential interferences. One point that is worth noting is that this voltammetric RECS to accomplish in vivo/online repetitive measurements included a time interval of more than 1 min, which is capable of tracking the physiological process happening over several minutes [152].
All in all, the integration of microdialysis with electrochemical systems offers advantages, such as simple equipment, low cost, and high sensitivity. Initially, the coupling of microdialysis with separation systems and integrated electrochemical detection was primarily used for detecting electroactive neurochemicals. As the coupling extended to biosensors, designing recognition units on the sensor surface allowed the detection of not only electroactive neurological substances but also non-electrochemically active neurochemicals. However, the sometimes slow electrochemical reaction rates and coupled biocatalytic processes limit the temporal resolution. Spectroscopic methods, such as laser-induced fluorescence (LIF) and visible light absorption, have fast reaction kinetics. The application of microdialysis in combination with spectroscopic techniques has been shown to provide a high temporal resolution for the detection of neurochemicals. Additionally, integrating microdialysis with mass spectrometry serves as a powerful tool for analyzing biologically active molecules. This integration provides sequence specificity and enhanced mass sensitivity, enabling detection across a broad range of analytes. These assays significantly compensate for the limitations of integrated microdialysis and electrochemical detection platforms.
3.3. Emerging Techniques
In recent years, a liquid/liquid interface microsensor (LLIM) using a nano-micropipette emerged, utilizing electrochemical techniques to monitor the charge transfer from the aqueous phase to the organic phase of an analyte. This innovation allows the monitoring of neurochemicals in the living brain, even those lacking redox activity. Electrochemistry at the liquid/liquid interface, or the interface between two immiscible electrolyte solutions, has advanced the direct analysis of ions by leveraging the difference in electrolytic energy between the ionic solvents of neighboring phases. Adjusting the pipette tip to the micrometer scale enhanced the spatial resolution of in vivo analyses. In recent decades, electrochemical sensing at liquid/liquid interfaces has garnered attention for analyzing non-electrochemically active substances, like neurochemicals, amino acids, peptides, and proteins. For example, choline (Ch) exhibits specific ion transfer potential and a distinct ion transfer current signal. Zhang’s group [153] employed 1,2-dichloroethane as the organic phase and choline-containing rat cerebrospinal fluid as the aqueous phase, utilizing the disparity in solvation energies between the liquid phases. This approach demonstrated a good linearity and selectivity in responding to Ch, achieving a detection limit of 0.37 μM. Also, nanotechnology and liquid–liquid interfacial sensing have combined to enable neuronal monitoring for neurochemicals. Novel sensing principles based on nanochannels, particularly ionic current rectification (ICR) technology, have been developed. Lanqun’s group explored ICR at the micrometer scale [154], unveiling a new strategy using the microscale to selectively sense ATP in the brain system. They employed polyimidazole [155] to modify the inner wall surface of the microtubule, offering a good linearity for ATP within the 5–100 nM concentration range through differential binding between the positively charged polyimidazole and negatively charged ATP aptamers.
In another study, field-effect transistor (FET) technology, highly sensitive and selective in biosensing, allows miniaturization and has gained prominence for real-time, high-throughput, and high-sensitivity assays. Among biosensing platforms, FET biosensors excel in enzyme-modified biosensors due to their precise analyte detection, small size, integrated compactness, and potentially cost-effective quality. These sensors detect hydrogen ions (H+), byproducts produced by enzymes and analytes, proportionate to the analyte concentration. Graphene, with its unique structural geometry, excellent electrical properties, biocompatibility, and sensitivity to surface charge alterations, serves as an active channel for immobilizing enzyme molecules in FET biosensors. Hwang et al. [156] developed a reduced graphene oxide-based enzyme-modified FET (RGO-EnFET) to study acetylcholinesterase enzyme kinetics and the impact of acetylcholinesterase inhibitors on AD therapy. Additionally, Fenoy’s group [157] proposed modifying the FET graphene channel with a copolymer poly(3-aminobenzylaminobenzidine-co-phenylene amine) (PABA) film, enhancing the electrostatic charge and creating a non-denaturing environment for enzyme immobilization. This improved the FETs’ pH sensitivity, enabling real-time detection of acetylcholinesterase within the 5–1000 μM range for acetylcholine sensing.
FET technology has also integrated aptamers. Andrews’ group [158] obtained DA- and 5-HT-specific aptamers through SELEX screening, constructing flexible aptamer field-effect transistor sensors for DA and 5-HT detection. These sensors successfully detected target analytes in artificial cerebrospinal fluid and mouse brain slices.
The quality of behavioral correlates of neurochemical measurements can be improved as new wireless data transmission systems are able to perform the tests without tethering the animal [159]. As physics, neurophysiology, chemistry, and other disciplines continue to advance, microelectronic devices for wireless neurochemical sensing have been developed to detect neurochemicals in non-tethered animals. In addition, wireless transmission systems can also be used in human medicine. Roham et al. [160] proposed an integrated chip for wireless neurochemical measurements that provides both amperometry and fast scanning cyclic voltammetry. With the continued efforts of researchers, wireless transmission system test instruments are slowly becoming portable and wearable. Tonello et al. [161] have developed a low-cost, high-sensitivity, portable point-of-care (PoC) detection system based on a screen-printed electrochemical sensor that used a proprietary antibody to detect unfolded p53, enabling the detection of this biomarker in Alzheimer’s patients.
In recent years, Drakakis’ group [162] has developed a battery-powered potentiostat and wireless data transmission system that includes integrated biosensors and a microfluidic system for microdialysis. It can detect glucose and lactate in the brain of brain-injured patients using amperometry and potassium ions using potentiometry.
4. In Vitro Electrochemical Measurements of Biomolecules in the Brain Tissue
Both the in vivo and in vitro assays of neurochemicals play crucial roles in exploring brain science. Conducting detections on brain slices in vitro helps to avoid interference from the in vivo environment. Isolated brain slice cultures serve as common analytical models for studying neurophysiology, frequently employed for monitoring neurochemicals in the brain tissue in vitro. Moreover, multiple slices from the same brain can be obtained and measured, allowing access to deeper tissue regions. The experimental setup is typically more convenient with brain slices. However, brain-sectioning experiments come with limitations. The tissues undergo mechanical trauma during sectioning, raising concerns about whether measurements are taken from healthy or injured regions. Moreover, phenomena like Donnan swelling, due to the exposure of intracellular charged molecules during sectioning, are likely to alter the tissue’s mechanical properties.
