1. Introduction
Integrated disease management (IDM) incorporates the coordinated use of multiple approaches to reduce the impact of disease-causing agents (pathogens) on agricultural crops
[1]. When applied in parallel or consecutively, these tactics can achieve control of multiple pathogens using different and sometimes synergistic suppression tactics. IDM builds upon the concept of integrated pest management (IPM), which has been widely utilized for decades to target and manage insect pests on agricultural crops and requires different strategies to be employed in a coordinated manner, often with resounding success
[2][3]. When IDM approaches are considered for cannabis (
Cannabis sativa L., high THC-containing genotypes) grown under greenhouse conditions, several aspects need to be modified from traditional IDM programs. First and foremost is the fact that there are no synthetic fungicides available for use on cannabis crops, thus eliminating a widely-used disease management strategy. Instead, only reduced-risk “biological” and “biorational” products are permitted. These products are mostly protective in action, i.e., non-fungicidal, so they are best suited for preventative applications, although some products can also be deployed as sanitizers. While claims of product efficacy and applications for disease reduction in cannabis may be made, not all are necessarily supported by data from replicated research trials or third-party evaluations. This adds to the difficulty in identifying the specific IDM approaches that are best suited for each pathogen. The recent expansion of hemp cultivation (
C. sativa, low THC-containing cultivars) in the USA following federal government approval should provide useful information on disease and pest management approaches that could be extended to cannabis
[4]. The lack of synthetic fungicides for cannabis production has prompted the registration of several biological control products that can be used at different stages of production
[5][6]. However, efficacy data for these products are not always available, and the modes of action of the biocontrol agents are not often fully understood in the context of cannabis IDM, highlighting the need for further research in this area
[6][7]. Fortunately, efficacy data may exist for many of these products on other crops, e.g., for organic production, and therefore, IDM approaches utilized in these crops can likely be extrapolated to cannabis crops
[8]. A second challenge for IDM development in cannabis is that highly bred cultivars containing specific resistance genes against important pathogens are lacking. Instead, genetic selections (genotypes) that target higher yields of inflorescences and THC content and that display unique morphological traits have been made a priority
[9]. In most instances, these efforts have excluded the specific incorporation of disease resistance traits. Consequently, some high-yielding genotypes frequently show high susceptibility to various pathogens. Fortunately, the broad genetic variation that currently exists among cannabis genotypes has led to the identification of resistance in various genotypes to specific pathogens, such as powdery mildew
[6][10][11][12]. The mechanisms underlying this resistance are currently under investigation
[13].
A third challenge is that when cannabis is compared to other widely-grown greenhouse crops, such as tomatoes, cucumbers, and peppers, the optimal cultural and environmental conditions for cultivation have not yet been fully established. Since different cannabis greenhouse operations can experience variable growing conditions, standardized research trials are needed to establish these parameters. Recent research has identified integral aspects of controlled environment cultivation practices that can be used as a baseline reference
[14][15]. The prevalent pathogens affecting cannabis crops in greenhouses have been recently characterized and described
[7], providing diagnostic information that is required for IDM implementation. Accurate diagnosis of the pathogen(s) involved in a disease syndrome is an important component of IDM, and several diagnostic methods have been described
[4][7][16][17][18][19] (
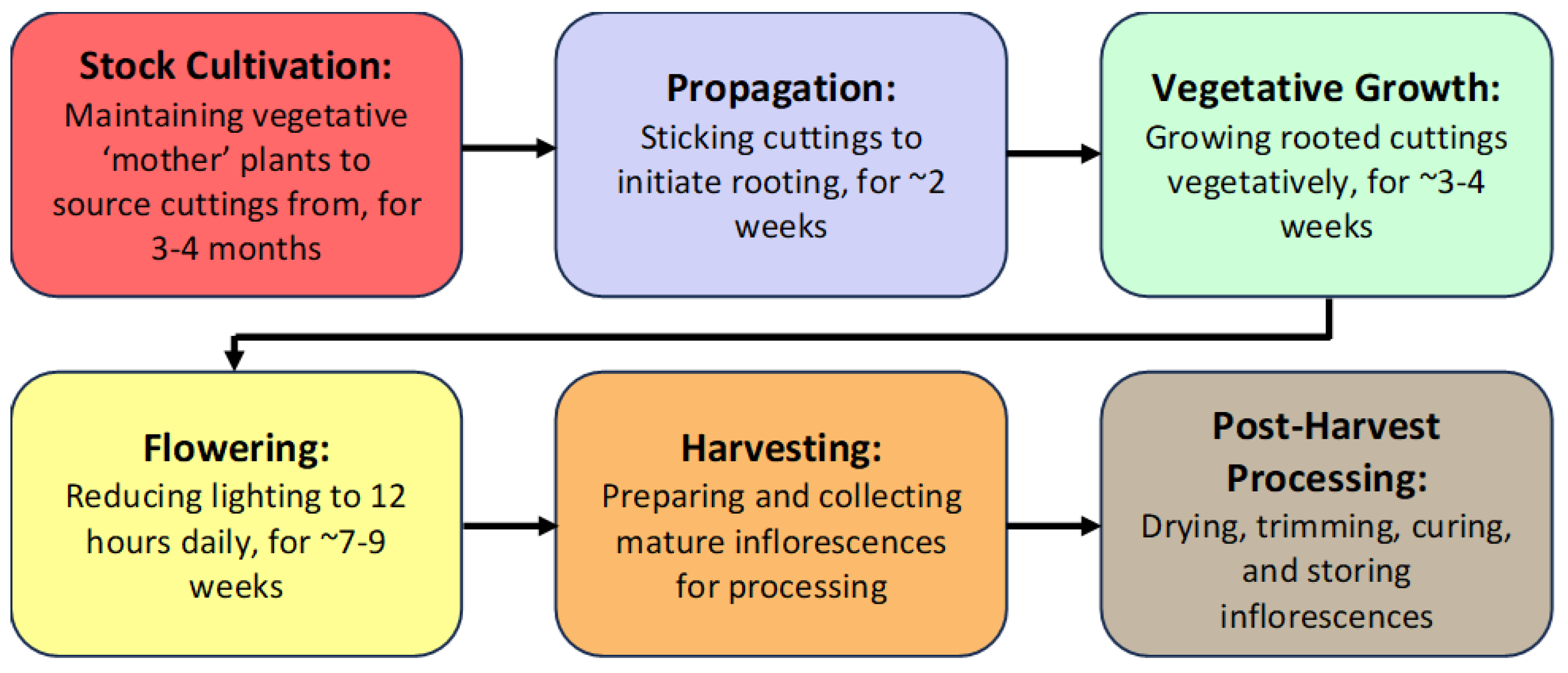
Figure 1).
