You're using an outdated browser. Please upgrade to a modern browser for the best experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Shelley Edwards | -- | 1491 | 2024-03-18 09:01:14 | | | |
| 2 | Mona Zou | Meta information modification | 1491 | 2024-03-19 10:16:20 | | | | |
| 3 | Mona Zou | -4 word(s) | 1487 | 2024-03-27 09:38:05 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Nicolau, G.K.; Edwards, S. Southern African Gekkonids Diversity. Encyclopedia. Available online: https://encyclopedia.pub/entry/56371 (accessed on 30 December 2025).
Nicolau GK, Edwards S. Southern African Gekkonids Diversity. Encyclopedia. Available at: https://encyclopedia.pub/entry/56371. Accessed December 30, 2025.
Nicolau, Gary K., Shelley Edwards. "Southern African Gekkonids Diversity" Encyclopedia, https://encyclopedia.pub/entry/56371 (accessed December 30, 2025).
Nicolau, G.K., & Edwards, S. (2024, March 18). Southern African Gekkonids Diversity. In Encyclopedia. https://encyclopedia.pub/entry/56371
Nicolau, Gary K. and Shelley Edwards. "Southern African Gekkonids Diversity." Encyclopedia. Web. 18 March, 2024.
Copy Citation
South Africa is recognised for its high reptile diversity and endemism, specifically among lizards. Phylogenetic diversity, endemism, and richness can have clear implications or raise important questions in a range of fields, and most urgently in conservation. Among squamate reptiles, these indices are very commonly associated with high temperatures and topographic heterogeneity. Indeed, mountainous biogeography has been a critical driver in the radiation of the family Gekkonidae within the subregion.
CANAPE
escarpment
phylogenetic diversity
phylogenetic endemism
1. Introduction
Historical climatic and geological events and phylogenetic biogeography have played essential roles in driving the large-scale distribution or isolation of organisms [1][2]. Highlighting specific regions harbouring exceptional phylogenetic diversity, endemism, and richness can have clear and fundamental conservation implications [3] or raise important questions for investigating major evolutionary and biogeographic events [4] and ecological drivers [5][6]. Squamate reptiles are a diverse group, with approximately 9850 species distributed throughout the globe [7]. Correlations of species richness and diversity among reptiles are commonly associated with high temperatures and topographic heterogeneity [8][9][10]. Squamate reptiles make excellent models for investigating the evolutionary and biogeographical drivers of species richness and diversification due to their significant range in habitat utilisation, habitat specialisation, and limited dispersal abilities [11][12].
South Africa is a megadiverse country with three global biodiversity hotspots: the Cape Floristic Region, the Maputaland-Pondoland-Albany Hotspot, and the Succulent Karoo. Regarding reptile distributions, compared to other African countries, South Africa has been relatively comprehensively sampled [13]. This area, including Lesotho and Eswatini, is also recognised for its high reptile diversity and endemism [14]. Mountainous biogeography has been a critical driver in the radiation of many reptiles within the subregion [15][16][17][18]. The complex topographic landscape has primarily been driven through two unrelated major geological events, namely the upliftment of the Great Escarpment and the Fold Mountains [19][20].
2. Gekkonid Diversity
The Southern African gekkonids are one of the most diverse and highly endemic groups of reptiles within the region, consisting of 86 recognised species from 12 genera (Figure 1). Of these, ~75% species and 5 genera are thought to be endemic or near-endemic to the region [14][15].
Afroedura Loveridge 1944 is a species-rich genus distributed throughout Southern Africa, extending northwards into Angola. Currently, there are 34 species [21], with several awaiting description. The genus primarily comprises rock-dwelling, montane species, except for a few arboreal species (e.g., Afroedura loveridgei and Afroedura marleyi) [14][21][22]. Three major clades are present within Afroedura, predominantly along the isolates of the Great Escarpment, with some members occupying coastal plains or the Cape Fold Mountains [15].
A monotypic genus, Afrogecko Bauer, Good & Branch, 1997, has a unique taxonomic past. Two subspecies, Phyllodactylus porphyreus cronwrighti and Phyllodactylus porphyreus namaquensis, neither of which is currently recognised [14], require further investigation due to strong genetic differences, thus making Afrogecko porphyreus a species complex [23]. There remains a strong likelihood of cryptic taxa within the Afrogecko porphyreus complex [23]. No new material on the P. p. namaquensis has been collected to confirm its status. Afrogecko porphyreus is restricted to southwestern South Africa. It is predominantly rupicolous; however, some populations occupy vegetation within the coastal plains [22].