In vivo assays are more time-sensitive compared to in vitro assays, enabling the real-time tracking of dynamic changes in target analyte concentrations throughout the organism. Yet, the complexity of the in vivo environment poses additional detection challenges. Both in vitro and in vivo assays possess strengths and weaknesses, continuously evolving and progressing in parallel, complementing each other with their respective strengths. In this regard, Wu’s group [163] enhanced electrode biocompatibility and stability by modifying the CFE with chitosan (CS) membranes, brain cell membranes, and the aptamer cholesterol amphiphile (DNA-Cho). Their electrode demonstrated a high sensitivity, specificity, and stability in detecting DA and was utilized to detect potassium-ion-induced DA release in brain slices and PC12 cells, unveiling the specific process of DA release inhibition by lipopolysaccharide. This robust electrode modification strategy facilitates the in vivo monitoring of DA sensing activities in complex environments. The development of microarray probes (MEAs) has offered new directions for researchers. Hossain et al. [164] designed and validated a platinum (Pt) microelectrode array-based GABA probe, utilized for in vitro measurements in brain slices. The probe includes two microbial sensors, with the concentration of GABA corrected by the difference in oxidation currents of hydrogen peroxide generated during measurements by the two microbial sensors. This approach minimizes the impact of the complex brain tissue environment. To enhance enzyme stability, Asri’s group [165] developed an enzyme-coating method that significantly optimized the mechanical stability, enabling its use in in vitro slices for selective acetylcholine detection. However, compared to in vivo monitoring, in vitro assays do not fully reflect the overall physiological trends in organisms, leading researchers to prefer in situ real-time monitoring in vivo.
References
- Teleanu, R.I.; Niculescu, A.G.; Roza, E.; Vladâcenco, O.; Grumezescu, A.M.; Teleanu, D.M. Neurotransmitters—Key Factors in Neurological and Neurodegenerative Disorders of the Central Nervous System. Int. J. Mol. Sci. 2022, 23, 5954.
- Zhang, X.; Hatamie, A.; Ewing, A.G. Nanoelectrochemical analysis inside a single living cell. Curr. Opin. Electrochem. 2020, 22, 94–101.
- Song, Q.; Li, Q.; Yan, J.; Song, Y. Echem methods and electrode types of the current in vivo electrochemical sensing. RSC Adv. 2022, 12, 17715–17739.
- Hatamie, A.; He, X.; Zhang, X.-W.; Oomen, P.E.; Ewing, A.G. Advances in nano/microscale electrochemical sensors and biosensors for analysis of single vesicles, a key nanoscale organelle in cellular communication. Biosens. Bioelectron. 2023, 220, 114899.
- Hatami, A.; Zhang, X.W.; Pieter, E.O.; Andrew, G.E. Nanoscale Electrochemical Sensors for Intracellular Measurements at the Single Cell. In Handbook of Nanobioelectrochemistry: Application in Devices and Biomolecular Sensing; Springer Nature: Singapore, 2023; pp. 131–152.
- Kissinger, P.T.; Hart, J.B.; Adams, R.N. Voltammetry in brain tissue—A new neurophysiological measurement. Brain Res. 1973, 55, 209–213.
- Cao, Q.; Shin, M.; Lavrik, N.V.; Venton, B.J. 3D-Printed Carbon Nanoelectrodes for In Vivo Neurotransmitter Sensing. Nano Lett. 2020, 20, 6831–6836.
- Dong, H.; Zhou, Q.; Zhang, L.; Tian, Y. Rational Design of Specific Recognition Molecules for Simultaneously Monitoring of Endogenous Polysulfide and Hydrogen Sulfide in the Mouse Brain. Angew. Chem. Int. Ed. 2019, 58, 13948–13953.
- Liang, H.; Zhu, M.; Ye, H.; Zeng, C.; Wang, S.; Niu, Y. Carbon fiber microelectrode array loaded with the diazonium salt-single-walled carbon nanotubes composites for the simultaneous monitoring of dopamine and serotonin in vivo. Anal. Chim. Acta 2021, 1186, 339086.
- Ganesana, M.; Trikantzopoulos, E.; Maniar, Y.; Lee, S.T.; Venton, B.J. Development of a novel micro biosensor for in vivo monitoring of glutamate release in the brain. Biosens. Bioelectron. 2019, 130, 103–109.
- Wei, H.; Wu, F.; Li, L.; Yang, X.; Xu, C.; Yu, P.; Ma, F.; Mao, L. Natural Leukocyte Membrane-Masked Microelectrodes with an Enhanced Antifouling Ability and Biocompatibility for In Vivo Electrochemical Sensing. Anal. Chem. 2020, 92, 11374–11379.
- Hu, K.; Le Vo, K.L.; Hatamie, A.; Ewing, A.G. Quantifying Intracellular Single Vesicular Catecholamine Concentration with Open Carbon Nanopipettes to Unveil the Effect of L-DOPA on Vesicular Structure. Angew. Chem. Int. Ed. 2022, 61, e202113406.
- Burmeister, J.J.; Gerhardt, G.A. Self-Referencing Ceramic-Based Multisite Microelectrodes for the Detection and Elimination of Interferences from the Measurement of l-Glutamate and Other Analytes. Anal. Chem. 2001, 73, 1037–1042.
- Xu, C.; Li, G.; Gan, L.; Yuan, B. In Situ Electrochemical Formation of Oxo-Functionalized Graphene on Glassy Carbon Electrode with Chemical Fouling Recovery and Antibiofouling Properties for Electrochemical Sensing of Reduced Glutathione. Antioxidants 2023, 12, 8.
- Feng, T.; Ji, W.; Tang, Q.; Wei, H.; Zhang, S.; Mao, J.; Zhang, Y.; Mao, L.; Zhang, M. Low-Fouling Nanoporous Conductive Polymer-Coated Microelectrode for In Vivo Monitoring of Dopamine in the Rat Brain. Anal. Chem. 2019, 91, 10786–10791.
- Kalant, H. A microdialysis procedure for extraction and isolation of corticosteroids from peripheral blood plasma. Biochem. J. 1958, 69, 99–103.
- Bito, L.; Davson, H.; Levin, E.; Murray, M.; Snider, N. The Concentrations of Free Amino acids and Other Electrolytes in Cerebrospinal Fluid, In Vivo Dialysate of Brain, and Blood Plasma of the DOG. J. Neurochem. 1966, 13, 1057–1067.
- Zhang, J.; Jaquins-Gerstl, A.; Nesbitt, K.M.; Rutan, S.C.; Michael, A.C.; Weber, S.G. In Vivo Monitoring of Serotonin in the Striatum of Freely Moving Rats with One Minute Temporal Resolution by Online Microdialysis–Capillary High-Performance Liquid Chromatography at Elevated Temperature and Pressure. Anal. Chem. 2013, 85, 9889–9897.
- Gong, M.; Zhang, N.; Maddukuri, N. Flow-gated capillary electrophoresis: A powerful technique for rapid and efficient chemical separation. Anal. Methods 2018, 10, 3131–3143.
- Qian, J.; Wu, Y.; Yang, H.; Michael, A.C. An Integrated Decoupler for Capillary Electrophoresis with Electrochemical Detection: Application to Analysis of Brain Microdialysate. Anal. Chem. 1999, 71, 4486–4492.