Figure 1. The different stages of cannabis production under greenhouse conditions. Each crop cultivation cycle from propagation to harvest spans ~12–15 weeks. This is followed by a final stage of post-harvest processing that includes drying, trimming, curing, and storage.
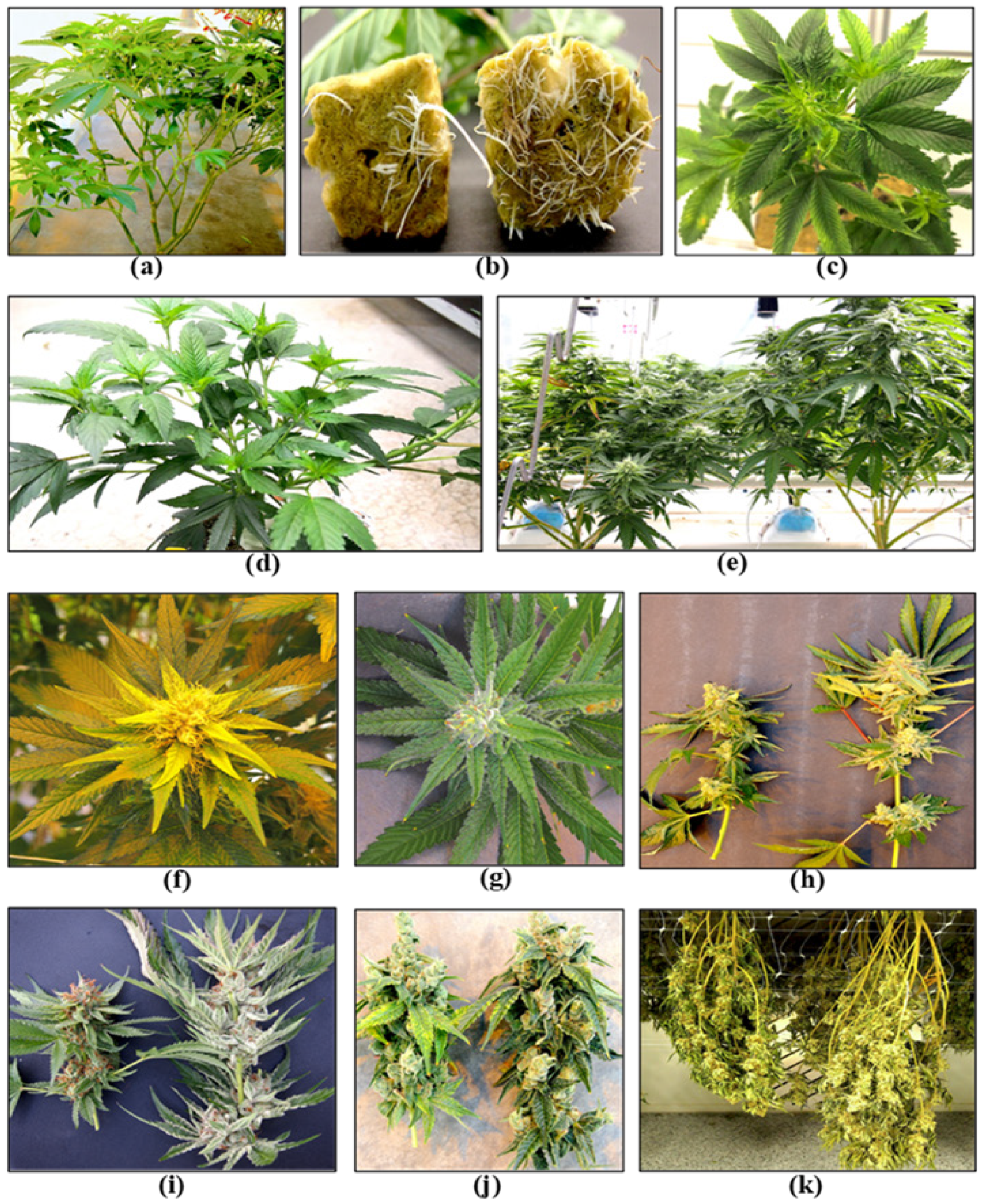
The first stage of production of a cannabis crop is the cultivation and maintenance of stock (mother) plants (Figure 2a), which provide a source of vegetative cuttings (Figure 2b). Once cuttings are rooted, they are transferred to greenhouse growing conditions for 2–3 weeks to continue vegetative growth (Figure 2c). The developing vegetative plants are then transferred to flowering rooms for 7–8 weeks (Figure 2d,e), after which time the inflorescences are harvested (Figure 2f).
