Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | FNU RAVINDER KUMAR | -- | 4607 | 2024-03-21 18:37:52 | | | |
| 2 | Camila Xu | Meta information modification | 4607 | 2024-03-22 02:46:58 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Srivastava, V.; Kumar, R.; Ahmad, A. Yeast-Based Screening of Anti-Viral Molecules. Encyclopedia. Available online: https://encyclopedia.pub/entry/56456 (accessed on 08 February 2026).
Srivastava V, Kumar R, Ahmad A. Yeast-Based Screening of Anti-Viral Molecules. Encyclopedia. Available at: https://encyclopedia.pub/entry/56456. Accessed February 08, 2026.
Srivastava, Vartika, Ravinder Kumar, Aijaz Ahmad. "Yeast-Based Screening of Anti-Viral Molecules" Encyclopedia, https://encyclopedia.pub/entry/56456 (accessed February 08, 2026).
Srivastava, V., Kumar, R., & Ahmad, A. (2024, March 21). Yeast-Based Screening of Anti-Viral Molecules. In Encyclopedia. https://encyclopedia.pub/entry/56456
Srivastava, Vartika, et al. "Yeast-Based Screening of Anti-Viral Molecules." Encyclopedia. Web. 21 March, 2024.
Copy Citation
Viruses are microscopic, subcellular biological entities that mainly consist of proteins and nucleic acid [ssDNA or dsDNA or (+) ssRNA or (−) ssRNA or both DNA and RNA (for example, Leukovirus)]. Yeast emerged as a model of choice for in vivo assays. Several features that compel the use of a yeast-based platform for screening purposes are briefly highlighted here.
screening assays
anti-viral
protease
drug discovery
virus
yeast
1. Introduction
Viruses are microscopic, subcellular biological entities that mainly consist of proteins and nucleic acid [ssDNA or dsDNA or (+) ssRNA or (−) ssRNA or both DNA and RNA (for example, Leukovirus)] [1]. Some viruses, such as enveloped viruses (or eViruses), also possess lipid membranes that are derived from host cells during the process of budding or at the time of cellular exit [2]. Viruses outside the host are known as virions, are biologically inert and unable to multiply, whereas upon entering the host, these inert biological structures exploit the resources from the host cell and start multiplying. Therefore, viruses are commonly referred to as cellular or molecular parasites [3]. Almost all cell types, including human cells, are susceptible to viral infections [4]. Of more importance, viruses are considered the most abundant biological entities in our environment as they are present almost everywhere life exists [5][6][7][8]. In another study, it was estimated that out of millions of viral species, only a few thousand are characterized in significant detail [9].
Although viruses are present externally on the skin, several viruses are reported to reside inside epithelial and other cells in the host body, for instance, in the respiratory tract, lungs, gut, and around body openings [10]. Generally, individuals with competent immune systems may better eliminate the pathogenic virus from the body compared to immunocompromised patients. Conditions such as the use of immunosuppressive drugs and chronic disease make individuals prone to clinical viral infections [11][12][13]. Apart from this, other factors that may increase the likelihood of pathogenic viral infections are the age of an individual (for example, poliovirus is mostly infectious in childhood, while smallpox virus is mostly infectious in adulthood), the surrounding population or community (vaccinated or unvaccinated), and genetic factors [14][15].
Viruses mostly have a fixed and limited range of hosts they can infect. For instance, the smallpox virus only has a human host [16], whereas rabies and coronaviruses can infect a wide range of mammals [17][18][19][20]. Moreover, some viruses such as influenza can cause pathogenic infection in both mammals and birds [21], whereas it is well known that viruses causing infection in plants do not infect animals and vice versa [22]. Similarly, the viruses of bacteria or prokaryotes do not infect eukaryotic cells [23]. Due to this limited host range and strict natural boundaries, most viruses are not pathogenic to humans and thus remain unnoticed, whereas some viruses cause a mild pathology that subsides within a few days or weeks even in the absence of significant clinical intervention. Notably, certain viruses such as ebola, hepatitis B, smallpox, dengue, poliovirus, HIV, rabies, human papillomavirus, mumps, and measles are of utmost concern [24]. In addition, the mortality rate associated with pathogenic viral infections varies widely and may range from 1–2% in the case of influenza to almost 100% in the case of rabies [25]. The clinical severity associated with pathogenic viral infections may range from mild to extreme, such as in the case of polio (caused by poliovirus), in which most people have no symptoms or mild symptoms (abortive poliomyelitis) with influenza-like and intestinal symptoms, while some individuals suffer serious symptoms, including paralysis [26][27]. Coronavirus infection can lead to lung or other organ damage, which can persist for several months to years [28][29][30].
In brief, apart from non-pathogenic viruses, there exist some viruses that cause serious threats to human health globally, and thus, all measures such as vaccination, use of anti-virals, and following safety protocols (personal hygiene, using a mask if required, social isolation or distancing) should be taken to prevent or combat these clinical viral infections [31][32][33].
2. Why Target Viral Enzymes?
To date, vaccines remain the most common and safest way to prevent pathogenic viral infections. The complete eradication of smallpox and the almost complete eradication of polio infections show the benefits and efficacy of vaccines globally [34][35]. Even in the ongoing fight to combat coronavirus infection, vaccines remain the most reliable tool to save human lives [36][37][38]. Despite this, almost all the commercially available vaccines, including recently introduced mRNA vaccines against coronavirus infection, have several issues, including their thermolabile nature [39][40]. Different issues associated with presently available vaccines and different ways to tackle them are discussed elsewhere [39][41][42][43][44]. Although their thermolabile nature and the strict requirement of following the cold chain (the need to store and transport vaccines either frozen or at 4 °C) are of concern, frequent mutations in the surface immunogenic proteins further complicate the future of subunit vaccines. For instance, the world has witnessed frequent changes in the spike (RBD) protein of SARS-CoV-2 and the emergence of several variants of the virus during the two years of the pandemic [45][46][47][48].
Apart from this, the bio-waste generated in the form of vaccine vials, needles, and syringes is another challenge. Similarly, a mismatch of syringes/needles and vaccine vials can also be a concern, as seen during coronavirus vaccination in several countries [39]. Of importance, a single dose of vaccine/s is not always 100% effective, and several doses may be required; e.g., up to 3–4 doses of coronavirus vaccines are recommended for older people and/or front-line healthcare professionals [49]. Additionally, another significant and unavoidable concern with vaccines is the associated allergies and side effects. The ability of a given vaccine to prevent pathogenic viral infections from different variants or species of the same genus remains doubtful [50]. Furthermore, it is difficult to predict at what pace vaccine development and availability on a global scale will take place in any future pandemic. Apart from this wide gap in vaccine availability in different parts of the world, economic and political issues remain a concern affecting the success of vaccination programs [51][52][53][54]. Further to this, there are instances when vaccination can be dangerous if not given under proper recommendation. For example, the dengue vaccine is recommended only to individuals who were previously infected with the dengue virus [55][56].
Hence, there is strong evidence to support the idea that relying solely on vaccines may not be sufficient, and it is imperative to explore alternative approaches for preventing or managing clinical viral infections. In light of this, the urgent requirement for the development of novel and safer anti-viral medications becomes apparent, as it will help safeguard against future outbreaks and pandemics [57][58]. Owing to this demand, non-structural viral proteins (mostly enzymes) such as proteases remain the most suitable targets for anti-viral drug development. It is important to mention that targeting viral enzymes has its own advantages as these enzymes are relatively conserved among different variants of the same virus as well as between different species and therefore can act as important targets for clinical interventions. For example, the protease of the coronavirus is conserved in SARS-CoV-2 and MERS. Even within the different strains of SARS-CoV-2, proteases show a higher degree of conservation of key amino acid residues in the active site than non-catalytic sites in the rest of the protein [59]. The degrees of conservation of different human SARS-CoV-2 and MERS-CoV-2 proteins are shown in Table 1. The viral enzymes show a higher degree of conservation compared to other structural or surface proteins [60]. Apart from proteases, other viral enzymes such as integrase and RNA-dependent RNA polymerase can also be used as targets in drug development [61][62][63].