- Zhang, J.; Liu, Y.; Jaquins-Gerstl, A.; Shu, Z.; Michael, A.C.; Weber, S.G. Optimization for speed and sensitivity in capillary high performance liquid chromatography. The importance of column diameter in online monitoring of serotonin by microdialysis. J. Chromatogr. A 2012, 1251, 54–62.
- König, M.; Thinnes, A.; Klein, J. Microdialysis and its use in behavioural studies: Focus on acetylcholine. J. Neurosci. Methods 2018, 300, 206–215.
- Kho, C.M.; Enche Ab Rahim, S.K.; Ahmad, Z.A.; Abdullah, N.S. A Review on Microdialysis Calibration Methods: The Theory and Current Related Efforts. Mol. Neurobiol. 2016, 54, 3506–3527.
- Saylor, R.A.; Lunte, S.M. A review of microdialysis coupled to microchip electrophoresis for monitoring biological events. J. Chromatogr. A 2015, 1382, 48–64.
- Zhang, Z.; Hao, J.; Xiao, T.; Yu, P.; Mao, L. Online electrochemical systems for continuous neurochemical measurements with low-potential mediator-based electrochemical biosensors as selective detectors. Analyst 2015, 140, 5039–5047.
- Lin, Y.; Yu, P.; Mao, L. A multi-enzyme microreactor-based online electrochemical system for selective and continuous monitoring of acetylcholine. Analyst 2015, 140, 3781–3787.
- Zhang, M.; Mao, L. Enzyme-based amperometric biosensors for continuous and on-line monitoring of cerebral extracellular microdialysate. FBL 2005, 10, 345–352.
- Bobin, S.; Popot, M.A.; Bonnaire, Y.; Tabet, J.C. Approach to the determination of insulin-like-growth-factor-I (IGF-I) concentration in plasma by high-performance liquid chromatography-ion trap mass spectrometry: Use of a deconvolution algorithm for the quantification of multiprotonated molecules in electrospray ionization. Analyst 2001, 126, 1996–2001.
- Dagher, A.; Robbins, T.W. Personality, addiction, dopamine: Insights from Parkinson’s disease. Neuron 2009, 61, 502–510.
- Volkow, N.D.; Fowler, J.S.; Wang, G.J.; Swanson, J.M. Dopamine in drug abuse and addiction: Results from imaging studies and treatment implications. Mol. Psychiatry 2004, 9, 557–569.
- Brennan, A.R.; Arnsten, A.F.T. Neuronal mechanisms underlying attention deficit hyperactivity disorder—The influence of arousal on prefrontal cortical function. Ann. N. Y. Acad. Sci. 2008, 1129, 236–245.
- Kurian, M.A.; Gissen, P.; Smith, M.; Heales, S.J.R.; Clayton, P.T. The monoamine neurotransmitter disorders: An expanding range of neurological syndromes. Lancet Neurol. 2011, 10, 721–733.
- Marecos, C.; Ng, J.; Kurian, M.A. What is new for monoamine neurotransmitter disorders? J. Inherit. Metab. Dis. 2014, 37, 619–626.
- Hamdan, S.K.; Mohd Zain, A. In vivo Electrochemical Biosensor for Brain Glutamate Detection: A Mini Review. Malays. J. Med. Sci. 2014, 21, 12–26.
- Lau, A.; Tymianski, M. Glutamate receptors, neurotoxicity and neurodegeneration. Pflügers Arch. Eur. J. Physiol. 2010, 460, 525–542.
- Martin, W.R.W. MR Spectroscopy in Neurodegenerative Disease. Mol. Imaging Biol. 2007, 9, 196–203.
- Stone, J.M.; Morrison, P.D.; Pilowsky, L.S. Review: Glutamate and dopamine dysregulation in schizophrenia—A synthesis and selective review. J. Psychopharmacol. 2007, 21, 440–452.
- Fouani, L.; Menezes, S.V.; Paulson, M.; Richardson, D.R.; Kovacevic, Z. Metals and metastasis: Exploiting the role of metals in cancer metastasis to develop novel anti-metastatic agents. Pharmacol. Res. 2017, 115, 275–287.
- Jentsch, T.J.; Stein, V.; Weinreich, F.; Zdebik, A.A. Molecular Structure and Physiological Function of Chloride Channels. Physiol. Rev. 2002, 82, 503–568.
- Graefe, A.; Stanca, S.E.; Nietzsche, S.; Kubicova, L.; Beckert, R.; Biskup, C.; Mohr, G.J. Development and Critical Evaluation of Fluorescent Chloride Nanosensors. Anal. Chem. 2008, 80, 6526–6531.
- Ashton, T.D.; Jolliffe, K.A.; Pfeffer, F.M. Luminescent probes for the bioimaging of small anionic species in vitro and in vivo. Chem. Soc. Rev. 2015, 44, 4547–4595.
- Zhao, H.; Carney, K.E.; Falgoust, L.; Pan, J.W.; Sun, D.; Zhang, Z. Emerging roles of Na+/H+ exchangers in epilepsy and developmental brain disorders. Prog. Neurobiol. 2016, 138–140, 19–35.
- Rossi, D.J.; Brady, J.D.; Mohr, C. Astrocyte metabolism and signaling during brain ischemia. Nat. Neurosci. 2007, 10, 1377–1386.
- Vollmer, L.L.; Strawn, J.R.; Sah, R. Acid–base dysregulation and chemosensory mechanisms in panic disorder: A translational update. Transl. Psychiatry 2015, 5, e572.
- Takei, Y.; Ando, H.; Tsutsui, K. Chapter 103—Gasotransmitter Family. In Handbook of Hormones; Academic Press: San Diego, CA, USA, 2016; pp. 601–602.
- Pałasz, A.; Menezes, I.C.; Worthington, J.J. The role of brain gaseous neurotransmitters in anxiety. Pharmacol. Rep. 2021, 73, 357–371.
- Han, Y.; Qin, J.; Chang, X.; Yang, Z.; Bu, D.; Du, J. Modulating effect of hydrogen sulfide on gamma-aminobutyric acid B receptor in recurrent febrile seizures in rats. Neurosci. Res. 2005, 53, 216–219.
- Garcia-Bereguiain, M.A.; Samhan-Arias, A.K.; Martin-Romero, F.J.; Gutierrez-Merino, C. Hydrogen Sulfide Raises Cytosolic Calcium in Neurons Through Activation of L-Type Ca2+ Channels. Antioxid. Redox Signal. 2008, 10, 31–42.
- Schreier, S.M.; Muellner, M.K.; Steinkellner, H.; Hermann, M.; Esterbauer, H.; Exner, M.; Gmeiner, B.M.K.; Kapiotis, S.; Laggner, H. Hydrogen Sulfide Scavenges the Cytotoxic Lipid Oxidation Product 4-HNE. Neurotox. Res. 2010, 17, 249–256.
