Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Flora Ventsislavova Tsvetanova | -- | 4128 | 2024-03-19 08:24:47 | | | |
| 2 | Mona Zou | Meta information modification | 4128 | 2024-03-21 08:26:25 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Tsvetanova, F. The Plethora of Microbes with Anti-Inflammatory Activities. Encyclopedia. Available online: https://encyclopedia.pub/entry/56399 (accessed on 07 February 2026).
Tsvetanova F. The Plethora of Microbes with Anti-Inflammatory Activities. Encyclopedia. Available at: https://encyclopedia.pub/entry/56399. Accessed February 07, 2026.
Tsvetanova, Flora. "The Plethora of Microbes with Anti-Inflammatory Activities" Encyclopedia, https://encyclopedia.pub/entry/56399 (accessed February 07, 2026).
Tsvetanova, F. (2024, March 19). The Plethora of Microbes with Anti-Inflammatory Activities. In Encyclopedia. https://encyclopedia.pub/entry/56399
Tsvetanova, Flora. "The Plethora of Microbes with Anti-Inflammatory Activities." Encyclopedia. Web. 19 March, 2024.
Copy Citation
Inflammation, which has important functions in human defense systems and in maintaining the dynamic homeostasis of the body, has become a major risk factor for the progression of many chronic diseases. Although the applied medical products alleviate the general status, they still exert adverse effects in the long term. For this reason, the solution should be sought in more harmless and affordable agents. Microorganisms offer a wide range of active substances with anti-inflammatory properties. They confer important advantages such as their renewable and inexhaustible nature.
anti-inflammatory activity
microorganisms
lactic acid bacteria
fungi
marine bacteria
1. Bacteria and Yeasts with Anti-Inflammatory Properties in Food
Microbes are on one side responsible for food contamination and spoilage, and on the other—they are at the core of obtaining beneficial products that make a major contribution to the food industry. Yeast and bacteria are the most widely used microorganisms in the food industry. They produce different foods through fermentation, the main contributor among bacteria being lactic acid bacteria (LAB) [1].
LAB represent a heterogeneous group of microorganisms from different genera—Lactobacillus, Enterococcus, Carnobacterium, Lactococcus, Leuconostoc, Pediococcus, Oenococcus, Streptococcus, Vagococcus, Tetragenococcus, and Weissella. Some strains of LAB together with several strains from Bifidobacterium create probiotics. Probiotics are defined as “live microorganisms that when administered in adequate amounts confer a health benefit to the host” [2]. They have been traditionally used as starter cultures for the preparation of fermented foods where lactic acid is the main product of carbohydrate metabolism. Lactic acid is known to inhibit the growth of many pathogens. LAB are included in functional foods as they provide multiple benefits for the host organism and possess GRAS status. LAB exert immunomodulating effects through the stimulation of IL-10 production and/or decreasing pro-inflammatory cytokines [3]. There is plenty of evidence demonstrating the beneficial role of specific lactobacilli strains in inflammatory conditions including wound healing, skin protection, defense against pathogenic agents, etc. [4][5][6][7][8]. Figure 1 illustrates the beneficial effects of LAB on the human organism. However, to manifest their beneficial qualities, they should be able to survive in the gastrointestinal tract and be present in adequate quantities [9]. The probiotic bacteria possess the ability to adhere to the host‘s intestinal mucosa, which reveals its relationship with gut mucosa cells and the generated immunomodulatory effect on the host, and also the pathogen competitive exclusion [10]. Moreover, a study from 2021 demonstrated that the probiotic lactobacilli ameliorate the symptoms of inflammation that occur as a result of anti-inflammatory drugs. The administration of probiotics to mice led to an increase in the population of anaerobes and lactobacilli along with a serious decrease in the total enterobacteria. Therefore, probiotic supplementation in non-steroidal anti-inflammatory drug-induced inflammation increased the intestinal antimicrobial activity, thus maintaining intestinal homeostasis [11]. In another work, the team of Santiago-Lόpez highlighted the potential of Lactobacillus fermentum to improve indomethacin (a non-steroidal anti-inflammatory drug)-induced inflammation [12]. These examples indicate that the cited lactobacilli possess even stronger anti-inflammatory effects than the non-steroidal anti-inflammatory drugs.
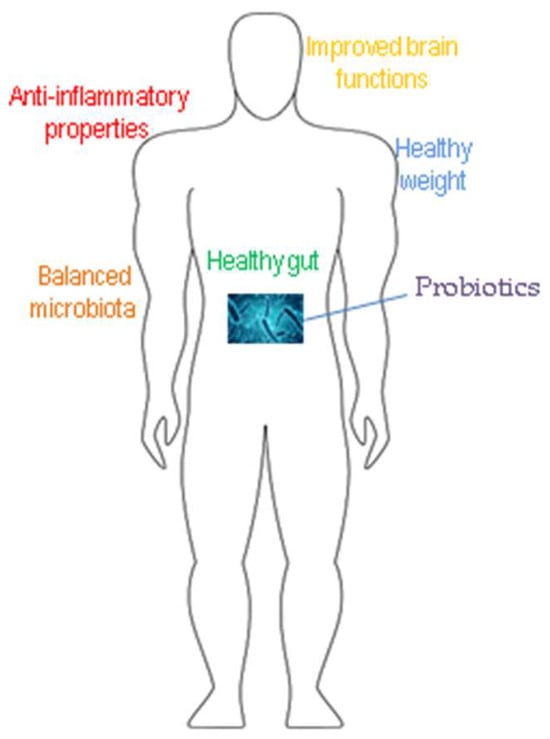
Figure 1. Main beneficial effects of probiotics.
Besides the production of lactic acid, LAB produce various beneficial substances including B-group vitamins (riboflavin, folates, cobalamin, thiamine), bacteriocins, bioactive peptides, biosurfactants, flavoring compounds, etc. Different strains of lactobacilli with anti-inflammatory activity are given in Table 1.
