Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Roshan Deen | -- | 1355 | 2024-03-25 08:07:54 | | | |
| 2 | Camila Xu | Meta information modification | 1355 | 2024-03-25 09:33:25 | | | | |
| 3 | Camila Xu | Meta information modification | 1355 | 2024-03-26 03:04:58 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Hasan, B.; Alansari, R.; Torsten, U.; Deen, G.R. Vaginal Infections and Treatment. Encyclopedia. Available online: https://encyclopedia.pub/entry/56469 (accessed on 07 February 2026).
Hasan B, Alansari R, Torsten U, Deen GR. Vaginal Infections and Treatment. Encyclopedia. Available at: https://encyclopedia.pub/entry/56469. Accessed February 07, 2026.
Hasan, Bushra, Renad Alansari, Uwe Torsten, G. Roshan Deen. "Vaginal Infections and Treatment" Encyclopedia, https://encyclopedia.pub/entry/56469 (accessed February 07, 2026).
Hasan, B., Alansari, R., Torsten, U., & Deen, G.R. (2024, March 25). Vaginal Infections and Treatment. In Encyclopedia. https://encyclopedia.pub/entry/56469
Hasan, Bushra, et al. "Vaginal Infections and Treatment." Encyclopedia. Web. 25 March, 2024.
Copy Citation
Vaginal infections are a global public health issue affecting worldwide up to 70% of women of reproductive age. The symptoms or clinical manifestations are itching, irritation, abnormal vaginal discharge, and discomfort when urinating and during sexual activity.
vaginal infections
biofilm
bacterial vaginosis
vaginal drug delivery
1. Introduction
Vaginal infections are a global public health issue affecting worldwide up to 70% of women of reproductive age [1][2]. The symptoms or clinical manifestations are itching, irritation, abnormal vaginal discharge, and discomfort when urinating and during sexual activity. In some cases, the infections may be asymptomatic or show mild symptoms, The most common infections caused by bacteria, fungus, and protozoa are bacterial vaginosis (BV), vulvovaginal candidiasis (VVC), and trichomoniasis [3][4]. Bacterial vaginosis is caused by the species Staphylococcus and Peptostreptococcus, Enterobacteriaceae, Gardnerella vaginalis, and Mycoplasma hominis. Vulvovaginal candidiasis is caused by Candida albicans, Candida tropicalis, Candida parapsilosos, Candida crusei, Candida glabrata, Candida stellatoidea, and Candida lusitaniae. Trichomoniasis is caused by the human protozoan pathogen Trichomonas vaginalis.
The female reproductive system is composed of the fallopian tubes, ovary, cervix, ectocervix, and vagina. The vagina is a distensible organ (~9 cm in length), which is characterized by a stratified epithelium, fibromuscular layer, lamina propria, and adventitia. The vagina is covered with cervical mucus which is composed of water (90%), urea, carbohydrates, mucins, fatty acids, proteolytic enzymes, and salts [5]. The consistency of cervical mucus and the thickness of the vaginal epithelium vary throughout the menstrual cycle and are regulated by hormones such as estrogen. The main functions of the mucus are to provide lubrication and protection against infections. The layers of vaginal tissue and compositions of mucus are illustrated in Figure 1.
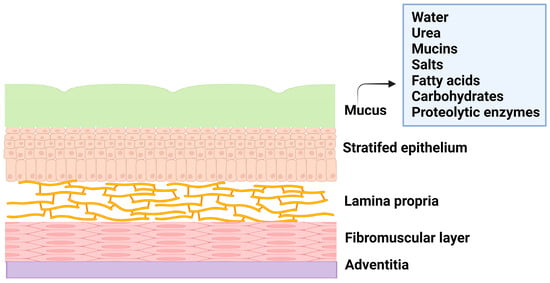
Figure 1. Illustration of layers of vagina tissue and mucus composition.
Various factors, such as age, menstrual cycle, hormone level etc., influence the physiology of the vagina, and these factors affect the natural vaginal flora or microbiota and the amount of vaginal fluids. The Lactobacillus species is the predominant microorganism that protects the vagina from the invasion of pathogens by producing lactic acid that provides the acidic environment of the vagina. When this normal symbiotic mutual relationship becomes imbalanced, the natural vaginal flora is heavily disturbed, leading to the onset of vaginal infections that promote the colonization of pathogens.