Figure 2. The stages of cannabis crop development. (a) Stock plants. (b) Rooting of cuttings. (c) Vegetative plants. (d,e) Flowering plants. (f) Harvested inflorescences being hung to dry. (g) Bucked and trimmed inflorescences.
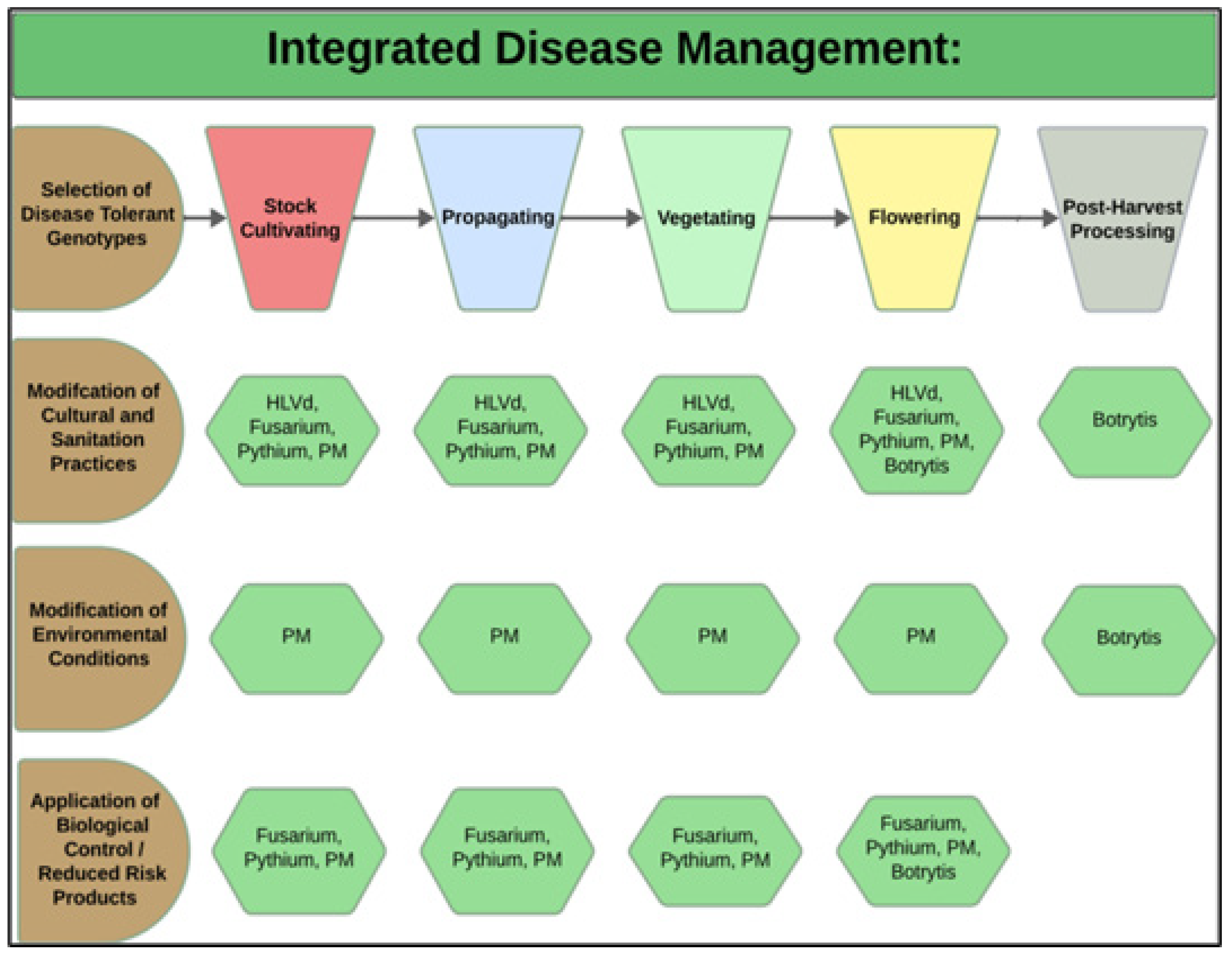
During each crop production year, up to 3–4 cropping cycles may take place per greenhouse compartment. The IDM approaches that can be developed include selection of disease-tolerant genotypes, implementation of cultural practices, modification of environmental climate settings, and application of reduced-risk products (Figure 3).
Figure 3. Integrated disease management strategies (left panel, in brown) are developed according to the crop development stage (top panel). The hexagons (in green) illustrate the specific diseases being targeted, which are discussed in more detail below. HLVd = hop latent viroid; PM = powdery mildew; Botrytis cinerea = bud rot.