Table 1. LAB with anti-inflammatory activity.
| Strain | Activity | Reference |
|---|---|---|
| L. reuteri DSM 17938 (lysate) | Decreased levels of IL-6 and IL-8 | [13] |
| L. reuteri ATCC PTA6475 | Synthesizes folate; suppression of TNF-α production in human monocytes |
[14] |
| L. reuteri MG9012 | Reduced NO production | [15] |
| L. paracasei CBA L74 | ||
| L. brevis | GABA production; inhibition of NO and iNOs production, and NF-kB activity | [16] |
| L. fermentum MG9014 | Reduced NO production | [15] |
| L. plantarum CRL2130 | Produced riboflavin; intestinal inflammation reduction via pro-inflammatory cytokines control |
[17] |
| L. plantarum OLL 2712 | Induced activity of IL-10 | [18] |
| L. plantarum M2, L. plantarum K09 | TNF-α suppression | [19] |
| L. paraplantarum BGCG11 | EPS production; decreased levels IL-1β, TNF-α, and iNOS, and enhanced levels IL-10 and IL-6 | [20] |
| L. rhamnosus RW-9595M | EPS production; IL-10 production inhibition | [21] |
| Lactobacillus rhamnosus | Reduced levels of IL-6 and C Reactive Protein | [22] |
| L. intestinalis LE1 and L. johnsonii LE2 | Reduced in vitro mercury toxicity on the intestinal mucosa | [23] |
| L. plantarum SGL 07, L. salivarius SGL 19 (lysates) | Stimulation of keratinocytes proliferation | [24] |
| L. plantarum CRL2130, Streptococcus thermophilus CRL807, and Streptococcus thermophilus CRL808 (blend) |
Riboflavin, folate production, immune-modulatory properties; decreased levels of IL-6, increase in TNF-α | [25] |
| L. casei, L. plantarum, L. acidophilus, L. delbrueckii subsp. bulgaricus, Bifidobacterium (B.) longum, B. breve, B. infantis, and S. salivarius (blend) |
Decreased levels of TNF-α and IL-6 in colon tissue | [26] |
| Lactobacillus, Lactococcus, Leuconostoc, Streptococcus, Acetobacter, Kluyveromyces, Torula, Candida, Saccharomyces (kefir consortium) |
Kefiran production; induced CD4+ and CD8+ T-lymphocytes populations | [27] |
| Kefiran production; normalized levels of IL4 and IL5 levels | [28] | |
| L. casei, L. acidophilus, Lactococcus lactis, Leuconostoc citrovorum, L. mesenteroides, Acetobacter aceti, A. rasens, Streptococcus thermophilus, S. lactis, Kluyveromuces sp., Sacharomyces sp. (Tibetan mushroom consortia) |
Granuloma formation inhibition | [29] |
| Lactobacillus sp., Acetobacter xylinoides, Gluconobacter oxydans, Komagataeibacter xylinum, Gluconacetobacter hansenii, Oenococcus oeni, Komagataeibacter europaeus, Schizosaccharomyces pombe, Zygosaccharomyces kombuchaensis, Torulaspora delbrueckii, Saccharomyces sp., Brettanomyces sp. (kombucha consortia) |
Riboflavin production; 87–91% improved anti-inflammatory activity; IC50 value close to the maximal inhibitory concentration of nordihydroguaiaretic acid | [30] |
| Leuconostoc mesenteroides BioE-LMD, Bacillus licheniformis BioE-BL11 (isolated from Korean kimchi) |
EPS production; inhibited secretion of IL-6; increased secretion of IL-10 | [31] |
| L. plantarum LM17 and LM19, L. rhamnosus LM07 (agave fermentation stage) |
Decreased intestinal permeability | [32] |
| L. casei EMRO 002, L. casei EMRO 213, L. plantarum EMRO 009, L. fermentum EMRO 211, L. rhamnosus EMRO 014, L. bulgaricus EMRO 212, Rhodopseudomonas palustris EMRO 201 (multi-strain extract) |
Inhibition of migration inhibitory factor tautomerase activity | [33] |
| L. mucosae AN1, L. fermentum SNR1 (encapsulated) |
Anti-inflammatory cytokines upregulation and pro-inflammatory cytokines downregulation | [9] |
1.1. Vitamins-Producing LAB
Riboflavin (vitamin B12) plays a major role in cellular metabolism as it is a precursor of flavin mononucleotide and adenine flavin dinucleotide, coenzymes that protect cells from ROS. Unbalanced ROS secretion is usually associated with chronic intestinal inflammation in the initial stages of IBDs. Several studies have demonstrated the anti-inflammatory potential of riboflavin which plays the role of an antioxidant enzyme cofactor. For example, in the study of Al-Harbi and co-workers, vitamin B12 intake inhibited the production of TNF-α [34]. On the other hand, its short-term deficiency was found to affect the ability of macrophages to induce adequate immune response while its supplementation reduced the macrophage pro-inflammatory activation [35]. This vitamin was also demonstrated to decrease the inflammation caused by oxidative stress in diabetes [36]. Riboflavin-producing Lactobacillus plantarum CRL2130 was reported to reduce intestinal inflammation in a model of colitis in mice through the control of pro-inflammatory cytokines. For the aims of the experiment, the strain was wrapped in a food matrix (fermented soymilk), or administered as a bacterial suspension [17][37]. All these studies show that riboflavin could be an adequate source of prevention of inflammation.
Except for riboflavin, folates and folic acid (vitamin B9) also take part in the reduction and prevention of inflammation. A study from 2017 explored the relationship between arterial hypertension and the decrease in serum homocysteine concentrations, and indirectly with the decrease in other marks of inflammation [38]. Pan and collaborators reported that the reason for IBDs may be associated with folate deficiency [39]. Moreover, folic acid supplementation could decrease the risk of colorectal cancer development in IBD-suffering individuals [40]. Lactobacillus reuteri ATCC PTA6475 indicated the ability to suppress the production of TNF—α in human monocytes—and exerted anti-inflammatory activity in a murine model of acute colitis. This quality is determined by the folC2 gene involved in folate biosynthesis. [14].
A probiotic blend of LAB-producing vitamins was described by Levit et al. The strains Lactobacillus plantarum CRL2130, Streptococcus thermophilus CRL807, and Streptococcus thermophilus CRL808 with immunomodulatory properties were studied for anti-inflammatory activities. The results displayed even more emphasized anti-inflammatory effects than in the case of individual strains administered to rodents with induced intestinal mucositis [25]. The successful application of microbial mixtures regarding anti-inflammatory effects was reported for Lactobacillus casei, Lactobacillus plantarum, Lactobacillus acidophilus, Lactobacillus delbrueckii subsp. bulgaricus, Bifidobacterium longum, Bifidobacterium breve, Bifidobacterium infantis, and Streptococcus salivarius where the tumor development associated with intestinal inflammation in mice was prevented [26]. These conclusions indicate that the employment of blends of microbes could enhance their individual anti-inflammatory properties.
In general, vitamin-producing LAB, especially those that secrete riboflavin and folate, could find application in the treatment of various diseases. Additionally, the blends of vitamin-producing LAB would not only enhance the efficiency of the primary treatments but would also fill the shortages of essential vitamins provoked by the disease or the treatment [41]. According to Albuquerque et al., vitamin production can be initiated by prebiotic supplementation [42]. A very important invention accessed in murine models, is that the vitamins produced by LAB are biologically active [43][44].