Table 1. Degrees of conservation between SARS-CoV-2 and MERS-CoV proteins.
| Proteins | Percentage Identity |
|---|---|
| nsp1 | No results after the BLAST search |
| nsp2 | 20.4 |
| nsp3 (PLpro) | 30.2 |
| nsp4 | 40 |
| nsp5 (Mpro) | 50.6 |
| nsp6 (putative transmembrane domain) | 34.4 |
| nsp7 (cofactor of nsp12) | 55.4 |
| nsp8 (cofactor of nsp12) | 53.0 |
| nsp9 (RNA replicase) | 52.2 |
| nsp10 | 59.4 |
| nsp11 | No results after the BLAST search |
| nsp12 (RNA-dependent RNA polymerase) | 71.3 |
| nsp13 (helicase) | 72.4 |
| nsp14 (proofreading exoribonuclease) | 62.9 |
| nsp15 (NendoU, endoribonuclease) | 50.6 |
| nsp16 (2′-O-methyltransferase) | 66.3 |
| S (spike glycoprotein) | 35.1 |
| orf3a | No results after the BLAST search |
| E (envelope small membrane protein) inferred from homology | 42.4 |
| M (membrane glycoprotein) inferred from homology |
42.6 |
| orf6 inferred from homology |
No results after the BLAST search |
| orf7a | No results after the BLAST search |
| orf8 | No results after the BLAST search |
| N (nucleocapsid protein) | 50.9 |
| orf9b | No results after the BLAST search |
| orf10 | No results after the BLAST search |
Notably, another reason that viral enzymes are potential targets to combat clinical viral infections is the lack of human homologs in viral genomes. This feature minimizes the risk of adverse effects and cytotoxicity in humans. In addition, the use of anti-viral compounds targeting these enzymes will offer several other advantages over vaccines such as easy application, less biomedical waste generation, and relatively easy handling [64][65][66]. Thus, the development and screening of lead molecules for their anti-viral properties will be worthwhile. It is important to mention that several viruses that infect humans use host cellular proteases or proteins for multiplication and hence small molecule protease inhibitors may not be useful in these cases; e.g., the Ebola virus uses human cathepsin B for processing its protein [67]. In such cases, vaccines remain the best option for the fight against pathogenic viral infections.
3. Assays for Screening Anti-Viral Molecules
With ever-increasing pathogenic viral infections and due to the lack of sufficient classes of anti-viral drugs, the identification of new anti-viral molecules bearing different modes of action, and which are safe for human use, is important from the clinical point of view. Therefore, significant efforts have been made to develop assays that allow rapid screening of anti-viral molecules. The screening is performed by employing either in vitro or in vivo assays. Although every assay is associated with some benefits and drawbacks, both types of assays are still frequently used by researchers for screening purposes. In vitro and/or in vivo assays provide possible lead candidates that are currently in different phases of clinical trials or have already entered clinical use. However, it is very important to know which screening technique is suitable for a particular type of enzyme or virus. Since the information available in the literature is limited, and the use of defined assays for specific pathogenic viruses or enzymes is not yet widely agreed upon, there is confusion amongst scientists.
3.1. In Vitro Assay for Screening of Anti-Viral Molecules
Before delving into significant concerns regarding in vitro assays, it is essential to acknowledge the factors that contribute to the popularity of these assays among researchers. The rapid availability of high-quality genome sequences and gene annotation facilitates fast cloning, expression, and purification of viral proteins including enzymes, especially proteases, and thus allows rapid screening of anti-viral molecules using in vitro assays. Apart from this, the availability of custom peptides (acting as substrates for the viral enzymes) favors rapid bursts in the available literature of in vitro assays performed for screening anti-viral molecules. Most of the in vitro assays for screening anti-viral molecules are based on FRET (Förster or fluorescence resonance energy transfer) or fluorescence intensity signal measurement using FACS (fluorescence-activated cell sorting) or a plate reader [68][69][70]. Other features that make these types of assays attractive include the ability to use or adapt such assays to a high-throughput platform (suitable for 96- or 384-well formats), high sensitivity, the ability to control reaction conditions, and minimizing the use of animals [71]. Besides these features, sometimes these assays can be relatively economical and more rapid compared to in vivo assays. Also, in vitro assays do not suffer the problem of off-targets (or nonspecific binding or interactions), which is a common occurrence in in vivo experiments involving cells or animals/organisms.
Despite several advantages, it is important to mention that sometimes in vitro techniques do not provide an exact result. For example, the result of an in vitro assay is highly dependent on the experimental conditions, including pH, molarity, or tonicity of buffers, and any deviation from the standard operating procedure (SOP) may change the result [72][73]. Therefore, it is of the utmost importance to take extra care when performing and analyzing data from in vitro assays. The in vitro assays are usually performed by using highly purified proteins and hence fail to mimic the actual molecular or cellular environment [66][67][68]. The reproducibility of in vitro assays is a big concern [74]. Another factor to consider in such studies is the protein quantity used for analysis. Generally, the concentration of enzymes used in in vitro assays falls in the µM to nM range, which is not appropriate when compared with their actual concentrations in cells or tissues [75][76]. Whether the concentrations of other chemicals (test compound or molecules) used in the assays can be attained in the cell is another concern. During in vitro assays, various chemicals or ligands interact with the target protein, and thus it may be possible that some chemical molecules may not even enter the cell (especially if molecules are charged or polar) [77]. Furthermore, even if the chemicals enter the cells successfully, their stability within the cells may be affected as most of the in vitro assays are performed at a temperature that is different from the physiological temperature (for humans, 37 °C) [75][76]. Apart from this, the expression and purification of certain proteins for an in vitro screening process can be challenging. It is important to note that the proteins purified from bacterial systems lack posttranslational modification (phosphorylation, glycosylation, etc.), which may alter the interaction between protein and ligand and hence may significantly affect the results of an experiment [78]. On the other hand, if the proteins used in such experiments are not of high purity, the repeat (duplicate, triplicate, or quadruplicate) sample results may be contradictory. Sometimes in vitro and in vivo assays do not correspond with one another. The reason could be the limitations of in vitro experiments; for instance, researchers have observed that in the case of FRET-based assays, quenching may be caused by reaction components, or they may cause increased fluorescence of reporter molecules [79]. Owing to the limitations of in vitro assays, the use of in vivo techniques for screening anti-viral molecules is highly recommended.
3.2. In Vivo Assay for Screening of Anti-Viral Molecules
Generally, in vivo assays are performed in animals or cell lines that mimic the microenvironment of the natural cells and tissues and thus provide researchers with more accurate platforms for investigating biological systems [80]. In vivo assays provide valuable information on the cellular toxicities of tested molecules, and the data generated from in vivo studies can be extrapolated to the respective biological systems, thereby providing confidence in decision making. Since in vivo assays mimic the cellular or molecular microenvironment, only molecules that can enter the cells and produce the desired effect are scored. The advantages of in vivo assays are that the protein purification step is not required and sometimes the final read-through can be very simple and easy (for example, growth or changes in optical density or fluorescence measurements).