2. Stock Cultivation Stage
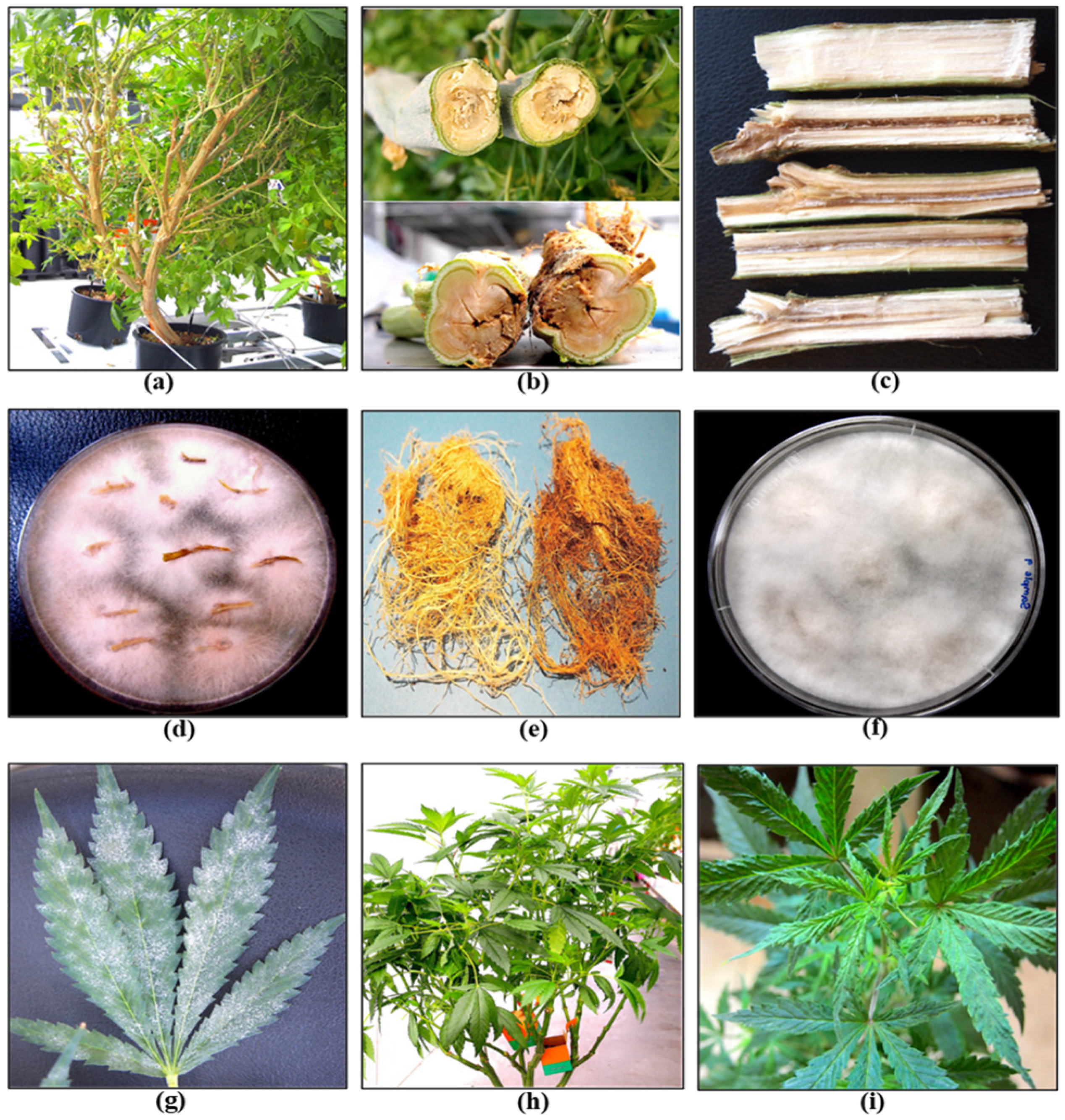
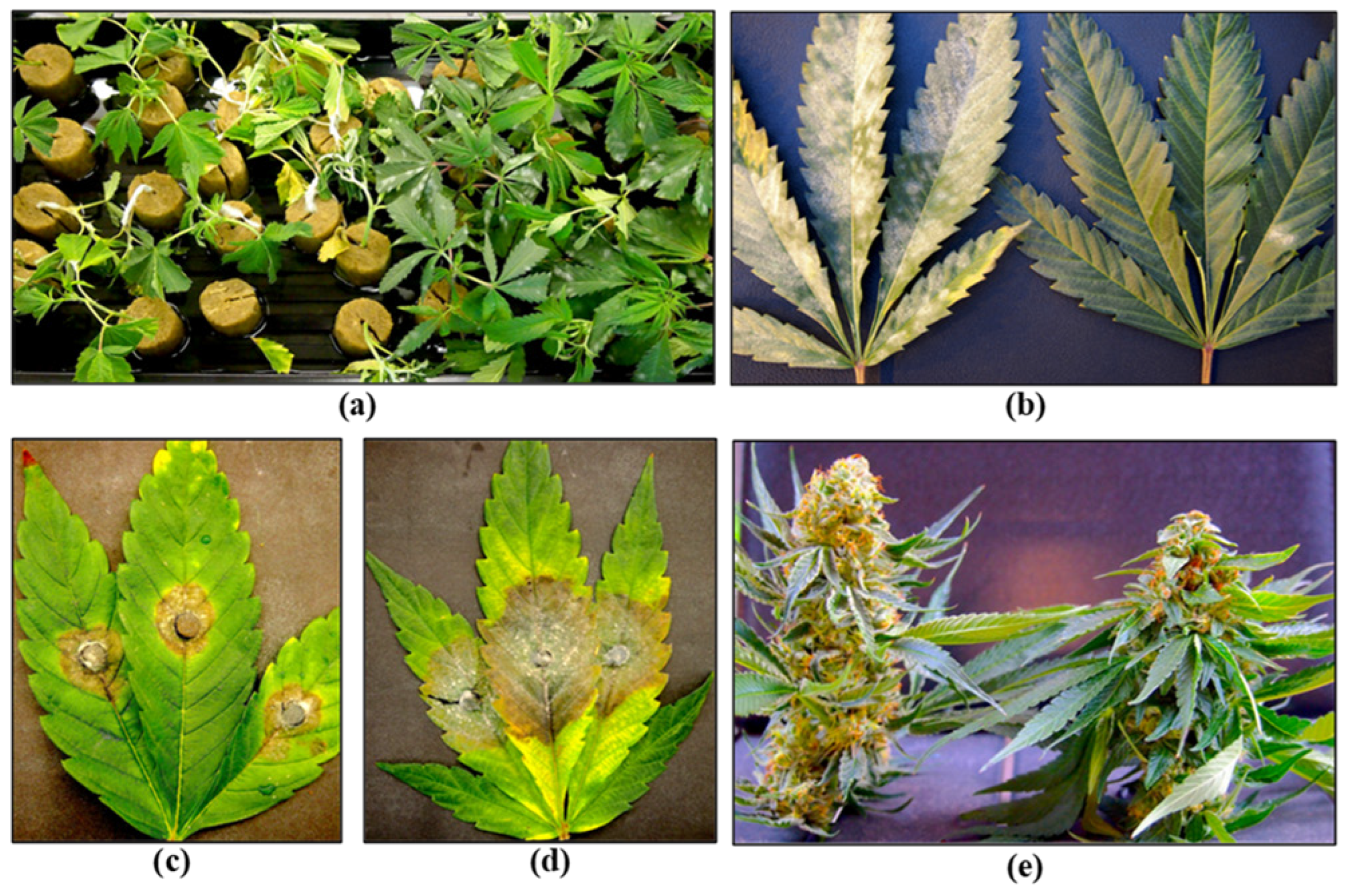
Stock (mother) plants provide a source of vegetative cuttings, which are commonly used in commercial cannabis production. These plants generally constitute a range of genotypes that are chosen for their desired phenotypic characteristics and biochemical profiles. They are grown in designated areas within the greenhouse or in separate indoor rooms. Physical separation of stock plants from larger-scale commercial production is important to prevent the spread of pathogens. The ages of these stock plants can vary and typically range from 3 to 12 months, depending on the facility. In the context of disease development, older plants often exhibit signs of declining growth, such as reduced shoot growth, leaf yellowing, and poor root development (Figure 4a). These symptoms may be indicative of sub-lethal infections by Fusarium and Pythium spp. or hop latent viroid (Figure 4 and Figure 5).
Figure 4. Symptoms of infection by a range of pathogens commonly observed in cannabis stock plants. (a) Declining growth with reduced vigor in a 7-month-old plant. (b,c) Internal stem discolouration due to F. oxysporum infection. (d) Isolation of colonies of F. oxysporum from diseased tissues. (e) Browning of roots due to Pythium infection. (f) Isolation of Pythium colonies from diseased roots. (g) Powdery mildew infection on the upper surface of leaves. (h,i) Infection by hop latent viroid causing reduced vigor and curling of young leaves.
Figure 5. Symptoms of hop latent viroid infection during propagation, vegetative growth and flowering stages of the cannabis crop cycle. (a) Infected stock plants may show unthrifty growth and smaller leaves. (b) Comparison of root development on cuttings derived from an HLVd-infected stock plant (left) and a healthy plant (right). (c) Vegetative plants may show curling and distortion of the youngest leaves. (d) Lateral branching may be seen on HLVd-infected vegetative plants. (e) Stunted growth of HLVd-infected flowering plant (left) compared to a healthy plant (right). (f,g) HLVd-infected inflorescence with yellowing compared to a healthy one, respectively. (h–j) Reduced inflorescence development in three different genotypes of cannabis resulting from HLVd infection. (k) Dried inflorescences from an HLVd-infected plant (left) compared to a healthy plant (right). In all comparison photos, the infected plant is shown on the left.