These pathogens thrive in the vagina by producing a biofilm that leads to the recurrence of the infection even after treatment [6][7], as the biofilm is an adaptation of the bacteria to resist any applied drugs and evade the immune system. The biofilm is composed of a group of pathogenic microbes adhered to one another on a surface that is, by extracellular polymeric substances, produced by the microbes. The formation of biofilm is illustrated in Figure 2. Biofilm is a physical barrier that limits the accessibility of drug molecules and enhances the thriving of pathogens. In this aspect, any drug formulation for vaginal delivery should not be harmful to the vaginal flora and its environment.
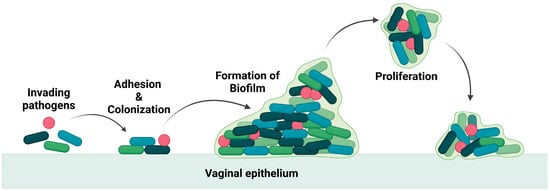
Figure 2. Illustration of invasion of pathogen and biofilm formation.
Vaginal infections, particularly bacterial vaginosis, are comparatively higher in non-pregnant women than in pregnant women and women at the age of 40 and above. This difference in the rate of infection is attributed to the imbalance of estrogen that affects the viability of the Lactobacillus species in the vagina.
2. Vaginal Infections and Treatment
Vaginal infections are a reproductive health issue, globally affecting women at their reproductive age, and the reported cases are of microbial origin. These include pathogenic bacteria, fungi, viruses, or parasites. The common types of vaginal infections are bacterial vaginosis (BV), vulva vaginal candidiasis (VVC) and trichomoniasis, human immunodeficiency virus (HIV) infections, and human papilloma virus (HPV) infections [1][2][3][4].
2.1. Bacterial Vaginosis (BV)
BV is the most prevalent infection globally, ranging from 23–29%, and the risk factors are sexual intercourse, douching, and poor hygiene. It is caused by an overgrowth of anaerobic bacteria and microaerophilic bacteria, such as Gardnerella vaginalis, Autopodium vaginae, Bacteroides spp., etc. The symptoms are thin white vaginal discharge with a fishy odour, irritation, and itchiness, and in general, the infection is diagnosed using the Nugent criteria, Amsel criteria, and Hay–Ison criteria [8]. The most common treatments for BV are antimicrobial formulations, such as oral metronidazole, oral clindamycin, oral tinidazole, metronidazole gel, clindamycin cream, and clindamycin ovules [9][10][11].
2.2. Vulva Vaginal Candidiasis (VVC)
VVC is primarily caused by the fungus Candida albicans, and it is estimated that nearly 75% of women will experience this infection at least once in their lifetime [12]. The symptoms of these infections are abnormal vaginal discharge, vaginal soreness, dyspareunia, and dysuria. Treatment of VVC is through oral and topical azole therapies, such as fluconazole, clotrimazole, miconazole, tioconazole, terconazole, and butoconazole [13][14][15].
2.3. Trichomoniasis
Trichomoniasis is a parasitic infection and is caused by a protozoan parasite, Trichomonas vaginalis. This is a nonviral sexually transmitted infection and, in 2016, has infected around 5.3% of women worldwide (the majority being asymptomatic). The symptoms of these infections are yellow–green vaginal discharge, lower abdominal pain, dysuria, and irritation in the vulva [16]. Infertility, poor pregnancy outcomes, and acquisition of sexually transmitted infections are associated with trichomoniasis [17]. The treatment is mainly through oral antibiotics, such as metronidazole and tinidazole. For complete eradication of the infection, topical formulations are not recommended due to the lower cure rate because of the poor residence time of the formulation on the vaginal tissues [18].
2.4. Human Immunodeficiency Viral Infection (HIV)
One of the most common viral sexually transmitted infections is HIV, with an estimate of about 38 million people being affected. Elevated risks are among intravenous drug users, sex workers, and the transgender community [19]. Symptoms of infections include liver dysfunctions, myalgias, swollen lymph nodes, tuberculosis, and AIDS. The currently available antiretroviral drugs are nucleoside or nucleotide reverse transcriptase inhibitors (tenofovir), non-nucleoside reverse transcriptase inhibitors (nevirapine), protease inhibitors (ritonavir, indinavir), integrase inhibitors (raltegravir), CCR5 antagonist (maraviroc), and fusion inhibitors (enfuvirtide) [20]. All of these drugs inhibit the various stages of the virus’ life cycle.