The only drawback faced with in vivo assays is the involvement of animals, which may be costly, have low throughput, and require ethical approval, which can be time-consuming [81]. However, several in vivo experiments only require the use of animal cell lines (including mammalian cell lines). Some major drawbacks of using cell lines are the high maintenance cost, the possibility of laboratory-acquired contamination, the requirement of a cell culture facility, and the requirement of sophisticated and costly instruments such as FACS. Furthermore, the generation of stable cell lines or animal models for in vivo studies may be lengthy and costly. In some instances, technical difficulties occur and assays involving whole animals cannot be adopted for high-throughput platforms, and the risk of being off-target is an important concern.
4. Yeast as a Screening Model
Since both in vitro and in vivo assays have several disadvantages, it is therefore important to find a system that can provide maximum benefits for both in vitro and in vivo systems. Yeast emerged as a model of choice for in vivo assays. Several features that compel the use of a yeast-based platform for screening purposes are briefly highlighted here. For example, yeasts are unicellular eukaryotic organisms that offer almost all the benefits offered by mammalian or animal cell-based assays (at least for screening purposes). Importantly, basic cell processes such as cell cycle regulation or programmed cell death and proteins are conserved or similar in almost all types of eukaryotic cells, including those of mammalian cell lines [82][83]. Therefore, yeasts are particularly suited to the study of the impact of those viral activities on related cellular activities during virus–host interactions [84]. Additionally, yeasts are easy to grow, and their maintenance is very economical compared with any eukaryotic system. In the past, yeast has been successfully used to study viruses from both basic as well as applied perspectives. For example, yeast has been used to study viral replication, structure–function analysis of viral enzymes, screening of anti-viral molecules, and development of anti-viral vaccines [85][86].
It is important to mention that, like animal or mammalian cells, yeast cells are also eukaryotic in nature. However, yeast cells differ from animal cells in several ways; for instance, yeast cells possess cell walls while animal cells do not, and generally yeast cells possess central and bigger vacuoles while their equivalent in animal cells are small but numerous lysosomes. Similar to animal cells, yeast cells also possess mitochondria and lack chloroplasts. Despite several similarities and differences, yeast offers several advantages, as mentioned above. Apart from this, yeast grows faster, with a short duplication time (90–120 min for budding yeast) compared to animal cells, which divide in 18 h or more. Yeast cells in general are smaller than animal cells [43].
The Saccharomyces cerevisiae yeast species is the most thoroughly studied organism or system at both the genetic and molecular levels. Since S. cerevisiae has been studied for years, a vast number of tools (for gene tagging, deletion, expression, and different libraries) are available for this species, which is advantageous in current and future research [87][88][89][90]. When required, metabolic pathway engineering or molecular modifications can be achieved easily. Yeast cells can be easily adapted to the needs of assays, due to their easy and responsive genetic manipulation. Thus, actual viral proteins that may be potential targets for anti-viral drug discovery can be easily expressed in this model. Yeast cells can also be used in 96-well plates and, therefore, this system is suitable for high-throughput screening processes. Similar to human cells, yeast cells also actively export toxic chemicals, but this can easily be resolved, and cells can be made more sensitive to the chemical under investigation; for instance, in the case of S. cerevisiae, cells can be made hypersensitive by deletion of the pleiotropic drug resistance (PDR) genes PDR1 and PDR3 [91]. These zinc cluster transcription factors mediate general drug resistance to many cytotoxic substances. Most yeast-based screening procedures are performed in PDR1- and PDR3-deleted strains. However, in some cases, other genes such as PDR5 or SNQ2 are also deleted along with PDR1 and PDR3 [92]. Because of all these advantages, yeasts have been successfully used over the years in several kinds of screening processes.
Technological improvements and the availability of advanced molecular tools such as CRISPR/Cas9 technology allow the manipulation of cell lines in an extraordinary way when compared with the situation a decade ago [93]. However, the complexity associated with this system, such as the need for high levels of expertise, high cost, and time consumption, gave an upper edge to the yeast-based platform for high-throughput screening. It is important to note that increasing complexity means increasing the frequency of off-target effects (due to the interaction of test molecules or compounds with non-targeted cellular proteins, these off-target effects also affect the interpretation of data and increase the chances of false positives). This, in turn, arises from the complex nature of multicellular organisms with a greater number of cellular proteins than unicellular yeast, which has fewer proteins. Unlike yeast, the tissue-specific proteome also increases this complexity, and is more common in multicellular organisms. This complexity increases from unicellular eukaryotes to multicellular eukaryotes (invertebrates) to vertebrates. Figure 1 illustrates various biological models employed to assess the effects of chemicals, in terms of whether they are beneficial or detrimental. Another reason to use alternative models which include yeast for screening of chemicals is the strict ethics regulations of different countries and government organizations aiming to reduce the use of animals in research [94]. For example, the United Kingdom recently achieved a reduction in the use of animals in research [95]. The American Environmental Protection Agency (USA EPA) also stresses reducing the use of animals in chemical testing [96]. Several other countries and organizations have adopted these regulations too, and it is anticipated that many other government organizations and countries will also follow these regulations and investigate more feasible alternatives. The other aspect that may impact drug discovery and the use of animals in the pharmaceutical sector is the use of technologies such as machine learning and artificial intelligence; however, little is known, and this subject needs further research [97].
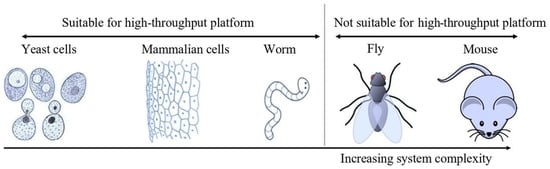
Figure 1. Schematic showing different models available for screening. (Note that another common model system (zebra fish) has not been included in the figure, but the overall message remains the same, namely that a complex model is not suitable for high-throughput screening). (Cartoons were taken from the mentioned site, which is properly acknowledged: http://clipart-library.com, accessed on 4 June 2022). Complexity increases from the left (yeast) to the right (mammals). So far, only yeast cells, animal cell lines, and worms are suitable for high-throughput screening (96-well format).
5. Screening of Viral Protease Inhibitors Using Yeast-Based Platforms
Interestingly, the use of yeast as an in vivo model for screening anti-viral molecules is very recent and only dates back to the early 21st century. The first study where yeast was used as an in vivo model was reported in 2003 from India, where the scientists used S. cerevisiae for screening anti-viral compounds [98]. The principle behind the method was to disturb the programmed ribosomal frameshift by molecules that lower viral multiplication in the yeast cells and thus rescue yeast growth in the presence of positive hits [98]. Later, in 2006, a study by Cottier and co-workers, from Switzerland, reported the use of S. cerevisiae for screening protease inhibitors against human cytomegalovirus (HCMV) protease [99]. The description of the assay is simple and easy to interpret as it is based on the growth inhibition of yeast in the presence of active protease. It is interesting to note that the yeast-based in vivo assay can identify the susceptibility of viral protease towards different inhibitors from different clinical isolates of HIV-1. This aids in determining whether a virus develops resistance against a given molecule or not [100]. Similarly, in another study, Benko and co-workers used fission yeast for screening protease inhibitors against HIV-1 protease. The assay is based on the rescue of yeast growth in the presence of a positive hit, and the read-through consists of increases in cell density and green fluorescent protein (GFP) intensity [101]. A list of studies where yeast was used for in vivo screening of viral enzyme inhibitors is shown in Table 2.