A closer inspection of the stems of diseased plants will often reveal internal discolouration in the pith and xylem tissues (
Figure 4b,c), a symptom of
Fusarium infection, and/or root browning that can be caused by
Fusarium or
Pythium species
[20][21][22][23]. Excessive waterlogging may also cause root browning on cannabis plants. Accurate pathogen diagnosis at this stage is critical to determine the most effective IDM strategies to implement. Stock plants are also susceptible to powdery mildew, which is clearly visible as white colonies on the upper surfaces of leaves (
Figure 4g). A significant challenge in maintaining healthy stock plants is the recent emergence of hop latent viroid (HLVd)
[24][25][26], which is mostly asymptomatic on stock plants but may cause occasional curling or mottling on the youngest leaves (
Figure 4h,i). The impact of HLVd infection in stock plants is seen when rooting frequency and vigor of cuttings derived from them are examined (
Figure 5). HLVd infection leads to poor root growth (
Figure 5b) that continues to impact plant growth at the vegetative stage (
Figure 5c,d) and can also impact flowering (
Figure 5e). HLVd-infected flowering plants derived from infected stock plants display reduced inflorescence growth as well as lower levels of cannabinoid production
[25]. This emphasizes the importance of maintaining pathogen-free stock plants during commercial production. Routine scouting for the presence of disease symptoms and testing stock plants for the presence of HLVd,
Fusarium, and
Pythium species is highly recommended.
3. IDM Approaches at the Stock Cultivation Stage
During the stock plant cultivation stage, various IDM strategies can be implemented to minimize the development of plant pathogens. The following are examples of some commonly used practices.
3.1. Biosecurity and Quarantine Inspection
Biosecurity practices, which include foot baths, wearing protective clothing, and removing pruned leaves and diseased plants, are standard in most horticultural greenhouse operations
[27]; these practices should be implemented for cannabis growing operations. In addition, it is important to establish a quarantine protocol in cases where plant materials, such as unrooted cuttings or whole plants, are brought in from an external source
[2]. Such precautionary measures can prevent pathogen introduction and are standard biosecurity protocols in commercial crop production
[28]. When applied to cannabis, this necessitates an isolation period of 3–4 weeks, during which plants are monitored for disease symptoms and tested for the presence of potential viruses and other pathogens
[6]. Testing for cannabis pathogens can be achieved by polymerase chain reaction (PCR) methodologies, and testing for viruses or viroids can be achieved using reverse transcription polymerase chain reaction (RT-PCR); many laboratories currently offer this service for an array of cannabis pathogens
[4][6][7]. After the plants are confirmed to be free of detectable pathogens, they can be used for commercial propagation. Infected plants should be destroyed.
3.2. Cultural and Environmental Management
Environmental management is a component of IDM across all stages of cannabis growth since climatic conditions can influence both plant growth as well as pathogen growth. Standard cannabis cultivation environmental setpoints, which are established for baseline pathogen management during low disease pressure periods, have been described
[15][29]. Conditions that are unfavorable for disease development while at the same time supporting optimal plant growth are required. This often necessitates lowering temperature and humidity levels below the optimal set points for plant development in order to reduce the vapour pressure deficit (VPD) in the crop environment during periods of high disease pressure. Optimal environmental conditions vary across the different developmental stages of cannabis. In the cloning or seedling stage, temperatures should be kept between 20 °C and 24 °C with relative humidity above 90%. For the stock plant and vegetative stages, temperatures should range from 25 °C to 28 °C to promote rapid growth, with relative humidity maintained between 65% and 75% (equating to a VPD of 1.1 to 0.94). During the flowering stage, temperatures should be set between 23 °C and 28 °C to facilitate the transfer of photosynthates to the flowers, with relative humidity between 50% and 70% (resulting in a VPD of 1.4 to 1.13). These environmental parameters can be achieved by modifying venting, heating, and air circulation strategies
[29]. Seasonal adjustments may also need to be made, as warmer temperatures with higher humidity in the summer months may increase the incidence of root-infecting pathogens, such as
Fusarium and
Pythium species. Similarly, cooler and more humid conditions during winter seasons may enhance the development of powdery mildew infections. The impact of environmental conditions on HLVd development is currently unknown.