1.2. Anti-Inflammatory Exopolysaccharides (EPSs) Producing Bacteria
Another substance with proven anti-inflammatory properties secreted by LAB, especially by Lactobacillus kefiranofaciens, is the exopolysaccharide kefiran, which is a branched gluconolactone. Kefiran is a constituent of the fermented beverage kefir. The consortium of microbes in kefir include the bacterial strains Lactobacillus, Lactococcus, Leuconostoc, Streptococcus, Acetobacter, and the yeasts Kluyveromyces, Torula, Candida, and Saccharomyces. According to Jenab et al., kefiran induced both CD4+ and CD8+ T-lymphocyte populations [27]. In another study, IL-4 and IL-5 levels were decreased to normal amounts after its administration in the ovalbumin-induced BALB/c mice asthma model. Therefore, kefiran has the potential to be used for pulmonary inflammatory treatment [28]. It is believed to be a promising candidate for the treatment of bronchial asthma and lung inflammation [45].
Besides the kefir consortium, symbiotic microorganisms of the Tibetan mushroom were also revealed to possess anti-inflammatory properties. This symbiotic population consists of bacteria and yeast living in polysaccharide grains secreted by them. Among the organisms of the consortia are Lactobacillus casei, Lactobacillus acidophilus, Lactococcus lactis, Leuconostoc citrovorum, Leuconostoc mesenteroides, Acetobacter aceti, Acetobacter rasens, Streptococcus thermophilus, Streptococcus lactis, Kluyveromuces sp., and Sacharomyces sp. [46]. The Tibetan mushroom suspension was tested on a cotton-induced granuloma and paw edema in rats resulting in a significant inhibition of 43% in the formation of granuloma tissue [29].
Exopolysaccharides isolated from the cell surface of the putative probiotic organism Lactobacillus paraplantarum BGCG11 were tested in a rat model induced by carrageenan injection in the hind paw. Reduced expression levels of pro-inflammatory mediators IL-1β, TNF-α, and iNOS, and enhanced levels of pro-inflammatory cytokines IL-10 and IL-6 were reached. This suggests that the antihyperalgesic and antiedematous effects of EPSs were associated with inflammatory response suppression [20]. EPSs from Lactobacillus rhamnosus RW-9595M were detected to induce higher levels of TNF-α, IL-6, and IL-12 though inhibited IL-10 production [21].
Bacillus licheniformis BioE-BL11 and Leuconostoc mesenteroides BioE-LMD 18 isolated from the Korean fermented kimchi were found to inhibit the secretion of pro-inflammatory cytokine IL-6 in the LPS-stimulated RAW 264.7 mouse macrophage. In addition, the secretion of the anti-inflammatory cytokine IL-10 was increased and it was established that the extent of the inhibition was dose-dependent. These results suggest that EPSs produced by B. licheniformis and L. mesenteroides could be proper ingredients for application in pharmaceutical products, cosmetics, and in the food industry [31]. In another study, L. plantarum LM17, L. plantarum LM19, and L. rhamnosus LM07 with probiotic characteristics were isolated from the agave fermentation stage in mezcal production [32]. The strains were shown to exert anti-inflammatory properties in TNF-α-stimulated HT-29 cells. These species were tested in vivo in a mouse model with dinitrobenzene sulfonic acid (DNBS)-induced chronic colitis. The inflammatory indicators were weight loss, intestinal permeability, and cytokine profiles. L. plantarum improved mice health as observed by reduced weight loss and significantly decreased intestinal permeability. These results confirmed the capability of LAB isolated from the agave fermentation to be used as probiotic supplements for IBD treatment [32]. The study of Hsieh and Allen manifested that the exopolysaccharides from the probiotic strain Bacillus subtilis protected mice from acute colitis caused by the pathogen Citrobacter rodentium. The polysaccharides inhibited the activation of T-cells and therefore controlled T-cell-mediated immune responses in multiple inflammatory diseases [47].
1.3. Biosurfactants with Anti-Inflammatory Properties-Producing Bacteria
Biosurfactants are natural products derived from bacteria, yeasts, or fungi, and represent amphiphilic compounds that contain polar-(water soluble) and non-polar-(insoluble in water) sections. The amphiphilic structure of biosurfactants is important for surface tension reduction. This property is applied in various industries such as pharmaceutical, cosmetics, food, and petroleum [48]. Biosurfactants are produced when the carbon source is an insoluble substrate; thus, microorganisms facilitate their diffusion into the cell by the production of different substances. They reduce surface tension in bacteria. Glycolipids, a polysaccharide–lipid complex, phospholipid, mycolic acid, lipoprotein, or lipopeptide are the possible structures of biosurfactants. Besides the availability of insoluble carbon sources, biosurfactant production may be induced by the culture conditions, such as temperature, pH, agitation, and concentration of P, Fe, N, Mg, and Mn ions in the media [49]. Among the biosurfactant-producing bacteria are Bacillus, Rhodococcus, Mycobacterium, Pseudomonas, Arthrobacter, etc. [50]. Bacillus subtilis produces the cyclic lipopeptide surfactin. Surfactin possesses several biological activities among which is its anti-inflammatory action. Its mechanism of action in the lipopolysaccharide (LPS)-stimulated macrophages displayed prevention of the secretion of anti-inflammatory agents (such as IL-1β and iNOs), and reduction in TNF-α and NO levels in response to septic shock [51]. The central LPS receptor is TLR4. It is a signal transduction pathway mediating LPS-induced inflammation. The studies have shown that surfactin downregulated the LPS-induced TLR4 protein expression of macrophages. Surfactin was also demonstrated to express anti-inflammatory activity by reducing the activation of nuclear factor-kB, which plays a role in the NF-κB cell signaling pathways [51][52]. Zhao et al. reported a Bacillus subtilis with anti-inflammatory properties owing to the possession of the cyclic lipopeptides, fengycin and iturin [52].
In conclusion, microbial biosurfactants possess various advantages over chemical ones; for example, they are less toxic, have higher foaming capability, have higher biodegradability, and exhibit specific activity at extreme pH and temperature [50].
1.4. Bacteriocins with Anti-Inflammatory Properties—Producing Lactobacilli
Lactobacilli produce bacteriocins, extracellular compounds with peptide structures synthesized by ribosomes. Their function is the defense against other microorganisms [53]. One example of a bacteriocin produced by lactobacilli is nisin which enhances the bacterial activity of mononuclear phagocytes. This action occurs through the increase in autophagy-inducing cytokine-like IFN-γ levels and decreasing IL-4 and IL-13 which is followed to downregulate the lung Th2 response, which is known to restrict autophagy. Therefore, therapy with probiotics could modulate the immune responses in the lung which augment the regulatory T-cell response in the therapy of peripheral blood mononuclear cells (PBMCs) and macrophages with combined M. tuberculosis and LAB [54]. Bacteriocins produced by Lactobacillus rhamnosus successfully reduced IL-6 and C-reactive protein levels in the serum accumulated after infectious diseases. Therefore, these biochemicals could be used in the prevention of postoperative infections [22].