Table 2. Studies where yeast was used as an in vivo model for screening of anti-viral molecules.
| Yeast Species | Virus | Protease | Assay Description | Reference |
|---|---|---|---|---|
| S. cerevisiae | SARS-CoV | Papain-like protease (PLP) | Growth inhibition of yeast in (the presence of protease) rescued by inhibitor | [102] |
| S. cerevisiae | SARS-CoV-2 | Mpro | Increases in fluorescence and cell number in the presence of protease inhibitor | [103] |
| S. cerevisiae | Human cytomegalovirus |
HCMV protease | Rescue of yeast by protease inhibitors by preventing cleavage of Trp1 | [99] |
| S. cerevisiae | SARS-CoV | Coronavirus RNA cap guanine-N7-methyltransferase | Growth of colonies on FAO plates | [104] |
| S. cerevisiae | HIV-1 | VP-1 | Growth inhibition of yeast in (the presence of protease) rescued by inhibitor | [100] |
| S. cerevisiae | HIV-1 | HIV-PR | Programmed—1 ribosomal frameshifting | [105] |
| S. cerevisiae | HIV-1 | HIV-PR | Programmed—1 ribosomal frameshifting | [98] |
| S. cerevisiae | SARS-CoV-2 | Mpro | FACS, FRET, growth inhibition | [106] |
| S. pombe | HIV-1 | HIV-PR | Rescue of growth in the presence of positive hits | [107] |
| S. pombe | HIV-1 | HIV-PR | Rescue of growth in the presence of positive hits | [108] |
| S. pombe | HIV-1 | HIV-PR | Rescue of growth in the presence of positive hits | [109] |
| S. pombe | HIV-1 | HIV-PR | Rescue of growth in the presence of positive hits | [101] |
Recently, Alalam and co-workers used budding yeast for screening of SARS-CoV-2 protease inhibitors and the rescue of yeast growth in the presence of positive hits, and increases in cell density and fluorescence intensity were determined for the assay [103]. In another study, SARS-CoV protease inhibitors were screened by measuring the rescue of yeast growth in the presence of positive hits with protease inhibitor activity [102]. It is important to note that yeast-based assays are not only used for screening viral protease inhibitors but also for screening inhibitors of other viral enzymes. For example, a yeast-based in vivo assay was able to identify the inhibitors of coronavirus RNA cap guanine-N7-methyltransferase [104]. This suggests that apart from the screening of protease inhibitors, yeast can be used for screening the inhibitors of other important viral enzymes. Although a flip or split GFP complementation-based in vivo assay has been used in yeast to study protein localization or interaction [110][111], the use of this approach for screening anti-viral molecules using yeast cells was not mentioned. However, split GFP-based in vivo screening was successfully performed in human cell lines [73][112][113]. Table 3 elaborates on some of the most common pathogenic viruses which infect humans along with viral proteases which have the potential to act as targets in drug discovery.
Table 3. List of proteases from common viruses.
| Virus | Disease | Nature of Genome | Enzyme Type | Protease | Reference |
|---|---|---|---|---|---|
| Polio | Polio (or poliomyelitis) | (+) ssRNA | Protease | 2Apro and 3 Cpro/3CDpro | [114][115] |
| Variola | Smallpox | dsDNA | Protease | K7L | [116] |
| MERS | Respiratory disease | (+) ssRNA | Protease | Mpro and PLpro | [117] |
| SARS-CoV | Respiratory disease | (+) ssRNA | Protease | Mpro and PLpro | [118] |
| Dengue | Dengue | (+) ssRNA (capped) | Protease | NS2B/3 | [119] |
| Herpes simplex virus | Cold sores, genital herpes | dsDNA (linear) | Protease | HSV protease | [120] |
| Varicella zoster virus | Chickenpox/varicella/shingles | dsDNA (linear) | Protease | VZV protease | [121] |
| Rubella | German measles or rubella | (+) ssRNA | Protease | NS-pro | [122] |
| Zika | Zika fever | (+) ssRNA | Protease | NS2B-NS3pro | [123] |
| HIV | AIDS | (+) ssRNA (linear) | Protease | HIV-PR | [124] |
6. The Bottleneck of In Vivo Assays for Viral Protease Inhibitors
Despite several studies reporting the use of whole cells (yeast or mammalian cells) for in vivo screening of viral protease inhibitors, an important associated challenge is the toxicity of expressed viral proteases. The expression of viral proteases within the cells (both yeast and mammalian cells) is highly toxic and even lethal. For example, the expression of proteases from HIV [125], poliovirus [126], hepatitis A [127], and SARS-CoV [102] has been found lethal for cells. Like eukaryotic cells, the expression of viral protease is toxic even to bacterial cells [128], and this further complicates the development and application of in vivo assays for the screening of viral protease inhibitors. Because of these complications, in vitro assays are more common for screening anti-viral molecules. Due to implications such as high cost, time, limited tools and resources, and complicated regulation of gene expression in mammalian cells, it is worth using a yeast-based system for screening purposes. In yeast, the genetic expression can be easily regulated due to responsive genetic manipulation and the availability of several promoters ranging from the strong inducible (GAL promoter, CUP promoter) to the moderate or weak constitutive (STE5 promoter). Further application of auxin degron-based protein depletion in yeast can assist in maintaining the growth of cells transformed for expressing viral protease [129][130]. Another possible approach is that the substrate of the protease can be overexpressed so that the expressed viral protease has less chance of cleaving host cell proteins, thereby minimizing the toxic effect of the viral protease. Since viral protease cleavage sequences are available, they can be utilized for determining the presence of these sequences in a yeast proteome, and also to choose species with proteomes lacking those particular sequences, or to present in the smallest number of native or endogenous proteins possible. All these reasons support the observation that yeast can overcome this bottleneck in the development of more novel in vivo assays for screening viral protease inhibitors.
References
- Roingeard, P. Viral detection by electron microscopy: Past, present and future. Biol. Cell. 2008, 100, 491–501.
- Dubois-Dalcq, M.; Holmes, K.V.; Rentier, B.; Kingsbury, D.W. Assembly of Enveloped RNA Viruses; Springer: New York, NY, USA, 1984.
- Kaján, G.L.; Doszpoly, A.; Tarján, Z.L.; Vidovszky, M.Z.; Papp, T. Virus–Host Coevolution with a Focus on Animal and Human DNA Viruses. J. Mol. Evol. 2020, 88, 41–56.
- Koonin, E.V.; Senkevich, T.G.; Dolja, V.V. The ancient Virus World and evolution of cells. Biol. Direct 2006, 1, 29.
- Lawrence, C.M.; Menon, S.; Eilers, B.J.; Bothner, B.; Khayat, R.; Douglas, T.; Young, M.J. Structural and functional studies of archaeal viruses. J. Biol. Chem. 2009, 284, 12599–12603.
- Chiu, C.Y.; Miller, S.A. Clinical metagenomics. Nat. Rev. Genet. 2019, 20, 341–355.
- Sanjuán, R.; Nebot, M.R.; Chirico, N.; Mansky, L.M.; Belshaw, R. Viral Mutation Rates. J. Virol. 2010, 84, 9733–9748.
- Crawford, D. Viruses: A Very Short Introduction; Oxford University Press: New York, NY, USA, 2022.
- Breitbart, M.; Rohwer, F. Here a virus, there a virus, everywhere the same virus? Trends Microbiol. 2005, 13, 278–284.
- Liang, G.; Bushman, F.D. The human virome: Assembly, composition and host interactions. Nat. Rev. Microbiol. 2021, 19, 514–527.
- Lei, V.; Petty, A.J.; Atwater, A.R.; Wolfe, S.A.; MacLeod, A.S. Skin Viral Infections: Host Antiviral Innate Immunity and Viral Immune Evasion. Front. Immunol. 2020, 11, 593901.
- Orlicka, K.; Barnes, E.; Culver, E.L. Prevention of infection caused by immunosuppressive drugs in gastroenterology. Ther. Adv. Chronic Dis. 2013, 4, 167–185.
- Tugizov, S.; Webster-Cyriaque, J.; Syrianen, S.; Chattopadyay, A.; Sroussi, H.; Zhang, L.; Kaushal, A. Mechanisms of Viral Infections Associated with HIV: Workshop 2B. Adv. Dent. Res. 2011, 23, 130–136.
