Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Emily Ming-Chieh Lu | -- | 2037 | 2024-03-14 10:18:14 | | | |
| 2 | Rita Xu | Meta information modification | 2037 | 2024-03-14 10:45:26 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Lu, E.M. Vitamin D Deficiency on Chronic Kidney Disease. Encyclopedia. Available online: https://encyclopedia.pub/entry/56251 (accessed on 08 February 2026).
Lu EM. Vitamin D Deficiency on Chronic Kidney Disease. Encyclopedia. Available at: https://encyclopedia.pub/entry/56251. Accessed February 08, 2026.
Lu, Emily Ming-Chieh. "Vitamin D Deficiency on Chronic Kidney Disease" Encyclopedia, https://encyclopedia.pub/entry/56251 (accessed February 08, 2026).
Lu, E.M. (2024, March 14). Vitamin D Deficiency on Chronic Kidney Disease. In Encyclopedia. https://encyclopedia.pub/entry/56251
Lu, Emily Ming-Chieh. "Vitamin D Deficiency on Chronic Kidney Disease." Encyclopedia. Web. 14 March, 2024.
Copy Citation
Vitamin D has important anti-inflammatory, anti-microbial properties and plays a central role in the host immune response. Due to the crucial role of the kidneys in the metabolism of vitamin D, patients with chronic kidney disease (CKD) are prone to vitamin D deficiency.
vitamin D
chronic kidney disease
periodontal disease
1. Introduction
Vitamin D deficiency is associated with various noncommunicable diseases, among which are chronic kidney disease (CKD) and periodontal disease. As well as its fundamental roles in calcium and phosphate homeostasis, vitamin D possesses important antibacterial, anti-inflammatory and host modulatory effects [1], which exert “renoprotective” and “perio-protective” effects.
CKD is a global health concern affecting 5–10% of the world population [2]. According to Kidney Disease Improving Global Outcomes (KDIGO), CKD is diagnosed on the basis of an estimated glomerular filtration rate (eGFR) and values less than 60 mL/min/1.73 m2 have been identified as the threshold for CKD [3][4], together with abnormalities of renal structure or function, present for more than 3 months with implications for health [4]. There are five stages in CKD, the higher the staging the lower the eGFR [4].
Periodontitis is a chronic multifactorial inflammatory disease with an overall prevalence of 11.2% and is the 6th most prevalent disease worldwide [5]. It is associated with dysbiosis of the oral flora, characterised by the progressive destruction of the periodontium, with loss of clinical attachment, loss of the alveolar bone, presence of periodontal pockets and gingival bleeding [6]. Periodontitis is a serious public health issue, as it can cause not only local symptoms, but it can also have a negative impact on the individual’s general health, contributing to the development, and to the worsening, of chronic non-communicable degenerative diseases, such as chronic kidney disease (CKD) [7].
Periodontal disease and CKD have shared pathophysiological mechanisms, namely an increased inflammatory state, impaired immune response, and oxidative stress [8]. Therefore, the occurrence of both conditions is likely to result in an amplification of adverse outcomes [9]. A recent large cohort study suggested a bidirectional, causal relationship between periodontal inflammation and renal function [10], such that a 10% increase in periodontal inflamed surface area (PISA) was associated with a 3.0% decrease in eGFR and a 10% decrease in eGFR led to a 25.0% increase in PISA [10].
2. Vitamin D Metabolic Pathways
Vitamin D is a fat-soluble hormone that can be obtained from two main sources. Firstly, it can be obtained from dietary sources such as fatty fish and mushrooms. It is found in the form of Ergocalciferol (D2) from plant sources or Cholecalciferol (D3) from animal sources [11]. Secondly, it can be obtained through the action of sunlight’s ultraviolet rays on skin in the form of 7-Dehydrocholesterol which is converted into the previtamin Cholecalciferol (D3) [12]. Due to the relatively small proportion of vitamin D from the diet, dermal synthesis accounts for 90% of vitamin D provision [13].