1.5. Other Substances with Anti-Inflammatory Effects Produced by Lactobacilli
LAB are known to produce γ-aminobutyric acid (GABA). GABA is a non-protein amino acid that regulates neuronal activity by nerve transmission inhibition. Its deficiency is associated with various neurological diseases, such as epilepsy, anxiety, depression, Alzheimer’s and Parkinson’s diseases, Huntington’s chorea, and schizophrenia [55]. According to several studies, GABA enhances plasma growth, the brain’s protein synthesis, hormone level, cognitive ability and memory, relaxes nerves, and lowers blood pressure [56][57]. The advantage of microbially produced GABA over a chemically synthesized one is that it is economically safe and friendly [16]. Soltani et al. reported that GABA increased the production of the anti-inflammatory mediator TGF-β1 and decreased the production of inflammatory mediators such as IL-β1, TNF-α, interferon-γ, and IL-12 in streptozotocin-treated mice [58]. Kim et al. isolated GABA-producing LAB from several fermented Korean foods and evaluated the anti-inflammatory effects of these strains on macrophages to determine their potential utilization as probiotics. The strains with pronounced GABA-producing ability were from the species L. brevis. The analysis revealed that almost all of the strains inhibited NO and iNOs production, and NF-kB activity, thus exhibiting anti-inflammatory activity [16]. Therefore, these GABA-producing lactobacilli can act as probiotics that control nerve excitement.
Kombucha, a fermentative drink, and the consortium involved in its production were the subject of an anti-inflammatory assay [30]. Kombucha, which is a beverage of Manchurian origin, is the fermentation product of various bacteria (Lactobacillus sp., Acetobacter xylinoides, Gluconobacter oxydans, Komagataeibacter xylinum, Gluconacetobacter hansenii, Oenococcus oeni, Komagataeibacter europaeus) and yeast strains (Schizosaccharomyces pombe, Zygosaccharomyces kombuchaensis, Torulaspora delbrueckii, Saccharomyces sp., Brettanomyces sp., etc.). In kombucha fermentation, the black tea leaves turn into valuable metabolites such as vitamins (C, B1, B2, B12), glucuronic and acetic acids, and ethanol [59]. The fermented drink was analyzed for different biological activities. The anti-inflammatory capacity against the enzyme lipoxygenase (LOX) was measured and it was defined as the percentage of inhibition of the 5-LOX enzyme. The control was nordihydroguaiaretic acid. The authors reported an improvement in the anti-inflammatory activity after the fermentation with the kombucha consortium (87–91%), while the non-fermented tea obtained 66% or even 0% of inhibition. An IC50 value of 9.0 ± 0.0 µg/mL was also registered, which is close to the maximal inhibitory concentration of 7.0 ± 0.2 µg/mL for the natural LOX inhibitor nordihydroguaiaretic acid. An optimization of the operational parameters was conducted, as well. According to the obtained results, the higher surface/height ratio of the fermentation vessel accelerated the fermentation kinetics and the anti-inflammatory properties. The observation reached in the aforementioned research indicates that kombucha extracts could be effective candidates for the development of non-steroidal drugs for the treatment of inflammation [30]. The relationship between the anti-inflammatory activity and the culturing conditions was also demonstrated by another group of scientists [18]. Culturing conditions were found to affect the anti-inflammatory effect of Lactobacillus plantarum OLL 2712 (with confirmed anti-inflammatory properties) in murine model cells where the levels of IL-10 and IL-12 were measured with OLL 2712 cells prepared under various culture conditions. The IL-10-inducing activities of OLL 2712 cells differed significantly between the groups at different culture phases, different mediums, temperatures, and pH. The cells in their exponential phase of growth exhibited higher IL-10-inducing activity than those in their stationary phase [18].
Metabolites of LAB isolated from equid milk were reported to exhibit anti-inflammatory activity. Kostelac and co-workers isolated two strains of Lactobacillus species, namely L. plantarum M2 and L. plantarum K09, from donkey and mare milk, respectively. They determined the TNF-α suppression associated to their extracellular metabolites in lipopolysaccharide (LPS)-stimulated human peripheral blood mononuclear cells (PBMCs). The extracellular metabolites with molecular mass under 2000 Da were found to suppress TNF-α production up to 67% in LPS-stimulated PBMCs. The latter finding confirmed the anti-inflammatory properties of the two lactobacilli strains. Moreover, no cyto/genotoxic effects toward PBMCs were ascertained as a result of the extracellular metabolite activity. Hence, the production of TNF-α in immune cells can be initialized in the presence of lipopolysaccharides, which are a building block of the outer membrane of G-bacteria [19].
In 2021, a team of scientists reported that the membrane vesicles (MVs) from lactobacilli exert anti-inflammatory therapeutic effects [60]. MVs are extracellular spherical particles shed from the cell surface. They are released in the extracellular space and carry parental cell cargo such as enzymes or nuclear acids responsible for the cell’s communication [61]. Additionally, the authors optimized the culture conditions with L. casei DSMZ 20011 and L. plantarum NCIMB 8826 to obtain an even more emphasized anti-inflammatory effect. MVs from L. casei cultured at a pH of 6.5 with agitation resulted in the strongest interleukin-10 release and tumor necrosis factor-α reduction. Concerning the MV from L. plantarum, pH = 5 had the most visible effect on the anti-inflammatory activity [60].
Macrophage migration inhibitory factor (MIF) is a homotrimeric protein, a key cytokine responsible for inflammatory progression. This cytokine binds to the CD74 receptor promoting the immune response cascade through the activation of macrophages and T-cells. In this way, it triggers the production of multiple cytokines like TNF, IFN-γ, IL-1β, IL-2, IL-6, IL-8, NO, COX-2, and PGE2 [62][63]. Nyotohadi and Kok aimed to evaluate the anti-inflammatory capacity of a multi-strain extract of LAB, specifically, MIF. The multi-strain extract consisted of Lactobacillus casei EMRO 002, L. casei EMRO 213, L. plantarum EMRO 009, L. fermentum EMRO 211, L. rhamnosus EMRO 014, L. bulgaricus EMRO 212, and Rhodopseudomonas palustris EMRO 201. The team intended to study the potential inhibitory effect of the bacterial consortium on the MIF tautomerase activity, the reversibility, and the mechanism of inhibition. MIF tautomerase activity was inhibited with an IC50 value of 7.80 ± 1.96 mg/L. It was confirmed that the reaction is reversible [33]. MIF was also reported to inhibit glucocorticoid production which is a basic factor for the anti-inflammatory effects [64]. Therefore, MIF activity inhibition may relieve the inflammation.