- Xie, C.; Luo, K.; Tan, L.; Yang, Q.; Zhao, X.; Zhou, L. A Review for In Vitro and In Vivo Detection and Imaging of Gaseous Signal Molecule Carbon Monoxide by Fluorescent Probes. Molecules 2022, 27, 8842.
- Li, W.; Li, R.; Chen, R.; Liang, X.; Song, W.; Lin, W. Activatable Photoacoustic Probe for In Situ Imaging of Endogenous Carbon Monoxide in the Murine Inflammation Model. Anal. Chem. 2021, 93, 8978–8985.
- Fu, G.-Q.; Xia, Y.-S.; Jiang, W.-L.; Wang, W.-X.; Tan, Z.-K.; Guo, K.-Y.; Mao, G.-J.; Li, C.-Y. A novel precipitating-fluorochrome-based fluorescent probe for monitoring carbon monoxide during drug-induced liver injury. Talanta 2022, 243, 123398.
- Zhang, C.; Peng, S.-Y.; Hong, S.; Chen, Q.-W.; Zeng, X.; Rong, L.; Zhong, Z.-L.; Zhang, X.-Z. Biomimetic carbon monoxide nanogenerator ameliorates streptozotocin induced type 1 diabetes in mice. Biomaterials 2020, 245, 119986.
- Yue, L.; Tang, Y.; Huang, H.; Song, W.; Lin, W. A fluorogenic probe for detecting CO with the potential integration of diagnosis and therapy (IDT) for cancer. Sens. Actuators B Chem. 2021, 344, 130245.
- Zhang, S.; Lachance, B.B.; Mattson, M.P.; Jia, X. Glucose metabolic crosstalk and regulation in brain function and diseases. Prog. Neurobiol. 2021, 204, 102089.
- Hollyer, T.R.; Bordoni, L.; Kousholt, B.S.; van Luijk, J.; Ritskes-Hoitinga, M.; Østergaard, L. The evidence for the physiological effects of lactate on the cerebral microcirculation: A systematic review. J. Neurochem. 2019, 148, 712–730.
- Xapelli, S.; Agasse, F.; Ferreira, R.; Silva, P.A.; Malva, O.J. Neuropeptide Y as an Endogenous Antiepileptic, Neuroprotective and Pro-Neurogenic Peptide. Recent Pat. CNS Drug Discov. 2006, 1, 315–324.
- Kalra, S.P.; Dube, M.G.; Pu, S.; Xu, B.; Horvath, T.L.; Kalra, P.S. Interacting Appetite-Regulating Pathways in the Hypothalamic Regulation of Body Weight. Endocr. Rev. 1999, 20, 68–100.
- O’Loughlin, E.K.; Pakan, J.M.P.; McDermott, K.W.; Yilmazer-Hanke, D. Expression of neuropeptide Y1 receptors in the amygdala and hippocampus and anxiety-like behavior associated with Ammon’s horn sclerosis following intrahippocampal kainate injection in C57BL/6J mice. Epilepsy Behav. 2014, 37, 175–183.
- Loewi, O. Über humorale übertragbarkeit der Herznervenwirkung. Pflüger’s Arch. Gesamte Physiol. Menschen Tiere 1921, 189, 239–242.
- Watson, C.J.; Venton, B.J.; Kennedy, R.T. In Vivo Measurements of Neurotransmitters by Microdialysis Sampling. Anal. Chem. 2006, 78, 1391–1399.
- Khan, A.S.; Michael, A.C. Invasive consequences of using micro-electrodes and microdialysis probes in the brain. TrAC Trends Anal. Chem. 2003, 22, 503–508.
- Khoshnevisan, K.; Maleki, H.; Honarvarfard, E.; Baharifar, H.; Gholami, M.; Faridbod, F.; Larijani, B.; Faridi Majidi, R.; Khorramizadeh, M.R. Nanomaterial based electrochemical sensing of the biomarker serotonin: A comprehensive review. Microchim. Acta 2019, 186, 49.
- Tiwari, J.N.; Vij, V.; Kemp, K.C.; Kim, K.S. Engineered Carbon-Nanomaterial-Based Electrochemical Sensors for Biomolecules. ACS Nano 2016, 10, 46–80.
- Shleev, S.; Tkac, J.; Christenson, A.; Ruzgas, T.; Yaropolov, A.I.; Whittaker, J.W.; Gorton, L. Direct electron transfer between copper-containing proteins and electrodes. Biosens. Bioelectron. 2005, 20, 2517–2554.
- Frey, O.; Holtzman, T.; McNamara, R.M.; Theobald, D.E.H.; van der Wal, P.D.; de Rooij, N.F.; Dalley, J.W.; Koudelka-Hep, M. Enzyme-based choline and l-glutamate biosensor electrodes on silicon microprobe arrays. Biosens. Bioelectron. 2010, 26, 477–484.
- Burmeister, J.J.; Davis, V.A.; Quintero, J.E.; Pomerleau, F.; Huettl, P.; Gerhardt, G.A. Glutaraldehyde Cross-Linked Glutamate Oxidase Coated Microelectrode Arrays: Selectivity and Resting Levels of Glutamate in the CNS. ACS Chem. Neurosci. 2013, 4, 721–728.
- Burmeister, J.J.; Pomerleau, F.; Palmer, M.; Day, B.K.; Huettl, P.; Gerhardt, G.A. Improved ceramic-based multisite microelectrode for rapid measurements of l-glutamate in the CNS. J. Neurosci. Methods 2002, 119, 163–171.
- Day, B.K.; Pomerleau, F.; Burmeister, J.J.; Huettl, P.; Gerhardt, G.A. Microelectrode array studies of basal and potassium-evoked release of l-glutamate in the anesthetized rat brain. J. Neurochem. 2006, 96, 1626–1635.
- Hascup, K.N.; Hascup, E.R.; Pomerleau, F.; Huettl, P.; Gerhardt, G.A. Second-by-second measures of L-glutamate in the prefrontal cortex and striatum of freely moving mice. J. Pharmacol. Exp. Ther. 2008, 324, 725.
- McLamore, E.S.; Mohanty, S.; Shi, J.; Claussen, J.; Jedlicka, S.S.; Rickus, J.L.; Porterfield, D.M. A self-referencing glutamate biosensor for measuring real time neuronal glutamate flux. J. Neurosci. Methods 2010, 189, 14–22.
- Rutherford, E.C.; Pomerleau, F.; Huettl, P.; Strömberg, I.; Gerhardt, G.A. Chronic second-by-second measures of l-glutamate in the central nervous system of freely moving rats. J. Neurochem. 2007, 102, 712–722.