- Kenney, A.D.; Dowdle, J.A.; Bozzacco, L.; McMichael, T.M.; Gelais, C.S.; Panfil, A.R.; Sun, Y.; Schlesinger, L.S.; Anderson, M.Z.; Green, P.L.; et al. Human Genetic Determinants of Viral Diseases. Annu. Rev. Genet. 2017, 51, 241–263.
- Singanayagam, A.; Hakki, S.; Dunning, J.; Madon, K.J.; Crone, M.A.; Koycheva, A.; Derqui-Fernandez, N.; Barnett, J.L.; Whitfield, M.G.; Varro, R.; et al. Community transmission and viral load kinetics of the SARS-CoV-2 delta (B.1.617.2) variant in vaccinated and unvaccinated individuals in the UK: A prospective, longitudinal, cohort study. Lancet Infect. Dis. 2022, 22, 183–195.
- Babkin, I.V.; Babkina, I.N. The Origin of the Variola Virus. Viruses 2015, 7, 1100–1112.
- Fisher, C.R.; Streicker, D.G.; Schnell, M.J. The spread and evolution of rabies virus: Conquering new frontiers. Nat. Rev. Microbiol. 2018, 16, 241–255.
- Damas, J.; Hughes, G.M.; Keough, K.C.; Painter, C.A.; Persky, N.S.; Corbo, M.; Hiller, M.; Koepfli, K.-P.; Pfenning, A.R.; Zhao, H.; et al. Broad host range of SARS-CoV-2 predicted by comparative and structural analysis of ACE2 in vertebrates. Proc. Natl. Acad. Sci. USA 2020, 117, 22311–22322.
- Sit, T.H.C.; Brackman, C.J.; Ip, S.M.; Tam, K.W.S.; Law, P.Y.T.; To, E.M.W.; Yu, V.Y.T.; Sims, L.D.; Tsang, D.N.C.; Chu, D.K.W.; et al. Infection of dogs with SARS-CoV-2. Nature 2020, 586, 776–778.
- Bosco-Lauth, A.M.; Hartwig, A.E.; Porter, S.M.; Gordy, P.W.; Nehring, M.; Byas, A.D.; VandeWoude, S.; Ragan, I.K.; Maison, R.M.; Bowen, R.A. Experimental infection of domestic dogs and cats with SARS-CoV-2: Pathogenesis, transmission, and response to reexposure in cats. Proc. Natl. Acad. Sci. USA 2020, 117, 26382–26388.
- Long, J.S.; Mistry, B.; Haslam, S.M.; Barclay, W.S. Host and viral determinants of influenza A virus species specificity. Nat. Rev. Microbiol. 2019, 17, 67–81.
- Balique, F.; Lecoq, H.; Raoult, D.; Colson, P. Can Plant Viruses Cross the Kingdom Border and Be Pathogenic to Humans? Viruses 2015, 7, 2074–2098.
- Koonin, E.V.; Dolja, V.V.; Krupovic, M. Origins and evolution of viruses of eukaryotes: The ultimate modularity. Virology 2015, 479–480, 2–25.
- Centers for Disease Control and Prevention. NCEZID: Deadly Infections. CDC. 2019. Available online: https://www.cdc.gov/ncezid/what-we-do/our-topics/deadly-unexplained-diseases.html (accessed on 4 September 2022).
- Paget, J.; Spreeuwenberg, P.; Charu, V.; Taylor, R.J.; Iuliano, A.D.; Bresee, J.; Simonsen, L.; Viboud, C.; Global Seasonal Influenza-Associated Mortality Collaborator Network and GLaMOR Collaborating Teams. Global mortality associated with seasonal influenza epidemics: New burden estimates and predictors from the GLaMOR Project. J. Glob. Health 2019, 9, 020421.
- Mehndiratta, M.M.; Mehndiratta, P.; Pande, R. Poliomyelitis: Historical facts, epidemiology, and current challenges in eradication. Neurohospitalist 2014, 4, 223–229.
- Centers for Disease Control and Prevention. What is Polio? CDC. 2022. Available online: https://www.cdc.gov/polio/what-is-polio/index.htm (accessed on 4 September 2022).
- Halawa, S.; Pullamsetti, S.S.; Bangham, C.R.M.; Stenmark, K.R.; Dorfmüller, P.; Frid, M.G.; Butrous, G.; Morrell, N.W.; Perez, V.A.d.J.; Stuart, D.I.; et al. Potential long-term effects of SARS-CoV-2 infection on the pulmonary vasculature: A global perspective. Nat. Rev. Cardiol. 2022, 19, 314–331.
- Sanchez-Ramirez, D.C.; Normand, K.; Zhaoyun, Y.; Torres-Castro, R. Long-Term Impact of COVID-19: A Systematic Review of the Literature and Meta-Analysis. Biomedicines 2021, 9, 900.
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. More than 50 long-term effects of COVID-19: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 16144.
- Centers for Disease Control and Prevention. Healthy Habits to Help Protect Against Flu. CDC. 2021. Available online: https://www.cdc.gov/flu/prevent/actions-prevent-flu.htm (accessed on 4 September 2022).
- MAYO CLINIC. Germs: Understand and Protect against Bacteria, Viruses and Infections. Mayo Clinic. 2022. Available online: https://www.mayoclinic.org/diseases-conditions/infectious-diseases/in-depth/germs/art-20045289 (accessed on 4 September 2022).
- Centers for Disease Control and Prevention. How to Protect Yourself and Others. 2022. Available online: https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/prevention.html (accessed on 4 September 2022).
- Fenner, F. Global Eradication of Smallpox. Rev. Infect. Dis. 1982, 4, 916–930.
- Norrby, E.; Uhnoo, I.; Brytting, M.; Zakikhany, K.; Lepp, T.; Olin, P. Polio close to eradication. Lakartidningen 2017, 114, EPDT.
- Tregoning, J.S.; Brown, E.S.; Cheeseman, H.M.; Flight, K.E.; Higham, S.L.; Lemm, N.-M.; Pierce, B.F.; Stirling, D.C.; Wang, Z.; Pollock, K.M. Vaccines for COVID-19. Clin. Exp. Immunol. 2020, 202, 162–192.
- Kaur, S.P.; Gupta, V. COVID-19 Vaccine: A comprehensive status report. Virus Res. 2020, 288, 198114.
- Callaway, E. The race for coronavirus vaccines: A graphical guide. Nature 2020, 580, 576–577.
- Kumar, R.; Srivastava, V.; Baindara, P.; Ahmad, A. Thermostable vaccines: An innovative concept in vaccine development. Expert Rev. Vaccines 2022, 21, 811–824.
- Kumar, R. Investigating the long-term stability of protein immunogen(s) for whole recombinant yeast-based vaccines. FEMS Yeast Res. 2018, 18, foy071.
- Kumar, R.; Kharbikar, B.N. Lyophilized yeast powder for adjuvant free thermostable vaccine delivery. Appl. Microbiol. Biotechnol. 2021, 105, 3131–3143.
- Kumar, R.; Kumar, P. Yeast-based vaccines: New perspective in vaccine development and application. FEMS Yeast Res. 2019, 19, foz007.
- Srivastava, V.; Nand, K.N.; Ahmad, A.; Kumar, R. Yeast-Based Virus-like Particles as an Emerging Platform for Vaccine Development and Delivery. Vaccines 2023, 11, 479.
- Kumar, R.; Srivastava, V. Application of anti-fungal vaccines as a tool against emerging anti-fungal resistance. Front. Fungal Biol. 2023, 4, 1241539.