The genus Chondrodactylus W. Peters, 1870 consists of large geckos, most of which are rupicolous, with some also displaying arboreal behaviour. Four species are present within South Africa [24], predominantly distributed inland of the Great Escarpment. A single member of the genus, Chondrodactylus angulifer, is a terrestrial burrower and has evolved accordingly [25][26].
The monotypic genus of leaf-toed geckos, Cryptactites Bauer et al., 1997 is a low-altitude coastal endemic. Its only species, Cryptactites peringueyi, is a small terrestrial and semi-arboreal gecko utilising coastal vegetation in a small range of the Eastern Cape province [27]. Its restricted range and poor phylogenetic diversity make this lineage the country’s most range-restricted gecko genus.
Another group of small leaf-toed geckos are from the genus Goggia Bauer, Good & Branch, 1997. This near-endemic group consists of 10 species restricted to southern and northwestern South Africa. The genus consists of rupicolous and often mountainous genera, except for two species, G. lineata and G. incognita, which are found in shrub or fynbos in open vegetation types [22][28].
The most species-rich genus, Hemidactylus Oken, 1817, is widely distributed throughout the globe. Despite high diversification of Hemidactylus across the Afrotropic and subtropical regions, only a single species, Hemidactylus mabouia, is found within the borders of South Africa. The species is a generalist and occupies mountainous, inland, and coastal habitats [14][22]. They are successful invaders throughout the country and on a global level [12][14]. It is predominantly rupicolous; however, as with most rupicolous species within the region, it displays arboreal behaviour, and is, additionally, well-adapted to urban environments [22].
The genus Homopholis Boulenger, 1885 consists of four large-bodied, soft-skinned species. They are widely distributed, except for Homopholis mulleri, which is restricted to the northern extent of the Soutpansberg Mountains in Limpopo, South Africa.
Within the region, Lygodactylus Gray, 1864 consists of 11 species [22]. Many species have limited ranges, with several restricted to a single massif or mountaintop [14][22]. Radiation among Lygodactylus took place in two major clades, an Afromontane (greater Drakensberg) clade and a savanna-dwelling clade (except for a single montane species, L. bernardi, from Zimbabwe) [16]. Lygodactylus capensis, a widespread species from the savanna clade, is one of the most successful invaders within the country. The species has successful colonies throughout many western cities and towns far outside its natural range [29].
The most species-rich gekkonid genus within the region is that of Pachydactylus Wiegmann, 1834, which consists of 29 species. Radiation and endemism within the group were likely driven due to substrate specialisation in many species and historical vicariant events [30]. This group displays major differences in size [31], morphology [25][32], and geographical and environmental niches among species [28].
A single species of Ptenopus Gray, 1866 occurs within South Africa, with the remaining two species restricted to Namibia. The genus is known to predominantly occupy savanna, scrubland, and desert habitats [14][22]. They are commonly known to utilise characteristic burrows, often within loose soils, from where males call [33]. Intraspecific diversity among South Africa’s only species, Ptenopus garrulus, is likely, with two subspecies currently recognised.
The genus Ramigekko Heinicke et al., 2014 consists of a single species, Ramigekko swartbergensis, which is a sizable rupicolous gekkonid restricted to the high mountain tops of the Klein and Groot Swartberg Mountains, within the Cape Fold mountain range [14][34][35][36]. The genus, which forms part of the circum-Indian Ocean leaf-toed geckos, was elevated from the now monotypic genus Afrogecko by Heinicke et al. [23]. Its closest living relative is the coastal endemic and monotypic genus, Cryptactites. The entire geographic range of the genus occurs in a predominantly inaccessible protected area, thus facing no major anthropogenic threats.
Another monotypic gecko, Rhoptropella Hewitt, 1937, a close relative of the Day Geckos—Lygodactylus, is another rupicolous genus. The small Namaqua Day Gecko—Rhoptropella ocellata, is restricted to mountain ranges in northwestern South Africa and southern Namibia [14]. This species is the only naturally-occurring diurnal gekkonid within this far-western arid region [22].
It is evident that there is exceptional diversity and endemism of gekkonids within the Southern African countries. Furthermore, with the group comprising several monotypic genera and genera with few species, it is essential that conservation measures are put in place to conserve phylogenetic diversity.