3.3. Sanitary Practices
Thorough sanitation of the growing environment before planting a new cannabis crop is important to reduce residual pathogen inoculum, which can be spread by water or air or on tools and potentially on clothing, gloves, or shoes. This is a common practice used on most greenhouse crops, especially where viruses are of concern
[6][27]. To reduce pathogen transmission, all surfaces and equipment, as well as gutters, tables, floors, drip emitters, and pots, should be cleaned by using reduced-risk sanitary products. These products include hydrogen peroxide with peracetic acid (Sanidate
® or Zerotol
®), dodecyl dimethyl ammonium chloride (Chemprocide
® or KleenGrow
®), isopropyl alcohol, and bleach
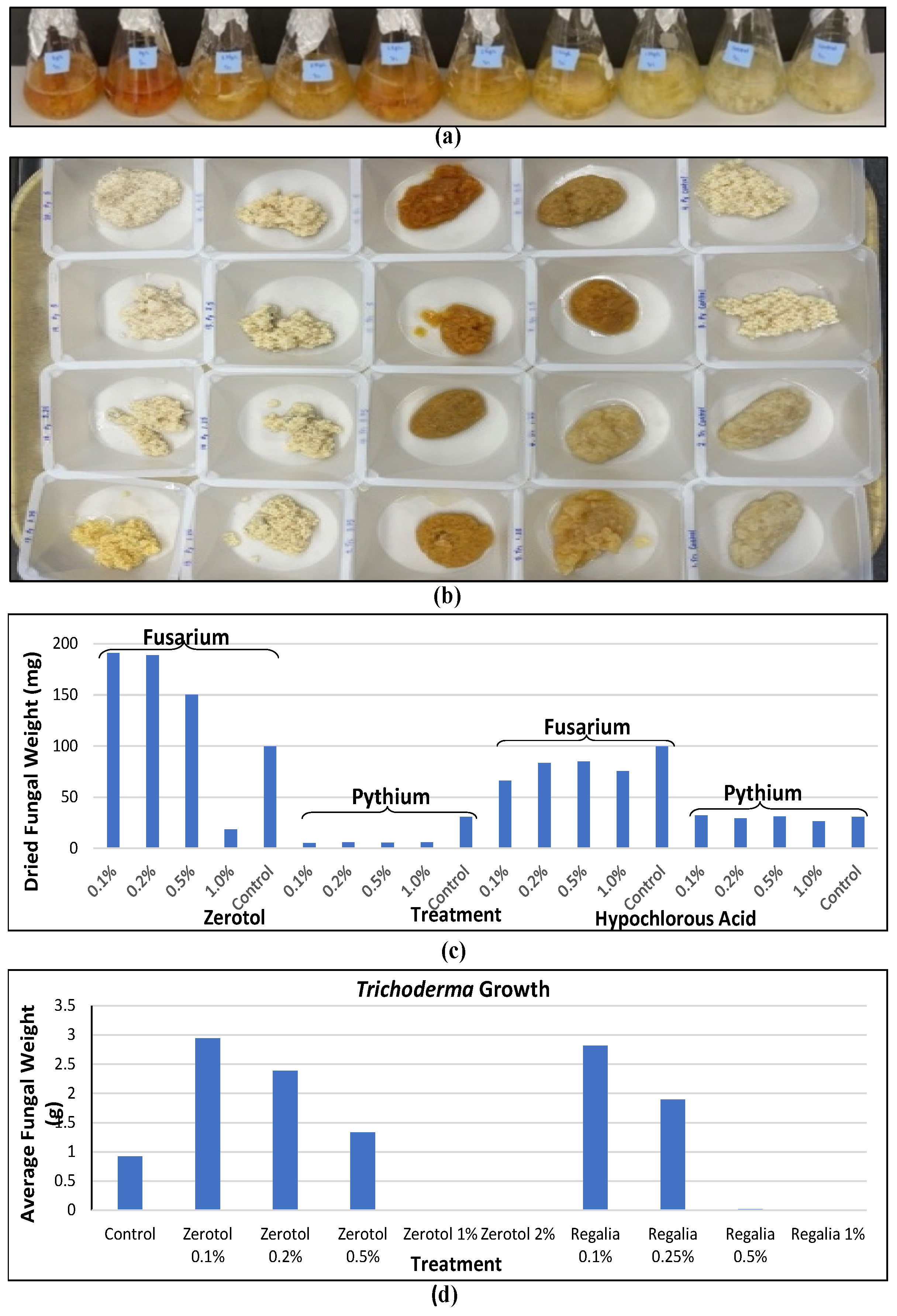
[6]. The efficacy of these products in inhibiting pathogen growth can vary depending on the pathogen, product, and concentrations used. A comparison of two products used at four concentrations against the growth of two pathogens is shown in
Figure 6a,b. At increasing concentrations, both Zerotol
® and hypochlorous acid (a product containing 1000 ppm that was diluted) reduced pathogen growth, but
Pythium showed a greater sensitivity compared to
Fusarium (
Figure 6c). These products can also potentially negatively affect the growth of beneficial
Trichoderma species applied as biocontrol agents (
Figure 6d). Therefore, care must be taken to consider the potential impact of applying reduced-risk products in conjunction with biocontrol products. These types of evaluations are important to conduct for any reduced-risk product targeted for the cannabis market to demonstrate efficacy and possible non-target effects.
Figure 6. The effect of reduced-risk products on pathogen growth can be evaluated under laboratory conditions by testing a range of concentrations in liquid culture medium. (a) Example of fungal growth in potato dextrose broth containing a range of concentrations of individual products. (b) Growth is measured by obtaining mycelium dry weights after a 7-day exposure. (c) The effect of Zerotol® and hypochlorous acid (1000 ppm) on growth of two pathogens at increasing concentrations from 0.1% to 1.0%. Both Fusarium and Pythium growth is reduced at higher concentrations, but growth of Pythium shows greater sensitivity compared to Fusarium. (d) Growth of Trichoderma can also be reduced by the presence of specific compounds when added to the culture medium.