- Korber, B.; Fischer, W.M.; Gnanakaran, S.; Yoon, H.; Theiler, J.; Abfalterer, W.; Hengartner, N.; Giorgi, E.E.; Bhattacharya, T.; Foley, B.; et al. Tracking Changes in SARS-CoV-2 Spike: Evidence that D614G Increases Infectivity of the COVID-19 Virus. Cell 2020, 182, 812–827.e19.
- Jalkanen, P.; Kolehmainen, P.; Häkkinen, H.K.; Huttunen, M.; Tähtinen, P.A.; Lundberg, R.; Maljanen, S.; Reinholm, A.; Tauriainen, S.; Pakkanen, S.H.; et al. COVID-19 mRNA vaccine induced antibody responses against three SARS-CoV-2 variants. Nat. Commun. 2021, 12, 3991.
- Ye, G.; Liu, B.; Li, F. Cryo-EM structure of a SARS-CoV-2 omicron spike protein ectodomain. Nat. Commun. 2022, 13, 1214.
- Saville, J.W.; Mannar, D.; Zhu, X.; Srivastava, S.S.; Berezuk, A.M.; Demers, J.-P.; Zhou, S.; Tuttle, K.S.; Sekirov, I.; Kim, A.; et al. Structural and biochemical rationale for enhanced spike protein fitness in delta and kappa SARS-CoV-2 variants. Nat. Commun. 2022, 13, 742.
- Magen, O.; Waxman, J.G.; Makov-Assif, M.; Vered, R.; Dicker, D.; Hernán, M.A.; Lipsitch, M.; Reis, B.Y.; Balicer, R.D.; Dagan, N. Fourth Dose of BNT162b2 mRNA COVID-19 Vaccine in a Nationwide Setting. N. Engl. J. Med. 2022, 386, 1603–1614.
- Hippisley-Cox, J.; Patone, M.; Mei, X.W.; Saatci, D.; Dixon, S.; Khunti, K.; Zaccardi, F.; Watkinson, P.; Shankar-Hari, M.; Doidge, J.; et al. Risk of thrombocytopenia and thromboembolism after COVID-19 vaccination and SARS-CoV-2 positive testing: Self-controlled case series study. BMJ 2021, 374, n1931.
- Yamey, G.; Garcia, P.; Hassan, F.; Mao, W.; McDade, K.K.; Pai, M.; Saha, S.; Schellekens, P.; Taylor, A.; Udayakumar, K. It is not too late to achieve global COVID-19 vaccine equity. BMJ 2022, 376, e070650.
- Tsagkaris, C.; Laubscher, L.; Papadakis, M.; Vladychuk, V.; Matiashova, L. Immunization in state of siege: The importance of thermostable vaccines for Ukraine and other war-torn countries and territories. Expert Rev. Vaccines 2022, 21, 1007–1008.
- Kumar, R.; Srivastava, V.; Baindara, P.; Ahmad, A. Response to: “immunization in state of siege: The importance of thermostable vaccines for Ukraine and other war-torn countries and territories”. Expert Rev. Vaccines 2022, 21, 1009–1010.
- Kumar, R.; Srivastava, V.; Nand, K.N. The Two Sides of the COVID-19 Pandemic. COVID 2023, 3, 1746–1760.
- Burke, D.S.; Scott, R.M.; Johnson, D.E.; Nisalak, A. A Prospective Study of Dengue Infections in Bangkok. Am. J. Trop. Med. Hyg. 1988, 38, 172–180.
- Kliks, S.C.; Nimmanitya, S.; Nisalak, A.; Burke, D.S. Evidence That Maternal Dengue Antibodies Are Important in the Development of Dengue Hemorrhagic Fever in Infants. Am. J. Trop. Med. Hyg. 1988, 38, 411–419.
- Monto, A.S. Vaccines and Antiviral Drugs in Pandemic Preparedness. Emerg. Infect. Dis. 2006, 12, 55–60.
- Pardi, N.; Weissman, D. Development of vaccines and antivirals for combating viral pandemics. Nat. Biomed. Eng. 2020, 4, 1128–1133.
- Lee, J.T.; Yang, Q.; Gribenko, A.; Perrin, B.S.; Zhu, Y.; Cardin, R.; Liberator, P.A.; Anderson, A.S.; Hao, L. Genetic Surveillance of SARS-CoV-2 M pro Reveals High Sequence and Structural Conservation Prior to the Introduction of Protease Inhibitor Paxlovid. mBio 2022, 13, e0086922.
- Melo-Filho, C.C.; Bobrowski, T.; Martin, H.J.; Sessions, Z.; Popov, K.I.; Moorman, N.J.; Baric, R.S.; Muratov, E.N.; Tropsha, A. Conserved coronavirus proteins as targets of broad-spectrum antivirals. Anti-Viral Res. 2022, 204, 105360.
- Craigie, R. The molecular biology of HIV integrase. Future Virol. 2012, 7, 679–686.
- Delelis, O.; Carayon, K.; Saïb, A.; Deprez, E.; Mouscadet, J.-F. Integrase and integration: Biochemical activities of HIV-1 integrase. Retrovirology 2008, 5, 114.
- Aftab, S.O.; Ghouri, M.Z.; Masood, M.U.; Haider, Z.; Khan, Z.; Ahmad, A.; Munawar, N. Analysis of SARS-CoV-2 RNA-dependent RNA polymerase as a potential therapeutic drug target using a computational approach. J. Transl. Med. 2020, 18, 275.
- Al-Omran, K.; Khan, E.; Ali, N.; Bilal, M. Estimation of COVID-19 generated medical waste in the Kingdom of Bahrain. Sci. Total Environ. 2021, 801, 149642.
- Phadke, R.; Costa, A.C.d.S.; Dapke, K.; Ghosh, S.; Ahmad, S.; Tsagkaris, C.; Raiya, S.; Maheswari, M.S.; Essar, M.Y.; Ahmad, S. Eco-friendly vaccination: Tackling an unforeseen adverse effect. J. Clim. Chang. Health 2021, 1, 100005.
- Das, A.K.; Islam, N.; Billah, M.; Sarker, A. COVID-19 pandemic and healthcare solid waste management strategy—A mini-review. Sci. Total. Environ. 2021, 778, 146220.
- Nishimura, H.; Yamaya, M. A Synthetic Serine Protease Inhibitor, Nafamostat Mesilate, Is a Drug Potentially Applicable to the Treatment of Ebola Virus Disease. Tohoku J. Exp. Med. 2015, 237, 45–50.
- Yamamoto, K.Z.; Yasuo, N.; Sekijima, M. Screening for Inhibitors of Main Protease in SARS-CoV-2: In Silico and In Vitro Approach Avoiding Peptidyl Secondary Amides. J. Chem. Inf. Model. 2022, 62, 350–358.
- Cihlova, B.; Huskova, A.; Böserle, J.; Nencka, R.; Boura, E.; Silhan, J. High-Throughput Fluorescent Assay for Inhibitor Screening of Proteases from RNA Viruses. Molecules 2021, 26, 3792.
- Coelho, C.; Gallo, G.; Campos, C.B.; Hardy, L.; Würtele, M. Biochemical screening for SARS-CoV-2 main protease inhibitors. PLoS ONE 2020, 15, e0240079.
- Graudejus, O.; Wong, R.D.P.; Varghese, N.; Wagner, S.; Morrison, B. Bridging the gap between in vivo and in vitro research: Reproducing in vitro the mechanical and electrical environment of cells in vivo. Front. Cell. Neurosci. 2018, 12.
- Ma, C.; Hu, Y.; Townsend, J.A.; Lagarias, P.I.; Marty, M.T.; Kolocouris, A.; Wang, J. Ebselen, Disulfiram, Carmofur, PX-12, Tideglusib, and Shikonin Are Nonspecific Promiscuous SARS-CoV-2 Main Protease Inhibitors. ACS Pharmacol. Transl. Sci. 2020, 3, 1265–1277.