2.1. Classical Pathway
The classical pathway for the activation of vitamin D involves two stages of hydroxylation. Firstly, the Vitamin D2 and D3 precursors are transported to the liver by vitamin D-binding protein (DBP) [14]. Precursors D2 and D3 are then converted into inactive 25-hydroxvitamin D (25(OH)D) by hydroxylation at the C25 position by 25-hydroxylase, coded by cytochrome P2R1 (CYP2R1) [15]. 25(OH)D acts as the main circulating and storage form of vitamin D in the body. 25(OH)D is then circulated in blood as the 25(OH)-DBP complex and undergoes glomerular filtration and uptake into the kidney proximal tubule cell by the receptor megalin. 25(OH)D-DBP then undergoes 1-α-hydroxylation by the Cytochrome P450 Family 27 Subfamily B Member 1 gene (CYP27B1) to its most activated state, 1,25-dihydroxyvitamin D (1,25(OH)2D), also known as calcitriol [15]. This is illustrated in Figure 1.
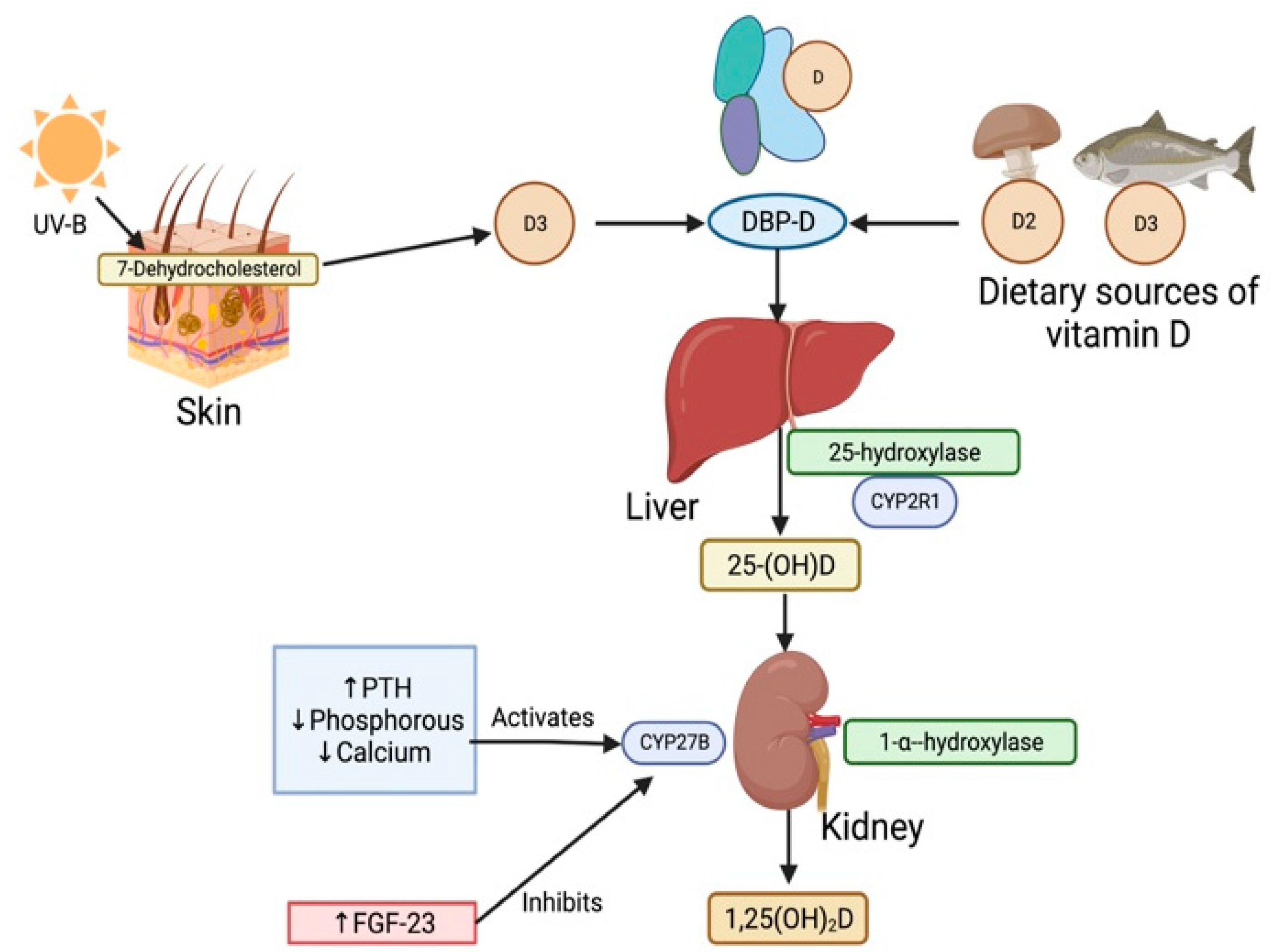
Figure 1. Vitamin ’s classical activation pathway in the human body. Sources of Vitamin D such as UV rays and diet deliver vitamin D in their precursor forms, D2 and D3. These precursors then bind to the Vitamin D-binding protein (DBP) at the site of synthesis and form the DBP-D protein complex. Vitamin D is then carried in the DBP-D complex through the blood plasma to the liver. Precursors D2 and D3 are hydroxylated into inactive 25-hydroxvitamin D [25(OH)D] by 25-hydroxylase, coded by the cytochrome P2R1 (CYP2R1) gene. 25(OH)D is taken up into the kidney from the blood and activated via 1-α-hydroxylation by the CYP27B gene to the 1,25-dihydroxyvitamin D (1,25(OH)2D) activated state. An increase in parathyroid hormone (PTH), reductions in phosphorous and calcium upregulates CYP27B gene activity, while an increase in fibroblast growth factor (FGF-23) downregulates CYP27B gene activity.