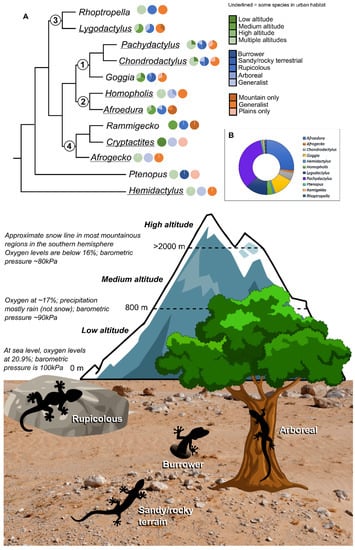
Figure 1. Representations of gekkonid genera within South Africa: (A) Simplified phylogenetic representation of the genera, constructed from the phylogeny produced in this study and from various published gekkonid phylogenies [15][37][38][39][40][41]. Numbers at the nodes indicate the Clade number. Pie charts at the tips indicate the number of species within the genus that inhabit various altitudes (green pie charts), the general habitat in which the species are found (biotopical preferences, blue pie charts), and the habitat specialisation of the species (orange pie charts). Genera that are underlined have species that enter into the urban environment. Information for the pie charts was obtained from the species accounts in the IUCN Red List (https://www.iucnredlist.org/; accessed on 17 December 2022). (B) Donut chart showing the number of species from each genus present in South Africa, Lesotho, and Eswatini. The illustration below details the biotopical preferences and altitudinal zone distinctions.
3. Conservation
A comprehensive assessment [42] estimating the extinction risk of reptiles found that ~21% are threatened with extinction. Conservation measures are often implicated in areas of high diversity and species richness [43][44], or specifically implemented for species of conservation concern (e.g., the establishment of the Mountain Zebra National Park (South Africa) in 1937, to protect the Mountain Zebra). However, phylogenetic diversity is often overlooked when assessing and planning conservation networks. Protected areas are critical for mitigating further biodiversity loss [45][46]. South Africa is a global leader in science-based conservation strategies [47][48][49][50]. The protected area network covers approximately 9% of South Africa’s mainland surface area [51], and it is essential for conserving the diverse fauna and flora, maintaining livelihoods, economic development, and preserving many ecological services. Despite the sizable protected area network and protected area expansion plan [52], it is insufficient in protecting South Africa’s threatened reptiles [53]. Fortunately, despite the high endemism and restricted distribution of many South African gekkonids [14], only a few taxa are listed under a threatened category in the IUCN Red List, these being Afroedura multiporis [54] and Homopholis mulleri [55], which are listed as Near Threatened, and a single Endangered species, Lygodactylus methueni [56].
References
- Hoorn, C.; Wesselingh, F.P.; ter Steege, H.; Bermudez, M.A.; Mora, A.; Sevink, J.; Sanmartín, I.; Sanchez-Meseguer, A.; Anderson, C.L.; Figueiredo, J.P.; et al. Amazonia through time: Andean uplift, climate change, landscape evolution and biodiversity. Science 2010, 330, 927–931.
- Smith, B.T.; McCormack, J.E.; Cuervo, A.M.; Hickerson, M.J.; Aleixo, A.; Cadena, C.D.; Pérez-Emán, J.; Burney, C.W.; Xie, X.; Harvey, M.G.; et al. The drivers of tropical speciation. Nature 2014, 515, 406–409.
- Hurlbert, A.H.; Jetz, W. Species richness, hotspots, and the scale dependence of range maps in ecology and conservation. Proc. Natl. Acad. Sci. USA 2007, 104, 13384–13389.
- Wiens, J.J.; Donoghue, M.J. Historical biogeography, ecology and species richness. Trends Ecol. Evol. 2004, 19, 639–644.
- Waide, R.B.; Willig, M.R.; Steiner, C.F.; Mittelbach, G.; Gough, L.; Dodson, S.I.; Juday, G.P.; Parmenter, R. The relationship between productivity and species richness. Annu. Rev. Ecol. Evol. Syst. 1999, 30, 257–300.
- Gotelli, N.J.; Colwell, R.K. Quantifying biodiversity: Procedures and pitfalls in the measurement and comparison of species richness. Ecol. Lett. 2001, 4, 379–391.
- Uetz, P.; Freed, P.; Aguilar, R.; Hošek, J. (Eds.) The Reptile Database. 2022. Available online: https://www.reptile-database.org (accessed on 5 December 2022).