2.5. Human Papilloma Viral Infection (HPV) (Low Risk and High Risk)
This viral infection occurs in the cervix and squamous epithelium (the basal layer) of the vagina. The virus is in the form of episomes in the basal layer and multiplies through the differentiation of epithelial cells [21]. Many patients in the initial stage of infection experience mild symptoms (development of genital warts) due to the production of antimicrobial peptides by the epithelial cells and the natural chemicals present in the vaginal mucus. Lasting HPV infections may result in the integration of the high risk HPV DNA into the host DNA and may lead to cervical, anogenital, and oropharyngeal cancer [22]. Vaccination and cryotherapy are the most effective approaches suggested by the World Health Organization (WHO) for the prevention of cervical cancer and the management of precancerous lesions [23]. The chemical structures of some important drugs used in the treatment of vaginal infections and sexually transmitted infections are shown in Figure 3.
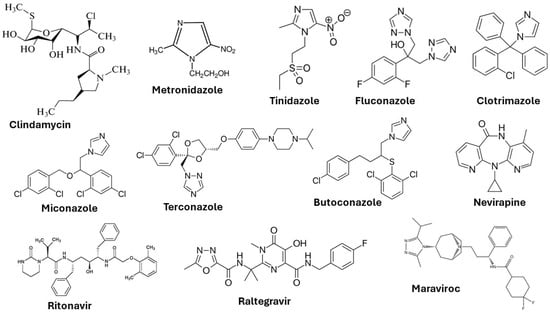
Figure 3. Chemical structures of drugs used to treat vaginal infections and sexually transmitted infections.
In the management of vaginal infections, the oral administration of drugs and topical applications are some of the current therapeutic approaches. However, both forms of drug-delivery approaches suffer from numerous systemic adverse effects [24]. Oral administration of the antibiotic metronidazole causes nausea, insomnia, dizziness, and dry mouth. The long-term oral administration of this antibiotic may lead to leukopenia and neutropenia [25]. Oral administration of miconazole nitrate, an antifungal drug leads to thrombocytopenic purpura, a blood disorder [26]. Apart from these, oral administration of such drugs may have serious side effects in pregnant women and women suffering from gastrointestinal disorders.
Given the high exposure contact surface and dense vascularization, the vagina is an alternative site for local and systemic drug delivery. The main advantage is the avoidance of the acidic gastrointestinal environment, fewer side effects and bypassing of the hepatic first-phase effect. Several conventional formulations, in the form of capsules, creams, solutions, gels, and vaginal suppositories, are used in the treatment of vaginal infections. These pharmaceutical products for vaginal drug delivery also suffer from a few limitations, such as poor adhesion, short retention or residence time, and poor release of the drug [26][27]. To address these limitations, formulations based on hydrogels have been developed. Hydrogels are well-suited for controlled delivery of biologically active therapeutics owing to their biocompatibility, high porosity, and high water-retention properties.
References
- Loveless, M.; Myint, O. Vulvovaginitis—Presentation of more common problems in pediatric and adolescent gynecology. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 48, 14–27.
- Gosecka, M.; Gosecki, M. Antimicrobial-polymer-based hydrogels for the intravaginal therapies—Engineering considerations. Pharmaceutics 2021, 13, 1393.
- Cook, M.; Brown, M.B. Polymeric gels for intravaginal drug delivery. J. Control. Release 2018, 270, 145–157.
- Palmeira-de-Oliveira, R.; Palmeira-de-Oliveira, A.; Martinez-de-Oliveira, J. New strategies for local treatment of vaginal infection. Adv. Drug Deli. Rev. 2015, 92, 105–122.
- Chappell, C.A.; Rohan, L.C.; Moncia, B.J.; Wang, I.; Meyn, L.A.; Bunge, K.; Hillier, S.L. The effects of reproductive hormones on the physical properties of cervicovaginal fluid. Am. J. Obstet. Gynecol. 2014, 211, 226e1–226e7.
- Martins dos Santos, A.; Carvalho, S.G.; Sousa Araujo, V.H.; Carvalho, G.C.; Daflon, G.; Chorilli, M. Recent advances in hydrogels as a strategy for drug delivery intended to vaginal infections. Int. J. Pharmaceut. 2020, 590, 119867–119878.