- Stephens, M.L.; Pomerleau, F.; Huettl, P.; Gerhardt, G.A.; Zhang, Z. Real-time glutamate measurements in the putamen of awake rhesus monkeys using an enzyme-based human microelectrode array prototype. J. Neurosci. Methods 2010, 185, 264–272.
- Konradsson-Geuken, Å.; Gash, C.R.; Alexander, K.; Pomerleau, F.; Huettl, P.; Gerhardt, G.A.; Bruno, J.P. Second-by-second analysis of alpha 7 nicotine receptor regulation of glutamate release in the prefrontal cortex of awake rats. Synapse 2009, 63, 1069–1082.
- Burmeister, J.J.; Palmer, M.; Gerhardt, G.A. l-lactate measures in brain tissue with ceramic-based multisite microelectrodes. Biosens. Bioelectron. 2005, 20, 1772–1779.
- Burmeister, J.J.; Pomerleau, F.; Huettl, P.; Gash, C.R.; Werner, C.E.; Bruno, J.P.; Gerhardt, G.A. Ceramic-based multisite microelectrode arrays for simultaneous measures of choline and acetylcholine in CNS. Biosens. Bioelectron. 2008, 23, 1382–1389.
- Ledo, A.; Lourenço, C.F.; Laranjinha, J.; Brett, C.M.A.; Gerhardt, G.A.; Barbosa, R.M. Ceramic-Based Multisite Platinum Microelectrode Arrays: Morphological Characteristics and Electrochemical Performance for Extracellular Oxygen Measurements in Brain Tissue. Anal. Chem. 2017, 89, 1674–1683.
- Peng, Q.; Yan, X.; Shi, X.; Ou, S.; Gu, H.; Yin, X.; Shi, G.; Yu, Y. In vivo monitoring of superoxide anion from Alzheimer’s rat brains with functionalized ionic liquid polymer decorated microsensor. Biosens. Bioelectron. 2019, 144, 111665.
- Wang, Q.; Wei, H.; Zhang, Z.; Wang, E.; Dong, S. Nanozyme: An emerging alternative to natural enzyme for biosensing and immunoassay. TrAC Trends Anal. Chem. 2018, 105, 218–224.
- Lubin, A.A.; Plaxco, K.W. Folding-Based Electrochemical Biosensors: The Case for Responsive Nucleic Acid Architectures. Acc. Chem. Res. 2010, 43, 496–505.
- Farjami, E.; Campos, R.; Nielsen, J.S.; Gothelf, K.V.; Kjems, J.; Ferapontova, E.E. RNA Aptamer-Based Electrochemical Biosensor for Selective and Label-Free Analysis of Dopamine. Anal. Chem. 2013, 85, 121–128.
- Chávez, J.L.; Hagen, J.A.; Kelley-Loughnane, N. Fast and Selective Plasmonic Serotonin Detection with Aptamer-Gold Nanoparticle Conjugates. Sensors 2017, 17, 681.
- Santos-Cancel, M.; Simpson, L.W.; Leach, J.B.; White, R.J. Direct, Real-Time Detection of Adenosine Triphosphate Release from Astrocytes in Three-Dimensional Culture Using an Integrated Electrochemical Aptamer-Based Sensor. ACS Chem. Neurosci. 2019, 10, 2070–2079.
- Xiao, Y.; Piorek, B.D.; Plaxco, K.W.; Heeger, A.J. A Reagentless Signal-On Architecture for Electronic, Aptamer-Based Sensors via Target-Induced Strand Displacement. J. Am. Chem. Soc. 2005, 127, 17990–17991.
- Li, H.; Arroyo-Currás, N.; Kang, D.; Ricci, F.; Plaxco, K.W. Dual-Reporter Drift Correction to Enhance the Performance of Electrochemical Aptamer-Based Sensors in Whole Blood. J. Am. Chem. Soc. 2016, 138, 15809–15812.
- Swensen, J.S.; Xiao, Y.; Ferguson, B.S.; Lubin, A.A.; Lai, R.Y.; Heeger, A.J.; Plaxco, K.W.; Soh, H.T. Continuous, Real-Time Monitoring of Cocaine in Undiluted Blood Serum via a Microfluidic, Electrochemical Aptamer-Based Sensor. J. Am. Chem. Soc. 2009, 131, 4262–4266.
- Taylor, I.M.; Du, Z.; Bigelow, E.T.; Eles, J.R.; Horner, A.R.; Catt, K.A.; Weber, S.G.; Jamieson, B.G.; Cui, X.T. Aptamer-functionalized neural recording electrodes for the direct measurement of cocaine in vivo. J. Mater. Chem. B 2017, 5, 2445–2458.
- Stoltenburg, R.; Reinemann, C.; Strehlitz, B. SELEX—A (r)evolutionary method to generate high-affinity nucleic acid ligands. Biomol. Eng. 2007, 24, 381–403.
- Shaver, A.; Kundu, N.; Young, B.E.; Vieira, P.A.; Sczepanski, J.T.; Arroyo-Currás, N. Nuclease Hydrolysis Does Not Drive the Rapid Signaling Decay of DNA Aptamer-Based Electrochemical Sensors in Biological Fluids. Langmuir 2021, 37, 5213–5221.
- Leung, K.K.; Downs, A.M.; Ortega, G.; Kurnik, M.; Plaxco, K.W. Elucidating the Mechanisms Underlying the Signal Drift of Electrochemical Aptamer-Based Sensors in Whole Blood. ACS Sens. 2021, 6, 3340–3347.
- Arroyo-Currás, N.; Somerson, J.; Vieira, P.A.; Ploense, K.L.; Kippin, T.E.; Plaxco, K.W. Real-time measurement of small molecules directly in awake, ambulatory animals. Proc. Natl. Acad. Sci. USA 2017, 114, 645–650.
- Li, H.; Dauphin-Ducharme, P.; Arroyo-Currás, N.; Tran, C.H.; Vieira, P.A.; Li, S.; Shin, C.; Somerson, J.; Kippin, T.E.; Plaxco, K.W. A Biomimetic Phosphatidylcholine-Terminated Monolayer Greatly Improves the In Vivo Performance of Electrochemical Aptamer-Based Sensors. Angew. Chem. Int. Ed. 2017, 56, 7492–7495.
- Zhao, F.; Zhang, L.; Zhu, A.; Shi, G.; Tian, Y. In vivo monitoring of local pH values in a live rat brain based on the design of a specific electroactive molecule for H+. Chem. Commun. 2016, 52, 3717–3720.
- Zhang, Z.; Li, M.; Zuo, Y.; Chen, S.; Zhuo, Y.; Lu, M.; Shi, G.; Gu, H. In Vivo Monitoring of pH in Subacute PD Mouse Brains with a Ratiometric Electrochemical Microsensor Based on Poly(melamine) Films. ACS Sens. 2022, 7, 235–244.