Ayyanna et al. tested two probiotic strains (Lactobacillus mucosae AN1 and Lactobacillus fermentum SNR1) for anti-inflammatory capacity. Two types of experiments were conducted—with encapsulated and unencapsulated strains in rat paw tissues. The strains were in small capsules which released the contents after a prolonged period. They were administered orally. The first group revealed 85 ± 13% of inhibition while the latter revealed 77 ± 25%. The strains exhibited anti-inflammatory cytokine upregulation and pro-inflammatory cytokine downregulation [9].
Five strains of animal origin, including Lactobacillus reuteri MG9012(YH9012), Lactobacillus fermentum MG9014(YH9014), Pediococcus pentosaceus MG9015(YH9015), Enterococcus faecium MG9003(YH9003), and Enterococcus faecium MG9007(YH9007), were evaluated for potential utilization as probiotics and for their anti-inflammatory effects. The investigation demonstrated that the strains exerted inhibition of NO and inhibited the expression of inducible nitric oxide synthase and cyclooxygenase. These results indicated that the selected strains are appropriate probiotic candidates for animal hosts. However, their further utilization must be confirmed for safety and effectiveness in in vivo investigations [15].
In a study with milk for infant formula, it was confirmed that the anti-inflammatory properties are not related to the inactivated bacteria but to their fermentation products. In vitro and ex vivo experiments with L. paracasei CBA L74 were carried out and it was determined that the fermentation products inhibited the pro-inflammatory cytokine secretion leaving anti-inflammatory cytokines either unaffected or even enhanced in response to Salmonella typhimurium [65].
The anti-inflammatory properties of whey fermented by Enterococcus faecalis M157 against oral cavity pathogens were estimated [66]. Bacterial pathogens cause chronic inflammation leading to periodontitis in the oral cavity, Porphyromonas gingivalis being the main causative. Lipopolysaccharides from its cell wall represent the basic virulence factor for chronic periodontitis as they were demonstrated as inducing the production of the inflammatory mediators IL-1β, IL-6, and NO [67]. The M157 strain successfully inhibited IL-1β, IL-6, and induced NO of Porphyromonas gingivalis in RAW 264.7 cells. IL-6 and IL-8 in human periodontal ligament cells were also inhibited [66].
2. Fungi with Anti-Inflammatory Potential
As an origin of valuable substances and biological activities, fungi are a promising bioresource employed both in the production of functional foods as well as in the pharmaceutical industry [68]. Talaromyces wortmanii is an endophytic fungi that was tested for anti-inflammatory activity against the causative of acne vulgaris—Propionibacterium acnes. Acne vulgaris is the most common skin complaint leading to serious psychosocial problems such as anxiety and depression. The anaerobic microbe P. acnes plays a role in the inflammatory phase of this condition through the activation of the pro-inflammatory mediators IL-8 via the NF-kB and mitogen-activated phosphokinase (MAPK) pathways. As is known, T. wortmanii is a producer of bioactive compounds; thus, its crude extract was studied to have the capacity to inhibit the TNF-α-induced ICAM-1 expression and P. acnes-induced IL-8 release. Compound C from T. wortmanii inhibited the P. acnes-mediated activation of NF-kB and AP-1 by means of inhibition of 1 kB degradation and the phosphorylation of ERK and JNK MAP kinases and IL-secretion in a dose-dependent manner. Two compounds were found to be anti-inflammatory substances—BB and C—with an inhibitory activity of 46 and 55%, respectively. However, the mechanism of inhibition remains unclear [69].
A compound named Physcion (C1) was recently isolated from another endophytic fungus, Aspergillus versicolor SB5 [70]. This substance exhibited inhibitory activity against COX-2 and LOX-1 with IC50 values of 43.10 and 17.54 µg/mL, respectively, making it a potential anti-inflammatory agent [70]. New polyketides from Aspergillus rugulosa demonstrated anti-inflammatory activity. Asperulosin A and aspertetronin A significantly inhibited NO production with IC50 values of 1.49 ± 0.31 and 3.41 ± 0.85 μM, respectively. In addition, the secretion of anti-inflammatory cytokine IL-10 was visibly enhanced, while the secretion of the pro-inflammatory cytokines IL6, TNF-α, IFN-γ, MCP-1, and IL12 was suppressed. The positive control in the assays was dexamethasone, a glucocorticoid drug used for inflammatory relief. Asperulosin A and aspertetronin A were found to possess better or comparable anti-inflammatory effects in comparison to dexamethasone [71].
Several substances with anti-inflammatory properties were isolated from the endophytic fungus Colletotrichum gloeosprioides JS0419. Colletogloeopyrone A, monocillin II, and monocillin II glycoside effectively reduced NO production without cytotoxicity and inhibited the secretion of IL-6 and TNF-α. Monocillin II, and monocillin II glycoside inhibited the protein expression of the NF-κB pathway, inducible NO synthase, and COX-2 while colletogloeopyrone A only inhibited COX-2 expression [72].
Phellinbaumins A and D from the fungus Phellinus baumii were found to display moderate inhibitory activity on NO production in a murine model, with IC50 values of 31.7 and 24.3 μM, respectively [73]. Two new ergosterol derivatives (chlamydosterols A and B) and three known to have anti-inflammatory activity were isolated from another endophytic fungus—Fusarium chlamydosporum. Chlamydosterol A showed a moderate 5-LOX inhibitory capacity with IC50 values of 3.06 and 3.57 µM, respectively (compared to indomethacin with an IC50 1.13 µM) [74].
β-glucans in fungi are believed to be one of the sources of its anti-inflammatory properties. β-glucans are polysaccharides, glucose polymers differing based on their length and branching structure. Several studies have demonstrated their beneficial effects on IBDs [75].
References
- Gholami-Shabani, M.; Shams-Ghahfarokhi, M.; Razzaghi-Abyaneh, M. Food Microbiology: Application of microorganisms in food industry. IntechOpen 2023, 1–27.
- FAO/WHO. Guidelines for the Evaluation of Probiotics in Food; Food and Agriculture Organization of the United Nations/World Health Organization: London, ON, Canada, 2002.
- Choi, E.-J.; Lee, H.; Kim, W.; Han, K.; Iwasa, M.; Kobayashi, K.; Debnath, T.; Tang, Y.; Kwak, Y.; Yoon, J.; et al. Enterococcus faecalis EF-2001 protects DNBS-induced inflammatory bowel disease in mice model. PLoS ONE 2019, 14, e0210854.
- Roudsari, M.R.; Karimi, R.; Sohrabvandi, S.; Mortazavian, A.M. Health effects of probiotics on the skin. Crit. Rev. Food Sci. Nutr. 2015, 55, 1219–1240.
- Knackstedt, R.; Knackstedt, T.; Gatherwright, J. The role of topical probiotics on skin conditions: A systematic review of animal and human studies and implications for future therapies. Exp. Dermatol. 2020, 29, 15–21.