- Ma, C.; Sacco, M.D.; Xia, Z.; Lambrinidis, G.; Townsend, J.A.; Hu, Y.; Meng, X.; Szeto, T.; Ba, M.; Zhang, X.; et al. Discovery of SARS-CoV-2 Papain-like Protease Inhibitors through a Combination of High-Throughput Screening and a FlipGFP-Based Reporter Assay. ACS Central Sci. 2021, 7, 1245–1260.
- Hirsch, C.; Schildknecht, S. In Vitro Research Reproducibility: Keeping Up High Standards. Front. Pharmacol. 2019, 10, 1484.
- Smith, E.; Davis-Gardner, M.E.; Garcia-Ordonez, R.D.; Nguyen, T.-T.; Hull, M.; Chen, E.; Yu, X.; Bannister, T.D.; Baillargeon, P.; Scampavia, L.; et al. High throughput screening for drugs that inhibit 3C-like protease in SARS-CoV-2. SLAS Discov. 2023, 28, 95–101.
- Owen, D.R.; Allerton, C.M.N.; Anderson, A.S.; Aschenbrenner, L.; Avery, M.; Berritt, S.; Boras, B.; Cardin, R.D.; Carlo, A.; Coffman, K.J.; et al. An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19. Science 2021, 374, 1586–1593.
- Sun, D.; Gao, W.; Hu, H.; Zhou, S. Why 90% of clinical drug development fails and how to improve it? Acta Pharm. Sin. B 2022, 12, 3049–3062.
- Perkins, J.R.; Diboun, I.; Dessailly, B.H.; Lees, J.G.; Orengo, C.A. Transient Protein-Protein Interactions: Structural, Functional, and Network Properties. Structure 2010, 18, 1233–1243.
- Leavesley, S.J.; Rich, T.C. Overcoming limitations of FRET measurements. Cytom. Part A 2016, 89, 325–327.
- Nierode, G.; Kwon, P.S.; Dordick, J.S.; Kwon, S.-J. Cell-Based Assay Design for High-Content Screening of Drug Candidates. J. Microbiol. Biotechnol. 2016, 26, 213–225.
- Rovida, C.; Hartung, T. Re-evaluation of animal numbers and costs for in vivo tests to accomplish REACH legislation requirements for chemicals—A report by the transatlantic think tank for toxicology (t(4)). ALTEX 2009, 26, 187–208.
- Hartwell, L.H.; Szankasi, P.; Roberts, C.J.; Murray, A.W.; Friend, S.H. Integrating Genetic Approaches into the Discovery of Anticancer Drugs. Science 1997, 278, 1064–1068.
- Peterson, T.A.; Park, D.; Kann, M.G. A protein domain-centric approach for the comparative analysis of human and yeast phenotypically relevant mutations. BMC Genom. 2013, 14 (Suppl. S3), S5.
- Zhao, R.Y. Yeast for virus research. Microb. Cell 2017, 4, 311–330.
- Galao, R.P.; Scheller, N.; Alves-Rodrigues, I.; Breinig, T.; Meyerhans, A.; Díez, J. Saccharomyces cerevisiae: A versatile eukaryotic system in virology. Microb. Cell Factories 2007, 6, 32.
- Glingston, R.S.; Yadav, J.; Rajpoot, J.; Joshi, N.; Nagotu, S. Contribution of yeast models to virus research. Appl. Microbiol. Biotechnol. 2021, 105, 4855–4878.
- Giaever, G.; Nislow, C. The Yeast Deletion Collection: A Decade of Functional Genomics. Genetics 2014, 197, 451–465.
- Dubreuil, B.; Sass, E.; Nadav, Y.; Heidenreich, M.; Georgeson, J.M.; Weill, U.; Duan, Y.; Meurer, M.; Schuldiner, M.; Knop, M.; et al. YeastRGB: Comparing the abundance and localization of yeast proteins across cells and libraries. Nucleic Acids Res. 2019, 47, D1245–D1249.
- Duina, A.A.; Miller, M.E.; Keeney, J.B. Budding Yeast for Budding Geneticists: A Primer on the Saccharomyces cerevisiae Model System. Genetics 2014, 197, 33–48.
- Longtine, M.S.; McKenzie, A., 3rd; Demarini, D.J.; Shah, N.G.; Wach, A.; Brachat, A.; Philippsen, P.; Pringle, J.R. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 1998, 14, 953–961.
- Stepanov, A.; Nitiss, K.C.; Neale, G.; Nitiss, J.L. Enhancing Drug Accumulation in Saccharomyces cerevisiae by Repression of Pleiotropic Drug Resistance Genes with Chimeric Transcription Repressors. Mol. Pharmacol. 2008, 74, 423–431.
- Michalkova-Papajova, D.; Obernauerova, M.; Subik, J. Role of the PDR Gene Network in Yeast Susceptibility to the Antifungal Antibiotic Mucidin. Antimicrob. Agents Chemother. 2000, 44, 418–420.
- Zhang, F.; Wen, Y.; Guo, X. CRISPR/Cas9 for genome editing: Progress, implications and challenges. Hum. Mol. Genet. 2014, 23, R40–R46.
- Doke, S.K.; Dhawale, S.C. Alternatives to animal testing: A review. Saudi Pharm. J. 2015, 23, 223–229.
- Cressey, D. UK ‘absolutely committed’ to reducing animal use in research. Nature 2014.
- United States Environmental Protection Agency. EPA New Approach Methods: Efforts to Reduce Use of Vertebrate Animals in Chemical Testing. EPA. 2022. Available online: https://www.epa.gov/research/epa-new-approach-methods-efforts-reduce-use-vertebrate-animals-chemical-testing (accessed on 4 September 2022).
- Bender, A.; Cortés-Ciriano, I. Artificial intelligence in drug discovery: What is realistic, what are illusions? Part 1: Ways to make an impact, and why we are not there yet. Drug Discov. Today 2021, 26, 511–524.
- Srivastava, R.; Lal, S.K. A yeast assay for high throughput screening of natural anti-viral agents. Biochem. Biophys. Res. Commun. 2003, 301, 218–221.
- Cottier, V.; Barberis, A.; Lüthi, U. Novel Yeast Cell-Based Assay to Screen for Inhibitors of Human Cytomegalovirus Protease in a High-Throughput Format. Antimicrob. Agents Chemother. 2006, 50, 565–571.
- Ravaux, I.; Perrin-East, C.; Attias, C.; Cottalorda, J.; Durant, J.; Dellamonica, P.; Gluschankof, P.; Stein, A.; Tamalet, C. Yeast cells as a tool for analysis of HIV-1 protease susceptibility to protease inhibitors, a comparative study. J. Virol. Methods 2014, 195, 180–184.
- Benko, Z.; Elder, R.T.; Li, G.; Liang, D.; Zhao, R.Y. Fission yeast as a HTS platform for molecular probes of HIV-1 Vpr-induced cell death. Int. J. High Throughput Screen. 2010, 1, 151–162.
- Frieman, M.; Basu, D.; Matthews, K.; Taylor, J.; Jones, G.; Pickles, R.; Baric, R.; Engel, D.A. Yeast Based Small Molecule Screen for Inhibitors of SARS-CoV. PLoS ONE 2011, 6, e28479.
- Alalam, H.; Sigurdardóttir, S.; Bourgard, C.; Tiukova, I.; King, R.D.; Grøtli, M.; Sunnerhagen, P. A Genetic Trap in Yeast for Inhibitors of SARS-CoV-2 Main Protease. mSystems 2021, 6, e0108721.