- Arpa, M.D.; Yoltas, A.; Onay Tarlan, E.; Senyuz, C.S.; Siphai, H.; Aydin, A.; Ustundag Okur, N. New therapeutic system based on hydrogels for vaginal candidiasis management: Formulation-characterization and in in vitro evaluation based on vaginal irritation and direct contact test. Pharm. Dev. Technol. 2020, 25, 1238–1248.
- Vazquez, F.; Fernández-Blázquez, A.; García, B. Vaginosis. Vaginal microbiota. Enferm. Infec. Microbiol. Clin. 2019, 37, 592–601.
- Gonçalves, B.; Ferreira, C.; Alves, C.T.; Henriques, M.; Azeredo, J.; Silva, S. Vulvovaginal candidiasis: Epidemiology, microbiology and risk factors. Crit. Rev. Microbiol. 2016, 42, 905–927.
- Workowski, K. Bacterial Vaginosis-2015 STD Treatment Guidelines. 2015. Available online: https://www.cdc.gov/std/tg2015/bv.htm (accessed on 4 January 2024).
- Jones, A. Bacterial vaginosis: A review of treatment, recurrence, and disparities. J. Nurse Pract. 2019, 15, 420–423.
- Denning, D.W.; Kneale, M.; Sobel, J.D.; Rautemaa-Richardson, R. Global burden of recurrent vulvovaginal candidiasis: A systematic review. Lancet Infect. Dis. 2018, 18, e339–e347.
- Chatzivasileiou, P.; Vyzantiadis, T.A. Vaginal yeast colonisation: From a potential harmless condition to clinical implications and management approaches—A literature review. Mycoses 2019, 62, 638–650.
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1–e50.
- Vulvovaginal Candidiasis—2015 STD Treatment Guidelines. 2015. Available online: https://www.cdc.gov/std/tg2015/candidiasis.htm (accessed on 4 January 2024).
- Łaniewski, P.; Herbst-Kralovetz, M. Vagina. Encycl. Reprod. 2018, 2, 353–359.
- Kissinger, P. Trichomonas vaginalis: A review of epidemiologic, clinical and treatment issues. BMC Infect. Dis. 2015, 15, 307.
- Workowski, K.A.; Bolan, G.A. Sexually transmitted diseases treatment guidelines, 2015. Mmwr Recomm. Rep. 2015, 64, 137.
- Deeks, S.G.; Overbaugh, J.; Phillips, A.; Buchbinder, S. HIV Infection. Nat. Rev. Dis. Prim. 2015, 1, 1–22.
- Fitzmaurice, C.; Dicker, D.; Pain, A.; Hamavid, H.; Moradi-Lakeh, M.; MacIntyre, M.F.; Allen, C.; Hansen, G.; Woodbrook, R.; Wolfe, C.; et al. The global burden of cancer 2013. JAMA Oncol. 2015, 1, 505–527.
- . Torcia, M.G. Interplay among vaginal microbiome, immune response, and sexually transmitted viral infections. Int. J. Mol. Sci. 2019, 20, 266.
- Yarbrough, V.L.; Winkle, S.; Herbst-Kralovetz, M.M. Antimicrobial peptides in the female reproductive tract: A critical component of the mucosal immune barrier with physiological and clinical implications. Hum. Reprod. Update 2015, 21, 353–377.
- World Health Organisation. Human Papillomavirus (HPV) and Cervical Cancer. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer (accessed on 4 January 2024).
- Knuth, K.; Amiji, M.; Robinson, J.R. Hydrogel delivery systems for vaginal and oral applications—Formulation and biological considerations. Adv. Drug Deliv. Rev. 1993, 11, 137–167.
- Badulescu, O.V.; Mocanu, M.; Iancu, C.E.; Constantin, M.M.L.; Badescu, M. Assessment of hematological toxicity in case f oral administration of metronidazole. Rev. Chim. Buchar. 2016, 67, 1137–1139.
- Capparelli, E.V.; Bricker-Ford, R.; Rogers, M.J.; McKerrow, J.H.; Reed, S.L. Phase I clinical trial results of auranofin, a novel antiparasitic agent. Antimicrob. Agents Chemother. 2017, 61, 450–455.
- Narayanaswamy, R.; Torchilin, V.P. Hydrogels and their applications in targeted drug delivery. Molecules 2019, 24, 603.
More
Information
Subjects:
Health Care Sciences & Services
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
946
Revisions:
3 times
(View History)
Update Date:
26 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