- Baquerizo Nole, K.L.; Yim, E.; Keri, J. Probiotics and prebiotics in dermatology. J. Am. Acad. Dermatol. 2014, 71, 814–821.
- Al-Ghazzewi, F.H.; Tester, R.F. Impact of prebiotics and probiotics on skin health. Benef. Microbes 2014, 5, 99–107.
- Lukic, J.; Chen, V.; Strahinik, I.; Begovic, J.; Lev-Tov, H.; Davis, S.; Tomic-Canic, M. Pastar, Probiotics or pro-healers: The role of beneficial bacteria in tissue repair. Wound Repair Regen. 2017, 25, 912–922.
- Ayyanna, R.; Ankaiah, D.; Arul, V. Anti-inflammatory and antioxidant properties of probiotic bacterium Lactobacillus mucosae AN1 and Lactobacillus fermentum SNR1 in Wistar albino rats. Front. Microbiol. 2018, 9, 3063.
- Catherine, A.; Kurrey, N.; Halami, P. In vitro adhesion and anti-inflammatory properties of native Lactobacillus fermentum and Lactobacillus delbrueckii spp. J. Appl. Microbiol. 2018, 125, 243–256.
- Monteros, M.; Galdeano, C.; Ballcels, M.; Weill, R.; De Paula, J.; Perdigón, G.; Cazorla, S. Probiotic lactobacilli as a promising strategy to ameliorate disorders associated with intestinal inflammation induced by a non-steroidal anti-inflammatory drug. Sci. Rep. 2021, 11, 571.
- Santiago-López, L.; Mendoza, A.; Vallejo-Cordoba, B.; Mata-Haro, V.; Wall-Medrano, A.; González-Córdova, A. milk fermented with Lactobacillus fermentum ameliorates indomethacin-induced intestinal inflammation: An exploratory study. Nutrients 2019, 11, 1610.
- Khmaladze, I.; Butler, E.; Fabre, S.; Gillbro, J. Lactobacillus reuteri DSM 17938—A comparative study on the effect of probiotics and lysates on human skin. Exp. Dermatol. 2019, 28, 822–828.
- Thomas, C.; Saulnier, D.; Spinler, J.; Hemarajata, P.; Gao, C.; Jones, S.; Grimm, A.; Balderas, M.; Burstein, M.; Morra, C.; et al. FolC2-mediated folate metabolism contributesto suppression of inflammation by probiotic Lactobacillus reuteri. MicrobiologyOpen 2016, 5, 802–818.
- Kim, K.-T.; Kim, J.-W.; Kim, S.-I.; Kim, S.; Nguyen, T.; Kang, C.-H. Antioxidant and anti-inflammatory effect and probiotic properties of lactic acid bacteria isolated from canine and feline feces. Microorganisms 2021, 9, 1971.
- Kim, Y.-L.; Nguyen, T.; Kim, J.-S.; Park, J.-Y.; Kang, C.-H. Isolation of gamma-aminobutyric acid (GABA)-producing lactic acid bacteria with anti-inflammatory effects from fermented foods in Korea. Fermentation 2023, 9, 612.
- Levit, R.; Savoy de Giori, G.; de Moreno de LeBlanc, A.; LeBlanc, J. Effect of riboflavin-producing bacteria against chemically induced colitis in mice. J. Appl. Microbiol. 2017, 124, 232–240.
- Toshimitsu, T.; Ozaki, S.; Mochizuki, J.; Furuichi, K.; Asami, Y. Effects of Lactobacillus plantarum strain OLL2712 culture conditions on the anti-inflammatory activities for murine immune cells and obese and type 2 diabetic mice. Appl. Env. Microbiol. 2017, 83, e03001-16.
- Kostelac, D.; Geric, M.; Gajski, G.; Markov, K.; Domijan, A.; Jakopovic, I.Z.; Svetec, I.; Frece, J. Lactic acid bacteria isolated from equid milk and their extracellular metabolites show great probiotic properties and anti-inflammatory potential. Int. Dairy J. 2021, 112, 104828.
- Dinić, M.; Pecikoza, U.; Djokić, J.; Stepanović-Petrović, R.; Milenković, M.; Stevanović, M.; Filipović, N.; Begović, J.; Golić, N.; Lukić, J. Exopolysaccharide produced by probiotic strain Lactobacillus paraplantarum BGCG11 reduces inflammatory hyperalgesia in rats. Front. Pharmacol. 2018, 9, 1.
- Zaghian, S.; Shokri, D.; Emtiazi, G. Co-production of a UV-stable bacteriocin-like inhibitory substance (BLIS) and indole-3-acetic acid hormone (IAA) and their optimization by Taguchi design in Bacillus pumilus. Ann. Microbiol. 2012, 62, 1189–1197.
- Zhou, B.; Zhang, D. Antibacterial effects of bacteriocins isolated from Lactobacillus rhamnosus (ATCC 53103) in a rabbit model of knee implant infection. Exp. Ther. Med. 2018, 15, 2985–2989.
- Rodríguez-Viso, P.; Domene, A.; V´elez, D.; Devesa, V.; Zúniga, M.; Monedero, V. Lactic acid bacteria strains reduce in vitro mercury toxicity on the intestinal mucosa. Food Chem. Toxicol. 2023, 173, 113631.
- Brandi, J.; Cheri, S.; Manfredi, M.; Di Carlo, C.; Vita Vanella, V.; Federici, F.; Bombiero, E.; Bazaj, A.; Rizzi, E.; Manna, L.; et al. Exploring the wound healing, anti inflammatory, anti pathogenic and proteomic effects of lactic acid bacteria on keratinocytes. Sci. Rep. 2020, 10, 11572.
- Levit, R.; Savoy de Giori, G.; de Moreno de LeBlanc, A.; LeBlanc, J. Beneficial effect of a mixture of vitamin-producing and immunemodulating lactic acid bacteria as adjuvant for therapy in a recurrent mouse colitis model. Appl. Microbiol. Biotechnol. 2019, 103, 8937–8945.
- Wang, C.; Li, W.; Wang, H.; Ma, Y.; Zhao, X.; Yang, H.; Qian, J.; Li, J. VSL#3 can prevent ulcerative colitis-associated carcinogenesis in mice. World J. Gastroenterol. 2018, 24, 4254–4262.
- Jenab, A.; Roghanian, R.; Emtiazi, G. Encapsulation of platelet in kefiran polymer and detection of bioavailability of immobilized platelet in probiotic kefiran as a new drug for surface bleeding. J. Med. Microbiol. 2015, 4, 45–55.
- Kwon, O.; Ahn, K.; Lee, M.; Kim, S.; Park, B.; Kim, M.; Lee, I.; Oh, S.; Lee, H. Inhibitory effect of kefiran on ovalbumin-induced lung inflammation in a murine model of asthma. Arch. Pharm. Res. 2008, 31, 1590–1596.