- Cui, B.; Liu, P.; Liu, X.; Liu, S.; Zhang, Z. Molecularly imprinted polymers for electrochemical detection and analysis: Progress and perspectives. J. Mater. Res. Technol. 2020, 9, 12568–12584.
- Kröger, S.; Turner, A.P.F.; Mosbach, K.; Haupt, K. Imprinted Polymer-Based Sensor System for Herbicides Using Differential-Pulse Voltammetry on Screen-Printed Electrodes. Anal. Chem. 1999, 71, 3698–3702.
- Tsai, T.-C.; Han, H.-Z.; Cheng, C.-C.; Chen, L.-C.; Chang, H.-C.; Chen, J.-J.J. Modification of platinum microelectrode with molecularly imprinted over-oxidized polypyrrole for dopamine measurement in rat striatum. Sens. Actuators B Chem. 2012, 171–172, 93–101.
- Xin, Y.; Li, Z.; Wu, W.; Fu, B.; Wu, H.; Zhang, Z. Recognition unit-free and self-cleaning photoelectrochemical sensing platform on TiO2 nanotube photonic crystals for sensitive and selective detection of dopamine release from mouse brain. Biosens. Bioelectron. 2017, 87, 396–403.
- Si, B.; Song, E. Molecularly imprinted polymers for the selective detection of multi-analyte neurotransmitters. Microelectron. Eng. 2018, 187–188, 58–65.
- Li, Y.; Song, H.; Zhang, L.; Zuo, P.; Ye, B.-c.; Yao, J.; Chen, W. Supportless electrochemical sensor based on molecularly imprinted polymer modified nanoporous microrod for determination of dopamine at trace level. Biosens. Bioelectron. 2016, 78, 308–314.
- Yadav, P.K.; Martinov, M.; Vitvitsky, V.; Seravalli, J.; Wedmann, R.; Filipovic, M.R.; Banerjee, R. Biosynthesis and Reactivity of Cysteine Persulfides in Signaling. J. Am. Chem. Soc. 2016, 138, 289–299.
- Tan, B.H.; Wong, P.T.H.; Bian, J.-S. Hydrogen sulfide: A novel signaling molecule in the central nervous system. Neurochem. Int. 2010, 56, 3–10.
- Zhao, F.; Liu, Y.; Dong, H.; Feng, S.; Shi, G.; Lin, L.; Tian, Y. An Electrochemophysiological Microarray for Real-Time Monitoring and Quantification of Multiple Ions in the Brain of a Freely Moving Rat. Angew. Chem. Int. Ed. 2020, 59, 10426–10430.
- Bucher, E.S.; Wightman, R.M. Electrochemical Analysis of Neurotransmitters. Annu. Rev. Anal. Chem. 2015, 8, 239–261.
- Adamah-Biassi, E.B.; Almonte, A.G.; Blagovechtchenski, E.; Grinevich, V.P.; Weiner, J.L.; Bonin, K.D.; Budygin, E.A. Real time adenosine fluctuations detected with fast-scan cyclic voltammetry in the rat striatum and motor cortex. J. Neurosci. Methods 2015, 256, 56–62.
- Ross, A.E.; Venton, B.J. Sawhorse Waveform Voltammetry for Selective Detection of Adenosine, ATP, and Hydrogen Peroxide. Anal. Chem. 2014, 86, 7486–7493.
- Van Gompel, J.J.; Bower, M.R.; Worrell, G.A.; Stead, M.; Chang, S.-Y.; Goerss, S.J.; Kim, I.; Bennet, K.E.; Meyer, F.B.; Marsh, W.R.; et al. Increased cortical extracellular adenosine correlates with seizure termination. Epilepsia 2014, 55, 233–244.
- Ding, S.; Liu, Y.; Ma, C.; Zhang, J.; Zhu, A.; Shi, G. Development of Glass-sealed Gold Nanoelectrodes for in vivo Detection of Dopamine in Rat Brain. Electroanalysis 2018, 30, 1041–1046.
- Sanghavi, B.J.; Wolfbeis, O.S.; Hirsch, T.; Swami, N.S. Nanomaterial-based electrochemical sensing of neurological drugs and neurotransmitters. Microchim. Acta 2015, 182, 1–41.
- Thomas, T.; Mascarenhas, R.J.; Nethravathi, C.; Rajamathi, M.; Kumara Swamy, B.E. Graphite oxide bulk modified carbon paste electrode for the selective detection of dopamine: A voltammetric study. J. Electroanal. Chem. 2011, 659, 113–119.
- Chen, X.; Chen, J.; Dong, H.; Yu, Q.; Zhang, S.; Chen, H. Sensitive detection of dopamine using a platinum microelectrode modified by reduced graphene oxide and gold nanoparticles. J. Electroanal. Chem. 2019, 848, 113244.
- Taylor, I.M.; Patel, N.A.; Freedman, N.C.; Castagnola, E.; Cui, X.T. Direct in Vivo Electrochemical Detection of Resting Dopamine Using Poly(3,4-ethylenedioxythiophene)/Carbon Nanotube Functionalized Microelectrodes. Anal. Chem. 2019, 91, 12917–12927.
- He, E.; Xu, S.; Dai, Y.; Wang, Y.; Xiao, G.; Xie, J.; Xu, S.; Fan, P.; Mo, F.; Wang, M.; et al. SWCNTs/PEDOT:PSS-Modified Microelectrode Arrays for Dual-Mode Detection of Electrophysiological Signals and Dopamine Concentration in the Striatum under Isoflurane Anesthesia. ACS Sens. 2021, 6, 3377–3386.
- Hou, H.; Jin, Y.; Wei, H.; Ji, W.; Xue, Y.; Hu, J.; Zhang, M.; Jiang, Y.; Mao, L. A Generalizable and Noncovalent Strategy for Interfacing Aptamers with a Microelectrode for the Selective Sensing of Neurotransmitters In Vivo. Angew. Chem. Int. Ed. 2020, 59, 18996–19000.
- Li, X.; Jin, Y.; Zhu, F.; Liu, R.; Jiang, Y.; Jiang, Y.; Mao, L. Electrochemical Conjugation of Aptamers on a Carbon Fiber Microelectrode Enables Highly Stable and Selective In Vivo Neurosensing. Angew. Chem. Int. Ed. 2022, 61, e202208121.
- Plock, N.; Kloft, C. Microdialysis—Theoretical background and recent implementation in applied life-sciences. Eur. J. Pharm. Sci. 2005, 25, 1–24.
- Bito, L.Z.; Davson, H. Local variations in cerebrospinal fluid composition and its relationship to the composition of the extracellular fluid of the cortex. Exp. Neurol. 1966, 14, 264–280.