- Lewin, A.; Feldman, A.; Bauer, A.M.; Belmaker, J.; Broadley, D.G.; Chirio, L.; Itescu, Y.; LeBreton, M.; Maza, E.; Meirte, D.; et al. Patterns of species richness, endemism and environmental gradients of African reptiles. J. Biogeogr. 2016, 43, 2380–2390.
- Kissling, W.D.; Blach-Overgaard, A.; Zwaan, R.E.; Wagner, P. Historical colonization and dispersal limitation supplement climate and topography in shaping species richness of African lizards (Reptilia: Agaminae). Sci. Rep. 2016, 6, 1–14.
- Kafash, A.; Ashrafi, S.; Yousefi, M.; Rastegar-Pouyani, E.; Rajabizadeh, M.; Ahmadzadeh, F.; Grünig, M.; Pellissier, L. Reptile species richness associated to ecological and historical variables in Iran. Sci. Rep. 2020, 10, 1–11.
- Doan, T.M. A south-to-north biogeographic hypothesis for Andean speciation: Evidence from the lizard genus Proctoporus (Reptilia, Gymnophthalmidae). J. Biogeog. 2003, 30, 361–374.
- Agarwal, I.; Bauer, A.M.; Jackman, T.R.; Karanth, K.P. Insights into Himalayan biogeography from geckos: A molecular phylogeny of Cyrtodactylus (Squamata: Gekkonidae). Mol. Phyl. Evol. 2014, 80, 145–155.
- Tolley, K.A.; Alexander, G.J.; Branch, W.R.; Bowles, P.; Maritz, B. Conservation status and threats for African reptiles. Biol. Conserv. 2016, 204, 63–71.
- Bates, M.F.; Branch, W.R.; Bauer, A.M.; Burger, M.; Marias, J.; Alexander, G.J.; De Villiers, M.S. Suricata 1: Atlas and Red List of the Reptiles of South Africa, Lesotho, and Swaziland; South African Biodiversity Institute: Pretoria, South Africa, 2014; Volume 46, pp. 331–397.
- Jacobsen, N.H.; Kuhn, A.L.; Jackman, T.R.; Bauer, A.M. A phylogenetic analysis of the southern African gecko genus Afroedura Loveridge (Squamata: Gekkonidae), with the description of nine new species from Limpopo and Mpumalanga provinces of South Africa. Zootaxa 2014, 3846, 451–501.
- Travers, S.L.; Jackman, T.R.; Bauer, A.M. A molecular phylogeny of Afromontane dwarf geckos (Lygodactylus) reveals a single radiation and increased species diversity in a South African montane center of endemism. Mol. Phyl. Evol. 2014, 80, 31–42.
- Tolley, K.A.; Bowie, R.C.K.; Measey, G.J.; Price, B.W.; Forest, F. The shifting landscape of genes since the Pliocene: Terrestrial phylogeography in the greater cape floristic region. In Fynbos: Ecology, Evolution and Conservation of a Megadiverse Region; Allsopp, N., Colville, J.F., Verboom, G.A., Eds.; Oxford University Press: Oxford, UK, 2014; pp. 143–163.
- Tolley, K.A.; Tilbury, C.R.; Burger, M. Convergence and vicariance: Speciation of chameleons in the Cape Fold Mountains, South Africa, and the description of three new species of Bradypodion Fitzinger, 1843. Afr. J. Herpetol. 2022, 71, 14–38.
- Partridge, T.C.; Maud, R.R. Geomorphic evolution of southern Africa since the Mesozoic. S. Afr. J. Geol. 1987, 90, 179–208.
- McCarthy, T.; Rubridge, B. The Story of Earth and Life; Struik Publishers: Cape Town, South Africa, 2005.
- Conradie, W.; Schmitz, A.; Lobón-Rovira, J.; Becker, F.S.; Pinto, P.V.; Hauptfleisch, M.L. Rock island melody remastered: Two new species in the Afroedura bogerti Loveridge, 1944 group from Angola and Namibia. Zoosyst. Evol. 2022, 98, 435–453.
- Branch, B. Field Guide to Snakes and Other Reptiles of Southern Africa; Struik Publishers: Cape Town, South Africa, 1998.
- Heinicke, M.P.; Daza, J.D.; Greenbaum, E.; Jackman, T.R.; Bauer, A.M. Phylogeny, taxonomy and biogeography of a circum-Indian Ocean clade of leaf-toed geckos (Reptilia: Gekkota), with a description of two new genera. Syst. Biodiv. 2014, 12, 23–42.