- Diniz, R.; Garla, L.; Schneedorf, J.; Carvalho, J. Study of anti-inflammatory activity of Tibetan mushroom, a symbiotic culture of bacteria and fungi encapsulated into a polysaccharide matrix. Pharmacol. Res. 2003, 47, 49–52.
- Villarreal-Soto, S.; Beaufort, S.; Bouajila, J.; Souchard, J.-P.; Renard, T.; Rollan, S.; Taillandier, P. Impact of fermentation conditions on the production of bioactive compounds with anticancer, anti-inflammatory and antioxidant properties in kombucha tea extracts. Process Biochem. 2019, 83, 44–54.
- Kook, S.; Lee, Y.; Jeong, E.; Kim, S. Immunomodulatory effects of exopolysaccharides produced by Bacillus licheniformis and Leuconostoc mesenteroides isolated from Korean kimchi. J. Func. Foods 2019, 54, 211–219.
- Hernández-Delgado, N.; Torres-Maravilla, E.; Mayorga-Reyes, L.; Martín, R.; Langella, P.; Pérez-Pastén-Borja, R.; Sánchez-Pardo, M.; Bermúdez-Humarán, L. Antioxidant and anti-inflammatory properties of probiotic candidate strains isolated during fermentation of agave (Agave angustifolia Haw). Microorganisms 2021, 9, 1063.
- Nyotohadi, D.; Kok, T. Potential of multi-strain probiotics extract as an antiinflammatory agent through inhibition of macrophage migration inhibitory factor activity. Funct. Foods Health Dis. 2023, 13, 1–10.
- Al-Harbi, N.; Imam, F.; Nadeem, A.; Al-Harbi, M.; Iqbal, M.; Ahmad, S. Carbon tetrachloride-induced hepatotoxicity in rat is reversed by treatment with riboflavin. Int. Immunopharmacol. 2014, 21, 383–388.
- Mazur-Bialy, A.; Pochec, E.; Plytycz, B. Immunomodulatory effect of riboflavin deficiency and enrichment-reversible pathological response versus silencing of inflammatory activation. J. Physiol. Pharmacol. 2015, 66, 793–802.
- Alam, M.; Iqbal, S.; Naseem, I. Ameliorative effect of riboflavin on hyperglycemia, oxidative stress and DNA damage in type-2 diabetic mice: Mechanistic and therapeutic strategies. Arc. Biochem. Bioph. 2015, 584, 10–19.
- Levit, R.; Savoy de Giori, G.; de Moreno de LeBlanc, A.; LeBlanc, J. Evaluation of the effect of soymilk fermented by a riboflavin-producing Lactobacillus plantarum strain in a murine model of colitis. Ben. Microbes 2017, 8, 65–72.
- Baszczuk, A.; Thielemann, A.; Musialik, K.; Kopczynski, J.; Bielawska, L.; Dzumak, A.; Kopczynski, Z.; Wysocka, E. The impact of supplementation with folic acid on homocysteine concentration and selected lipoprotein parameters in patients with primary hypertension. J. Nutr. Sci. Vitaminol. 2017, 63, 96–103.
- Pan, Y.; Liu, Y.; Guo, H.; Jabir, M.; Liu, X.; Cui, W.; Li, D. Associations between folate and vitamin B12 levels and inflammatory bowel disease: A meta-analysis. Nutrients 2017, 9, 4.
- Burr, N.; Hull, M.; Subramanian, V. Folic acid supplementation may reduce colorectal cancer risk in patients with inflammatory bowel disease: A systematic review and meta-analysis. J. Clin. Gastroenterol. 2017, 51, 247–253.
- LeBlanc, J.G.; Levit, R.; de Giori, G.; de Moreno de LeBlanc, A. Application of vitamin-producing lactic acid bacteria to treat intestinal inflammatory diseases. Appl. Microbiol. Biotechnol. 2020, 104, 3331–3337.
- Albuquerque, M.; Bedani, R.; LeBlanc, J.; Saad, S. Passion fruit by-product and fructooligosaccharides stimulate the growth and folate production by starter and probiotic cultures in fermented soymilk. Int. J. Food Microbiol. 2017, 26, 35–41.
- Carrizo, S.; de Moreno de LeBlanc, A.; LeBlanc, J.; Rollan, G. Quinoa pasta fermented with lactic acid bacteria prevents nutritional deficiencies in mice. Food Res. Int. 2020, 127, 108735.
- Juarez del Valle, M.; Laiño, J.; Savoy de Giori, G.; LeBlanc, J. Factors stimulating riboflavin produced by Lactobacillus plantarum CRL 725 grown in a semi-definedmedium. J. Basic Microbiol. 2017, 57, 245–252.
- Jenab, A.; Roghanian, R.; Emtiazi, G. Bacterial natural compounds with anti-inflammatory and immunomodulatory properties (mini review). Drug Des. Dev. Ther 2020, 14, 3787–3801.
- Cardoso, L.; Schneedorf, J.; Fiorini, J.; de Oliveira, B.; Carvalho, J. Efeito da administração de cogumelo tibetano, um consórcio microbiano, sobre a peristalse intestinal em ratos. Braz. J. Pharmacog. 2005, 15, 212–214.
- Hsieh, S.; Allen, P. Immunomodulatory roles of polysaccharide capsules in the intestine. Front. Immunol. 2020, 11, 690.
- Giri, S.; Sen, S.; Jun, J.; Sukumaran, V.; Park, S. Role of Bacillus licheniformis VS16-derived biosurfactant in mediating immune responses in Carp Rohu and its application to the food industry. Front. Microbiol. 2017, 8, 514.
- Karanth, N.; Deo, P.; Veenanadig, N. Microbial production of biosurfactants and their importance. Curr. Sci. 1999, 10, 116–126.
- Fanaei, M.; Emtiazi, G. Microbial assisted (Bacillus mojavensis) production of bio-surfactant lipopeptide with potential pharmaceutical applications and its characterization by MALDI-TOF-MSanalysis. J. Mol. Liq. 2018, 268, 707–714.
- Zhang, Y.; Liu, C.; Dong, B.; Ma, X.; Hou, L.; Cao, X.; Wang, C. Anti-inflammatory activity and mechanism of surfactin in lipopolysaccharide-activated macrophages. Inflammation 2015, 38, 756–764.
- Zhao, H.; Shao, D.; Jiang, C.; Shi, J.; Li, Q.; Huang, Q.; Rajoka, M.; Yang, H.; Jin, M. Biological activity of lipopeptides from Bacillus. Appl. Microbiol. Biotechnol. 2017, 101, 5951–5960.