3.4. Testing for Pathogen Presence and Eradication
Early detection of disease symptoms in stock plants is important to prevent pathogens from spreading within the growing environment. There are several diagnostic approaches that have been described to detect cannabis pathogens, and a number of commercial laboratories provide testing services for a range of pathogens
[4][7][19][25]. The practice of culling and replenishing stock plants is a standard component of IDM programs when diseased plants are detected
[30]. Stock plants should be replaced after several (3–4) months of production with new, pathogen-free plants, which is key to maintaining a healthy and vigorous stock plant population. Plants infected with
Fusarium,
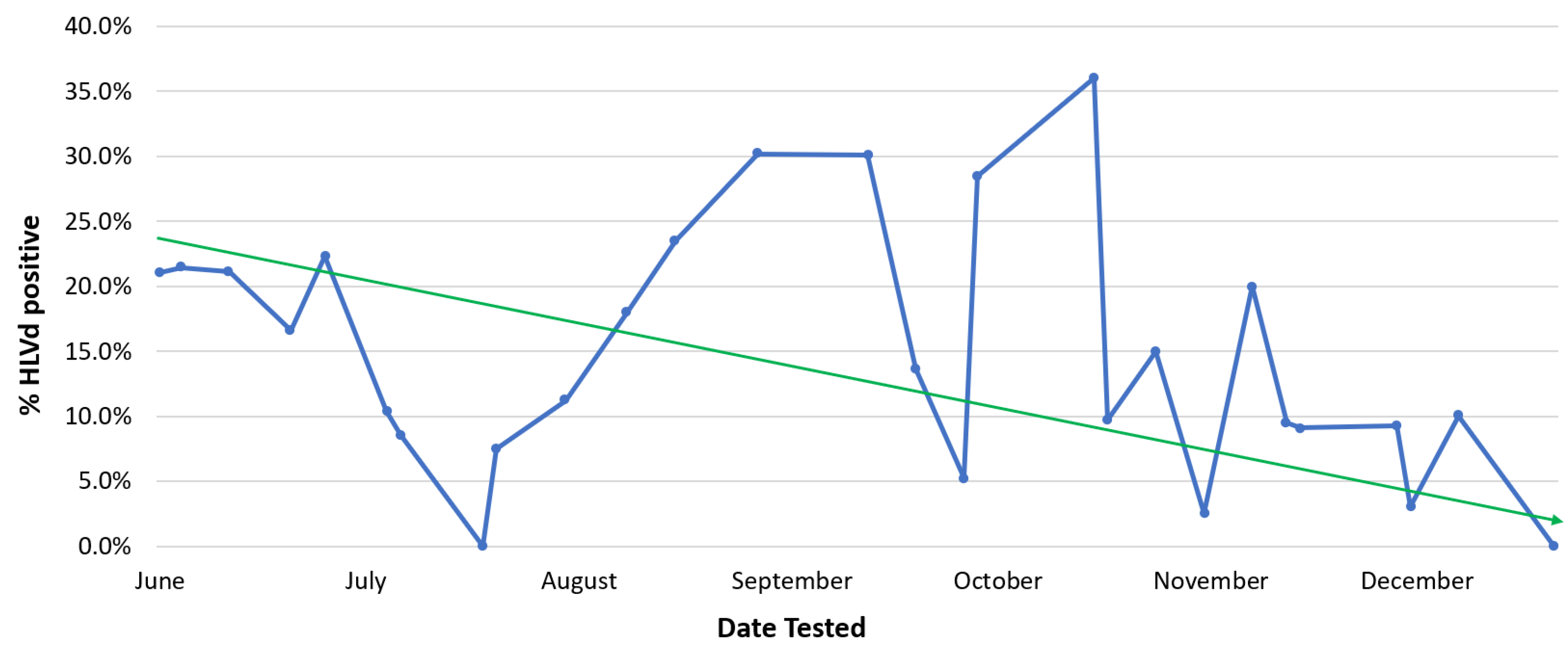
Pythium, or HLVd should be promptly removed from a facility. Eradication of diseased plants, particularly those infected with HLVd, is essential. When regular (weekly) pathogen testing is followed by the destruction of those plants infected by HLVd, a gradual decline in the occurrence of diseased stock plants can be achieved (
Figure 7). After many rounds of testing performed over a 6-month period, this strategy was shown to reduce HLVd frequency in stock plants from 22% to 1% (
Figure 7). Peaks of infection can still be seen, which are attributed to the re-introduction of diseased plant material that went undetected initially and was inadvertently used as a source of cuttings. Removing this material upon detection resulted in the continued downward trend of infection.
Figure 7. The impact of eradication of HLVd-infected stock plants on the frequency of positively infected plants over a 6-month duration. The blue line shows the actual incidence of infected plants, which fluctuates over time. The solid green line is the general trend that shows a decline in number of infected plants. Presence of the viroid in infected plants was confirmed by RT-PCR. The data shown are from the 2022 growing season.
3.5. Utilizing Disease-Tolerant Genotypes
The utility of disease-tolerant genotypes that may have been developed through selective breeding and genotype screening is an important aspect of IDM for stock plants. Disease-tolerant genotypes of cannabis have been identified for a number of pathogens, including root rot (
Fusarium oxysporum)
[22], powdery mildew (
Golovinomyces ambrosiae)
[10][11][12][31], leaf blight (
Neofusicoccum parvum)
[32], and bud rot (
B. cinerea)
[33][34] (
Figure 8). Recent research suggests that specific defense genes may play a role in certain host–pathogen interactions, leading to a resistant phenotype
[11][12][13][35]. Continued evaluation of cannabis genotypes for pathogen response is a critical component of an IDM program.
Figure 8. Examples of cannabis genotypes that exhibit a level of disease tolerance to different pathogens. (a) Fusarium damping-off, with susceptible genotype on the left and tolerant genotype on the right. (b) Powdery mildew, with susceptible genotype on the left and tolerant one on the right. (c,d) Alternaria leaf blight, with tolerant genotype on the left and susceptible one on the right. (e) B. cinerea bud rot, with tolerant genotype on the left and susceptible one on the right.