- Heinz, M.D.; Brennan, I.G.; Jackman, T.R.; Bauer, A.M. Phylogeny of the genus Chondrodactylus (Squamata: Gekkonidae) with the establishment of a stable taxonomy. Bull. Mus. Comp. 2021, 163, 151–210.
- Lamb, T.; Bauer, A.M. Footprints in the sand: Independent reduction of subdigital lamellae in the Namib-Kalahari burrowing geckos. Proc. Royal Soc. B 2006, 273, 855–864.
- Gamble, T.; Greenbaum, E.; Jackman, T.R.; Russell, A.P.; Bauer, A.M. Repeated origin and loss of adhesive toepads in geckos. PLoS ONE 2012, 7, e39429.
- Nicolau, G.K.; Petford, M.; Edwards, S.; Busschau, T.; Lynch, K.; Kemp, L.; Conradie, W. New insights into the geographical distribution, ecology and conservation status of South Africa’s endemic Coastal Leaf-toed Gecko, Cryptactites peringueyi (Boulenger, 1910). Herp. Notes 2021, 14, 439–450.
- Heinicke, M.P.; Jackman, T.R.; Bauer, A.M. The measure of success: Geographic isolation promotes diversification in Pachydactylus geckos. BMC Evol. Biol. 2017, 17, 1–17.
- Rebelo, A.D.; Bates, M.F.; Burger, M.; Branch, W.R.; Conradie, W. Range expansion of the Common Dwarf Gecko, Lygodactylus capensis: South Africa’s most successful reptile invader. Herp. Notes 2019, 12, 643–650.
- Bauer, A.M.; Lamb, T. Phylogenetic relationships of southern African geckos in the Pachydactylus group (Squamata: Gekkonidae). Afr. J. Herp. 2005, 54, 105–129.
- Branch, W.R.; Bauer, A.M.; Good, D.A. A review of the Namaqua gecko, Pachydactylus namaquensis (Reptilia: Gekkonidae) from southern Africa, with the description of two new species. Afr. Zool. 1996, 31, 53–69.
- Bauer, A.M.; Lamb, T.; Branch, W.R. A revision of the Pachydactylus serval and P. weberi groups (Reptilia: Gekkota: Gekkonidae) of Southern Africa, and with the description of eight new species. Proc. Calif. Acad. Sci. 2006, 57, 595–709.
- Hibbitts, T.J.; Whiting, M.J.; Stuart-Fox, D.M. Shouting the odds: Vocalization signals status in a lizard. Behav. Ecol. Sociobiol. 2007, 61, 1169–1176.
- Branch, W.R.; Bauer, A.M. Notes on two poorly-known Phyllodactylus (Squamata: Gekkonidae) from South Africa. Herpetol. Nat. Hist. 1996, 4, 127–134.
- Haacke, W.D. Description of a new species of Phyllodactylus Gray (Reptilia: Gekkonidae) from the Cape Fold Mountains, South Africa. Ann. Transvaal Mus. 1996, 36, 229–237.
- Bauer, A.M.; Good, D.A.; Branch, B. The taxonomy of the southern african leaf-toed geckos (Squamata: Gekkonidae), with a review of Old World “Phyllodactylus” and the description of five new genera. Proc. Calif. Acad. Sci. 1997, 49, 447–497.
- Pyron, R.A.; Burbrink, F.T.; Wiens, J.J. A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes. BMC Evol. Biol. 2013, 13, 1–54.
- Makhubo, B.G.; Tolley, K.A.; Bates, M.F. Molecular phylogeny of the Afroedura nivaria (Reptilia: Gekkonidae) species complex in South Africa provides insight on cryptic speciation. Mol. Phylogenet. Evol. 2015, 82, 31–42.
- Busschau, T.; Conradie, W.; Daniels, S.R. Evidence for cryptic diversification in a rupicolous forest-dwelling gecko (Gekkonidae: Afroedura pondolia) from a biodiversity hotspot. Mol. Phylogenet. Evol. 2019, 139, 106549.
- Branch, W.R.; Schmitz, A.; Lobón-Rovira, J.; Baptista, N.L.; António, T.; Conradie, W. Rock island melody: A revision of the Afroedura bogerti Loveridge, 1944 group, with descriptions of four new endemic species from Angola. Zoosyst. Evol. 2021, 97, 55.