- Voloshyna, I.; Soloshenko, K.; Krasinko, V.; Lych, I.; Shkotova, L. Bacteriocins Lactobacillus—An alternative to antimicrobial drugs. Biopolym. Cell Vol. 2021, 37, 85–97.
- Sivaraj, A.; Sundar, R.; Manikkam, R.; Parthasarathy, K.; Rani, U.; Kumar, V. Potential applications of lactic acid bacteria and bacteriocins in anti-mycobacterial therapy. Asian Pac. J. Trop. Med. 2018, 11, 453–459.
- Crittenden, D.; Chebib, M.; Jordan, M. Stabilization of zwitterions in solution: GABA Analogues. J. Phys. Chem. A 2005, 109, 4195–4201.
- Strain, G. Nutrition, brain function and behavior, Psychiatr. Clin. N. Am. 1981, 4, 253–268.
- Mitsushima, D.; Shwe, T.; Funabashi, T.; Shinohara, K.; Kimura, F. GABA release in the medial preoptic area of cyclic female rats. Neuroscience 2002, 113, 109–114.
- Soltani, N.; Qiu, H.; Aleksic, M.; Glinka, Y.; Zhao, F.; Liu, R.; Li, Y.; Zhang, N.; Chakrabarti, R.; Ng, T.; et al. GABA exerts protective and regenerative effects on islet beta cells and reverses diabetes. Proc. Natl. Acad. Sci. USA 2011, 108, 11692–11697.
- Coton, M.; Pawtowski, A.; Taminiau, B.; Burgaud, G.; Deniel, F.; Coulloumme-Labarthe, L.; Fall, A.; Daube, G.; Coton, E. Unraveling microbial ecology of industrialscale Kombucha fermentations by metabarcoding and culture-based methods. FEMS Microbiol. Ecol. 2017, 93, 1–16.
- Muller, L.; Kuhn, T.; Koch, M.; Fuhrmann, G. Stimulation of probiotic bacteria induces release of membrane vesicles with augmented anti-inflammatory activity. ACS Appl. Bio Mater. 2021, 4, 3739–3748.
- Hong, J.; Dauros-Singorenko, P.; Whitcombe, A.; Payne, L.; Blenkiron, C.; Phillips, A.; Swift, S. Analysis of the Escherichia coli extracellular vesicle proteome identifies markers of purity and culture conditions. J. Extracell. Vesicles 2019, 8, 1.
- Calandra, T.; Roger, T. Macrophage migration inhibitory factor: A regulator of innate immunity. Nat. Rev. Immunol. 2003, 3, 791–800.
- Aksakal, A.; Kerget, B.; Kerget, F.; Aşkın, S. Evaluation of the relationship between macrophage migration inhibitory factor level and clinical course in patients with COVID-19 pneumonia. J. Med. Virol. 2021, 93, 6519–6524.
- Aeberli, D.; Leech, M.; Morand, E. Macrophage migration inhibitory factor and glucocorticoid sensitivity. Rheumatology 2006, 45, 937–943.
- Zagato, E.; Mileti, E.; Massimiliano, L.; Fasano, F.; Budelli, A.; Penna, G.; Rescigno, M. Lactobacillus paracasei CBA L74 metabolic products and fermented milk for infant formula have antiinflammatory activity on dendritic cells in vitro and protective effects against colitis and an enteric pathogen in vivo. PLoS ONE 2014, 9, e87615.
- Song, D.; Lee, H.; Kim, G.-B.; Kang, S.-S. Whey fermented by Enterococcus faecalis M157 exhibits antiinflammatory and antibiofilm activities against oral pathogenic bacteria. J. Dairy Sci. 2022, 105, 1900–1912.
- Zhang, J.; Yu, C.; Zhang, X.; Chen, H.; Dong, J.; Lu, W.; Song, Z.; Zhou, W. Porphyromonas gingivalis lipopolysaccharide induces cognitive dysfunction, mediated by neuronal inflammation via activation of the TLR4 signaling pathway in C57BL/6 mice. J. Neuroinflam. 2018, 15, 37.
- Du, B.; Lin, C.; Bian, Z.; Xu, B. An insight into anti-inflammatory effects of fungal beta-glucans. Trends Food Sci. Technol. 2015, 41, 49–59.
- Pretsch, A.; Nagl, M.; Schwendinger, K.; Kreiseder, B.; Wiederstein, M.; Pretsch, D.; Genov, M.; Hollaus, R.; Zinssmeister, D.; Debbab, A.; et al. Antimicrobial and anti-inflammatory activities of endophytic fungi Talaromyces wortmannii extracts against acne-inducing bacteria. PLoS ONE 2014, 9, 6.
- Elawady, M.; Hamed, A.; Alsallami, W.; Gabr, E.; Abdel-Monem, M.; Hassan, M. Bioactive metabolite from endophytic Aspergillus versicolor sb5 with anti-acetylcholinesterase, anti-inflammatory and antioxidant activities: In vitro and in silico studies. Microorganisms 2023, 11, 1062.
- Xu, Q.; Qiao, Y.; Zhang, Z.; Deng, Y.; Chen, T.; Tao, L.; Xu, Q.; Liu, J.; Sun, W.; Ye, Y.; et al. New polyketides with anti-inflammatory activity from the fungus Aspergillus rugulosa. Front. Pharmacol. 2021, 12, 700573.
- Kim, J.; Baek, J.Y.; Bang, S.; Kim, J.Y.; Jin, Y.; Lee, J.W.; Jang, D.S.; Kang, K.S.; Shim, S.H. New anti-inflammatory β-resorcylic acid lactones derived from an endophytic fungus, Colletotrichum sp. ACS Omega 2023, 8, 3530–3538.
- Zhao, Y.-Q.; Dong, X.-Y.; Ning, J.-L.; Jin, Y.-X.; Wang, J.-K.; Feng, T. Chemical constituents from the fungus Phellinus baumii and their anti-inflammatory activity. Phytochem. Lett. 2023, 54, 18–22.
- Al-Rabia, M.; Mohamed, G.; Ibrahim, S.; Asfour, H. Anti-inflammatory ergosterol derivatives from the endophytic fungus Fusarium chlamydosporum. Nat. Prod. Res. 2020, 35, 5011–5020.
- Ye, M.B.; Bak, J.P.; An, C.S.; Jin, H.L.; Kim, J.M.; Kweon, H.J.; Choi, D.K.; Park, P.J.; Kim, Y.J.; Lim, B.O. Dietary β-glucan regulates the levels of inflammatory factors, inflammatory cytokines, and immunoglobulins in interleukin-10 knockout mice. J. Med. Food 2011, 14, 468–474.
More
Information
Subjects:
Biotechnology & Applied Microbiology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
514
Revisions:
2 times
(View History)
Update Date:
21 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