- Zhang, M.; Liu, K.; Gong, K.; Su, L.; Chen, Y.; Mao, L. Continuous On-Line Monitoring of Extracellular Ascorbate Depletion in the Rat Striatum Induced by Global Ischemia with Carbon Nanotube-Modified Glassy Carbon Electrode Integrated into a Thin-Layer Radial Flow Cell. Anal. Chem. 2005, 77, 6234–6242.
- Bert, L.; Robert, F.; Denoroy, L.; Stoppini, L.; Renaud, B. Enhanced temporal resolution for the microdialysis monitoring of catecholamines and excitatory amino acids using capillary electrophoresis with laser-induced fluorescence detection Analytical developments and in vitro validations. J. Chromatogr. A 1996, 755, 99–111.
- Hernandez, L.; Escalona, J.; Verdeguer, P.; Guzman, N.A. In Vivo Monitoring of Brain Glutamate by Microdialysis Coupled to Capillary Electrophoresis and Laser Induced Fluorescence Detection. J. Liq. Chromatogr. 1993, 16, 2149–2160.
- Hernandez, L.; Tucci, S.; Guzman, N.; Paez, X. In vivo monitoring of glutamate in the brain by microdialysis and capillary electrophoresis with laser-induced fluorescence detection. J. Chromatogr. A 1993, 652, 393–398.
- O’Shea, T.J.; Weber, P.L.; Bammel, B.P.; Lunte, C.E.; Lunte, S.M.; Smyth, M.R. Monitoring excitatory amino acid release in vivo by microdialysis with capillary electrophoresis- electrochemistry. J. Chromatogr. A 1992, 608, 189–195.
- Robert, F.; Bert, L.; Lambás-Señas, L.; Denoroy, L.; Renaud, B. In vivo monitoring of extracellular noradrenaline and glutamate from rat brain cortex with 2-min microdialysis sampling using capillary electrophoresis with laser-induced fluorescence detection. J. Neurosci. Methods 1996, 70, 153–162.
- Zhou, J.; Heckert, D.M.; Zuo, H.; Lunte, C.E.; Lunte, S.M. On-line coupling of in vivo microdialysis with capillary electrophoresis/electrochemistry. Anal. Chim. Acta 1999, 379, 307–317.
- Tao, L.; Kennedy, R.T. Measurement of antibody-antigen dissociation constants using fast capillary electrophoresis with laser-induced fluorescence detection. Electrophoresis 1997, 18, 112–117.
- Lada, M.W.; Vickroy, T.W.; Kennedy, R.T. High Temporal Resolution Monitoring of Glutamate and Aspartate in Vivo Using Microdialysis On-Line with Capillary Electrophoresis with Laser-Induced Fluorescence Detection. Anal. Chem. 1997, 69, 4560–4565.
- O’Shea, T.J.; Greenhagen, R.D.; Lunte, S.M.; Lunte, C.E.; Smyth, M.R.; Radzik, D.M.; Watanabe, N. Capillary electrophoresis with electrochemical detection employing an on-column Nafion joint. J. Chromatogr. A 1992, 593, 305–312.
- Wallingford, R.A.; Ewing, A.G. Separation of serotonin from catechols by capillary zone electrophoresis with electrochemical detection. Anal. Chem. 1989, 61, 98–100.
- Curry, P.D., Jr.; Engstro-Silverman, C.E.; Ewing, A.G. Electrochemical detection for capillary electrophoresis. Electroanalysis 1991, 3, 587–596.
- O’Shea, T.J.; Telting-Diaz, M.W.; Lunte, S.M.; Lunte, C.E.; Smyth, M.R. Capillary electrophoresis—Electrochemistry of microdialysis samples for pharmacokinetic studies. Electroanalysis 1992, 4, 463–468.
- Woolley, A.T.; Lao, K.; Glazer, A.N.; Mathies, R.A. Capillary Electrophoresis Chips with Integrated Electrochemical Detection. Anal. Chem. 1998, 70, 684–688.
- Herzog, G.; Damien, W.M.A. Electrochemical Strategies in Detection Science; Royal Society of Chemistry: London, UK, 2017; Volume 80, p. 1483.
- Nandi, P.; Lunte, S.M. Recent trends in microdialysis sampling integrated with conventional and microanalytical systems for monitoring biological events: A review. Anal. Chim. Acta 2009, 651, 1–14.
- Nuchtavorn, N.; Suntornsuk, W.; Lunte, S.M.; Suntornsuk, L. Recent applications of microchip electrophoresis to biomedical analysis. J. Pharm. Biomed. Anal. 2015, 113, 72–96.
- Oborny, N.J.; Costa, E.E.M.; Suntornsuk, L.; Abreu, F.C.; Lunte, S.M. Evaluation of a Portable Microchip Electrophoresis Fluorescence Detection System for the Analysis of Amino Acid Neurotransmitters in Brain Dialysis Samples. Anal. Sci. 2016, 32, 35–40.
- Saylor, R.A.; Lunte, S.M. PDMS/glass hybrid device with a reusable carbon electrode for on-line monitoring of catecholamines using microdialysis sampling coupled to microchip electrophoresis with electrochemical detection. Electrophoresis 2018, 39, 462–469.
- Gunawardhana, S.M.; Bulgakova, G.A.; Barybin, A.M.; Thomas, S.R.; Lunte, S.M. Progress toward the development of a microchip electrophoresis separation-based sensor with electrochemical detection for on-line in vivo monitoring of catecholamines. Analyst 2020, 145, 1768–1776.
- Lada, M.W.; Kennedy, R.T. Quantitative in Vivo Monitoring of Primary Amines in Rat Caudate Nucleus Using Microdialysis Coupled by a Flow-Gated Interface to Capillary Electrophoresis with Laser-Induced Fluorescence Detection. Anal. Chem. 1996, 68, 2790–2797.
- Shou, M.; Smith, A.D.; Shackman, J.G.; Peris, J.; Kennedy, R.T. In vivo monitoring of amino acids by microdialysis sampling with on-line derivatization by naphthalene-2,3-dicarboxyaldehyde and rapid micellar electrokinetic capillary chromatography. J. Neurosci. Methods 2004, 138, 189–197.
- Ciriacks Klinker, C.; Bowser, M.T. 4-Fluoro-7-nitro-2,1,3-benzoxadiazole as a Fluorogenic Labeling Reagent for the in Vivo Analysis of Amino Acid Neurotransmitters Using Online Microdialysis−Capillary Electrophoresis. Anal. Chem. 2007, 79, 8747–8754.
- McNeff, C.V.; Yan, B.; Stoll, D.R.; Henry, R.A. Practice and theory of high temperature liquid chromatography. J. Sep. Sci. 2007, 30, 1672–1685.
- Wu, N.; Clausen, A.M. Fundamental and practical aspects of ultrahigh pressure liquid chromatography for fast separations. J. Sep. Sci. 2007, 30, 1167–1182.
- Xiang, Y.; Liu, Y.; Lee, M.L. Ultrahigh pressure liquid chromatography using elevated temperature. J. Chromatogr. A 2006, 1104, 198–202.