- Lobon-Rovira, J.; Conradie, W.; Pinto, P.V.; Keates, C.; Edwards, S.; Plessis, A.D.; Branch, W.R. Systematic revision of Afrogecko ansorgii (Boulenger, 1907) (Sauria: Gekkonidae) from western Angola. Zootaxa 2022, 5124, 401–430.
- Cox, N.; Young, B.E.; Bowles, P.; Fernandez, M.; Marin, J.; Rapacciuolo, G.; Böhm, M.; Brooks, T.M.; Hedges, S.B.; Hilton-Taylor, C.; et al. A global reptile assessment highlights shared conservation needs of tetrapods. Nature 2022, 605, 285–290.
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858.
- Dinerstein, E.; Vynne, C.; Sala, E.; Joshi, A.R.; Fernando, S.; Lovejoy, T.E.; Mayorga, J.; Olson, D.; Asner, G.P.; Baillie, J.E.M.; et al. A global deal for nature: Guiding principles, milestones, and targets. Sci. Adv. 2019, 5, eaaw2869.
- Burgess, N.; Küper, W.; Mutke, J.; Brown, J.; Westaway, S.; Turpie, S.; Meshack, C.; Taplin, J.; McClean, C.; Lovett, J.C. Major gaps in the distribution of protected areas for threatened and narrow range Afrotropical plants. Biodivers. Conserv. 2005, 14, 1877–1894.
- Kearney, S.G.; Adams, V.M.; Fuller, R.A.; Possingham, H.P.; Watson, J.E. Estimating the benefit of well-managed protected areas for threatened species conservation. Oryx 2020, 54, 276–284.
- Carruthers, J. National Park Science: A Century of Research in South Africa; Cambridge University Press: Cambridge, UK, 2017.
- Sinclair, S.P.; Milner-Gulland, E.J.; Smith, R.J.; McIntosh, E.J.; Possingham, H.P.; Vercammen, A.; Knight, A.T. The use, and usefulness, of spatial conservation prioritizations. Conserv. Lett. 2018, 11, e12459.
- Botts, E.A.; Pence, G.; Holness, S.; Sink, K.; Skowno, A.; Driver, A.; Harris, L.R.; Desmet, P.; Escott, B.; Lötter, M.; et al. Practical actions for applied systematic conservation planning. Conserv. Biol. 2019, 33, 1235–1246.
- Tickner, D.; Opperman, J.J.; Abell, R.; Acreman, M.; Arthington, A.H.; Bunn, S.E.; Cooke, S.J.; Dalton, J.; Darwall, W.; Edwards, G.; et al. Bending the curve of global freshwater biodiversity loss: An emergency recovery plan. BioScience 2020, 70, 330–342.
- Stats, S.A. The Nature of South Africa’s Protected Area Estate. 2021. Available online: https://www.statssa.gov.za/?p=14732 (accessed on 14 January 2023).
- Department of Environmental Affairs. National Protected Areas Expansion Strategy for South Africa 2016; Department of Environmental Affairs: Pretoria, South Africa, 2016.
- Tolley, K.A.; Weeber, J.; Maritz, B.; Verburgt, L.; Bates, M.F.; Conradie, W.; Hofmeyr, M.D.; Turner, A.A.; da Silva, J.M.; Alexander, G.J. No safe haven: Protection levels show imperilled South African reptiles not sufficiently safe-guarded despite low average extinction risk. Biol. Conserv. 2019, 233, 61–72.
- Tolley, K.A.; Weeber, J.; Bates, M.F.; Bauer, A.M. Afroedura multiporis. The IUCN Red List of Threatened Species 2022: E.T115648679A197428768. Available online: https://www.iucnredlist.org/species/115648679/197428768 (accessed on 17 December 2022).
- Tolley, K.A.; Alexander, G.J.; Conradie, W.; Pietersen, D.; Weeber, J. Homopholis mulleri. The IUCN Red List of Threatened Species 2022: E.T10235A197398514. Available online: https://www.iucnredlist.org/species/10235/197398514 (accessed on 17 December 2022).
- Tolley, K.A.; Weeber, J.; Pietersen, D.; Conradie, W.; Alexander, G.J. Lygodactylus methueni. The IUCN Red List of Threatened Species 2022: E.T12439A197400102. Available online: https://www.iucnredlist.org/species/12439/197400102 (accessed on 17 December 2022).
More
Information
Subjects:
Biodiversity Conservation
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
687
Revisions:
3 times
(View History)
Update Date:
27 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