- Sun, Y.; Wang, Z.; Tao, J.; Wang, Y.; Wu, A.; Yang, Z.; Wang, K.; Shi, L.; Chen, Y.; Guo, D. Yeast-based assays for the high-throughput screening of inhibitors of coronavirus RNA cap guanine-N7-methyltransferase. Antivir. Res. 2014, 104, 156–164.
- Rakauskaitė, R.; Liao, P.-Y.; Rhodin, M.H.J.; Lee, K.; Dinman, J.D. A rapid, inexpensive yeast-based dual-fluorescence assay of programmed—1 ribosomal frameshifting for high-throughput screening. Nucleic Acids Res. 2011, 39, e97.
- Flynn, J.M.; Samant, N.; Schneider-Nachum, G.; Barkan, D.T.; Yilmaz, N.K.; Schiffer, C.A.; Moquin, S.A.; Dovala, D.; Bolon, D.N.A. Comprehensive fitness landscape of SARS-CoV-2 Mpro reveals insights into viral resistance mechanisms. eLife 2022, 11, e77433.
- Zhang, J.; Vernon, K.; Li, Q.; Benko, Z.; Amoroso, A.; Nasr, M.; Zhao, R.Y. Single-Agent and Fixed-Dose Combination HIV-1 Protease Inhibitor Drugs in Fission Yeast (Schizosaccharomyces pombe). Pathogens 2021, 10, 804.
- Benko, Z.; Elder, R.T.; Li, G.; Liang, D.; Zhao, R.Y. HIV-1 Protease in the Fission Yeast Schizosaccharomyces pombe. PLoS ONE 2016, 11, e0151286.
- Benko, Z.; Liang, D.; Li, G.; Elder, R.T.; Sarkar, A.; Takayama, J.; Ghosh, A.K.; Zhao, R.Y. A fission yeast cell-based system for multidrug resistant HIV-1 proteases. Cell Biosci. 2017, 7, 5.
- Bader, G.; Enkler, L.; Araiso, Y.; Hemmerle, M.; Binko, K.; Baranowska, E.; De Craene, J.-O.; Ruer-Laventie, J.; Pieters, J.; Tribouillard-Tanvier, D.; et al. Assigning mitochondrial localization of dual localized proteins using a yeast Bi-Genomic Mitochondrial-Split-GFP. eLife 2020, 9, e56649.
- Park, K.; Yi, S.Y.; Lee, C.-S.; Kim, K.E.; Pai, H.-S.; Seol, D.-W.; Chung, B.H.; Kim, M. A Split Enhanced Green Fluorescent Protein-Based Reporter in Yeast Two-Hybrid System. Protein J. 2007, 26, 107–116.
- Rothan, H.A.; Teoh, T.C. Cell-Based High-Throughput Screening Protocol for Discovering Antiviral Inhibitors Against SARS-CoV-2 Main Protease (3CLpro). Mol. Biotechnol. 2021, 63, 240–248.
- Ma, C.; Tan, H.; Choza, J.; Wang, Y.; Wang, J. Validation and invalidation of SARS-CoV-2 main protease inhibitors using the Flip-GFP and Protease-Glo luciferase assays. Acta Pharm. Sin. B 2022, 12, 1636–1651.
- Castelló, A.; Álvarez, E.; Carrasco, L. The Multifaceted Poliovirus 2A Protease: Regulation of Gene Expression by Picornavirus Proteases. J. Biomed. Biotechnol. 2011, 2011, 369648.
- Kean, K.M.; Teterina, N.; Girard, M. Cleavage specificity of the poliovirus 3C protease is not restricted to Gln-Gly at the 3C/3D junction. J. Gen. Virol. 1990, 71, 2553–2563.
- Aleshin, A.E.; Drag, M.; Gombosuren, N.; Wei, G.; Mikolajczyk, J.; Satterthwait, A.C.; Strongin, A.Y.; Liddington, R.C.; Salvesen, G.S. Activity, Specificity, and Probe Design for the Smallpox Virus Protease K7L. J. Biol. Chem. 2012, 287, 39470–39479.
- Lin, M.-H.; Chuang, S.-J.; Chen, C.-C.; Cheng, S.-C.; Cheng, K.-W.; Lin, C.-H.; Sun, C.-Y.; Chou, C.-Y. Structural and functional characterization of MERS coronavirus papain-like protease. J. Biomed. Sci. 2014, 21, 54.
- Zumla, A.; Chan, J.F.; Azhar, E.I.; Hui, D.S.; Yuen, K.Y. Coronaviruses—Drug discovery and therapeutic options. Nat. Rev. Drug Discov. 2016, 15, 327–347.
- Nitsche, C.; Holloway, S.; Schirmeister, T.; Klein, C.D. Biochemistry and Medicinal Chemistry of the Dengue Virus Protease. Chem. Rev. 2014, 114, 11348–11381.
- Hoog, S.S.; Smith, W.W.; Qiu, X.; Janson, C.A.; Hellmig, B.; McQueney, M.S.; O’Donnell, K.; O’Shannessy, D.; DiLella, A.G.; Debouck, C.; et al. Active Site Cavity of Herpesvirus Proteases Revealed by the Crystal Structure of Herpes Simplex Virus Protease/Inhibitor Complex. Biochemistry 1997, 36, 14023–14029.
- Qiu, X.; Janson, C.A.; Culp, J.S.; Richardson, S.B.; Debouck, C.; Smith, W.W.; Abdel-Meguid, S.S. Crystal structure of varicella-zoster virus protease. Proc. Natl. Acad. Sci. USA 1997, 94, 2874–2879.
- Liu, X.; Ropp, S.L.; Jackson, R.J.; Frey, T.K. The Rubella Virus Nonstructural Protease Requires Divalent Cations for Activity and Functions in trans. J. Virol. 1998, 72, 4463–4466.
- Hilgenfeld, R.; Lei, J.; Zhang, L. The Structure of the Zika Virus Protease, NS2B/NS3pro. Adv. Exp. Med. Biol. 2018, 1062, 131–145.
- Huang, L.; Chen, C. Understanding HIV-1 protease autoprocessing for novel therapeutic development. Future Med. Chem. 2013, 5, 1215–1229.
- Blanco, R.; Carrasco, L.; Ventoso, I. Cell Killing by HIV-1 Protease. J. Biol. Chem. 2003, 278, 1086–1093.
- Goldstaub, D.; Gradi, A.; Bercovitch, Z.; Grosmann, Z.; Nophar, Y.; Luria, S.; Sonenberg, N.; Kahana, C. Poliovirus 2A Protease Induces Apoptotic Cell Death. Mol. Cell. Biol. 2000, 20, 1271–1277.
- Komissarov, A.A.; Karaseva, M.A.; Roschina, M.P.; Shubin, A.V.; Lunina, N.A.; Kostrov, S.V.; Demidyuk, I.V. Individual Expression of Hepatitis A Virus 3C Protease Induces Ferroptosis in Human Cells In Vitro. Int. J. Mol. Sci. 2021, 22, 7906.
- Babél, L.M.; Linneversl, C.J.; Schmidt, B.F. Production of Active Mammalian and Viral Proteases in Bacterial Expression Systems. Biotechnol. Genet. Eng. Rev. 2000, 17, 213–254.
- Nishimura, K.; Fukagawa, T.; Takisawa, H.; Kakimoto, T.; Kanemaki, M. An auxin-based degron system for the rapid depletion of proteins in nonplant cells. Nat. Methods 2009, 6, 917–922.
- Kumar, R.; Dhali, S.; Srikanth, R.; Ghosh, S.K.; Srivastava, S. Comparative proteomics of mitosis and meiosis in Saccharomyces cerevisiae. J. Proteom. 2014, 109, 1–15.
More
Information
Subjects:
Microbiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
634
Revisions:
2 times
(View History)
Update Date:
22 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