- Liu, Y.; Zhang, J.; Xu, X.; Zhao, M.K.; Andrews, A.M.; Weber, S.G. Capillary Ultrahigh Performance Liquid Chromatography with Elevated Temperature for Sub-One Minute Separations of Basal Serotonin in Submicroliter Brain Microdialysate Samples. Anal. Chem. 2010, 82, 9611–9616.
- Castro, H.C.V.; Valenzuela, L.L.C.; Sanchez, C.S.J.; Pena, P.K.; Perez, J.L.S.; Ibarra, O.J.; Villagran, M.A. An Update of the Classical and Novel Methods Used for Measuring Fast Neurotransmitters During Normal and Brain Altered Function. Curr. Neuropharmacol. 2014, 12, 490–508.
- Wilson, G.S.; Gifford, R. Biosensors for real-time in vivo measurements. Biosens. Bioelectron. 2005, 20, 2388–2403.
- Thevenot, D.R.; Tóth, K.; Durst, R.A.; Wilson, G.S. Electrochemical Biosensors: Recommended Definitions and Classification. Pure Appl. Chem. 1999, 71, 2333–2348.
- Yu, Y.; Liu, X.; Jiang, D.; Sun, Q.; Zhou, T.; Zhu, M.; Jin, L.; Shi, G. -Au/Pt biosensor for glutamate sensing in vivo integrated with on-line microdialysis system. Biosens. Bioelectron. 2011, 26, 3227–3232.
- Yu, P.; He, X.; Zhang, L.; Mao, L. Dual Recognition Unit Strategy Improves the Specificity of the Adenosine Triphosphate (ATP) Aptamer Biosensor for Cerebral ATP Assay. Anal. Chem. 2015, 87, 1373–1380.
- Gu, H.; Hou, Q.; Liu, Y.; Cai, Y.; Guo, Y.; Xiang, H.; Chen, S. On-line regeneration of electrochemical biosensor for in vivo repetitive measurements of striatum Cu2+ under global cerebral ischemia/reperfusion events. Biosens. Bioelectron. 2019, 135, 111–119.
- Liu, F.; Dong, H.; Tian, Y. Real-time monitoring of peroxynitrite (ONOO−) in the rat brain by developing a ratiometric electrochemical biosensor. Analyst 2019, 144, 2150–2157.
- Jiang, Y.; Xiao, X.; Li, C.; Luo, Y.; Chen, S.; Shi, G.; Han, K.; Gu, H. Facile Ratiometric Electrochemical Sensor for In Vivo/Online Repetitive Measurements of Cerebral Ascorbic Acid in Brain Microdiaysate. Anal. Chem. 2020, 92, 3981–3989.
- Wang, X.; Xu, T.; Zhang, Y.; Gao, N.; Feng, T.; Wang, S.; Zhang, M. In Vivo Detection of Redox-Inactive Neurochemicals in the Rat Brain with an Ion Transfer Microsensor. ACS Sens. 2021, 6, 2757–2762.
- He, X.; Zhang, K.; Li, T.; Jiang, Y.; Yu, P.; Mao, L. Micrometer-Scale Ion Current Rectification at Polyelectrolyte Brush-Modified Micropipets. J. Am. Chem. Soc. 2017, 139, 1396–1399.
- Zhang, K.; He, X.; Liu, Y.; Yu, P.; Fei, J.; Mao, L. Highly Selective Cerebral ATP Assay Based on Micrometer Scale Ion Current Rectification at Polyimidazolium-Modified Micropipettes. Anal. Chem. 2017, 89, 6794–6799.
- Chae, M.-S.; Yoo, Y.K.; Kim, J.; Kim, T.G.; Hwang, K.S. Graphene-based enzyme-modified field-effect transistor biosensor for monitoring drug effects in Alzheimer’s disease treatment. Sens. Actuators B Chem. 2018, 272, 448–458.
- Fenoy, G.E.; Marmisollé, W.A.; Azzaroni, O.; Knoll, W. Acetylcholine biosensor based on the electrochemical functionalization of graphene field-effect transistors. Biosens. Bioelectron. 2020, 148, 111796.
- Nakatsuka, N.; Yang, K.-A.; Abendroth, J.M.; Cheung, K.M.; Xu, X.; Yang, H.; Zhao, C.; Zhu, B.; Rim, Y.S.; Yang, Y.; et al. Aptamer–field-effect transistors overcome Debye length limitations for small-molecule sensing. Science 2018, 362, 319–324.
- Tan, C.; Robbins, E.M.; Wu, B.; Cui, X.T. Recent Advances in In Vivo Neurochemical Monitoring. Micromachines 2021, 12, 208.
- Roham, M.; Daberkow, D.P.; Ramsson, E.S.; Covey, D.P.; Pakdeeronachit, S.; Garris, P.A.; Mohseni, P. A Wireless IC for Wide-Range Neurochemical Monitoring Using Amperometry and Fast-Scan Cyclic Voltammetry. IEEE Trans. Biomed. Circuits Syst. 2008, 2, 3–9.
- Tonello, S.; Abate, G.; Borghetti, M.; Marziano, M.; Serpelloni, M.; Uberti, D.L.; Lopomo, N.F.; Memo, M.; Sardini, E. Wireless Point-of-Care Platform with Screen-Printed Sensors for Biomarkers Detection. IEEE Trans. Instrum. Meas. 2017, 66, 2448–2455.
- Tageldeen, M.K.; Gowers, S.A.N.; Leong, C.L.; Boutelle, M.G.; Drakakis, E.M. Traumatic brain injury neuroelectrochemical monitoring: Behind-the-ear micro-instrument and cloud application. J. NeuroEngineering Rehabil. 2020, 17, 114.
- Wu, H.; Meng, Z.; Wang, J.; Yao, G.; Yang, L.; Zeng, Z.; She, K.; Zhao, S.; Wang, G.; Zhang, Y.; et al. Aptamer functionalized cell membrane for brain and nerve cell sensing with high sensitivity and stability. Biosens. Bioelectron. 2023, 227, 115149.
- Hossain, I.; Tan, C.; Doughty, P.T.; Dutta, G.; Murray, T.A.; Siddiqui, S.; Iasemidis, L.; Arumugam, P.U. A Novel Microbiosensor Microarray for Continuous ex Vivo Monitoring of Gamma-Aminobutyric Acid in Real-Time. Front. Neurosci. 2018, 12, 500.
- Asri, R.; O’Neill, B.; Patel, J.C.; Siletti, K.A.; Rice, M.E. Detection of evoked acetylcholine release in mouse brain slices. Analyst 2016, 141, 6416–6421.
More
Information
Subjects:
Chemistry, Analytical
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
742
Revisions:
2 times
(View History)
Update Date:
27 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