Calcitriol has wide ranging physiological and pharmacological effects [1]. Calcitriol is responsible for increasing intestinal calcium and phosphate absorption when serum calcium and phosphate levels are low. It also increases phosphorus resorption from bone and is involved in the production of antimicrobial peptides, epithelial defence mechanisms, host modulatory effects, the maintenance of the renin-angiotensin system, the inhibition of host tumour cells and suppression of parathyroid hormone (PTH) release [1]. Calcitriol exerts these affects by binding to intracellular vitamin D receptors (VDRs), which are steroid hormone nuclear receptors and function as transcription factors [16].
In health, the activation of the CYP27B gene is regulated by PTH, phosphorus, calcium, and fibroblast growth factor-23 (FGF-23) levels, and subsequently calcitriol levels [17]. Increased PTH levels combined with decreased phosphorus and calcium levels activate the CYP27B gene and lead to increased calcitriol levels [13]. Meanwhile, increased FGF-23 levels inhibit the CYP27B gene and decrease calcitriol levels [18].
2.2. Non-Classical Pathway
Aside from the classical metabolism of vitamin D and its role in calcium and phosphate homeostasis, a non-classical pathway of calcitriol synthesis appears to be present in various tissues, both including and peripheral to the kidneys. Additionally, 1-𝛼-hydroxylase (which is primarily expressed in the kidneys) may also be expressed in extrarenal cells and tissues [19]. Thus, the extra-renal production of calcitriol primarily functions as an autocrine or paracrine factor at extra-renal sites and thus plays a role in the non-classical actions of vitamin D [20][21].
Central to the non-classical function is the regulation of the renin–angiotensin–aldosterone system (RAAS). Calcitriol is regarded as a negative endocrine regulator of the renin gene, thereby inhibiting the RAAS and preserving renal function. The RAAS stimulates the production of renin, which cleaves angiotensin into angiotensin I, which is then processed into angiotensin II by the angiotensin-converting enzyme (ACE). Angiotensin II binds to the type 1 angiotensin II receptor (AT1R) to produce various deleterious effects for renal and cardiovascular tissues, including hypertension [22][23]. This is described in more detail in Figure 2.
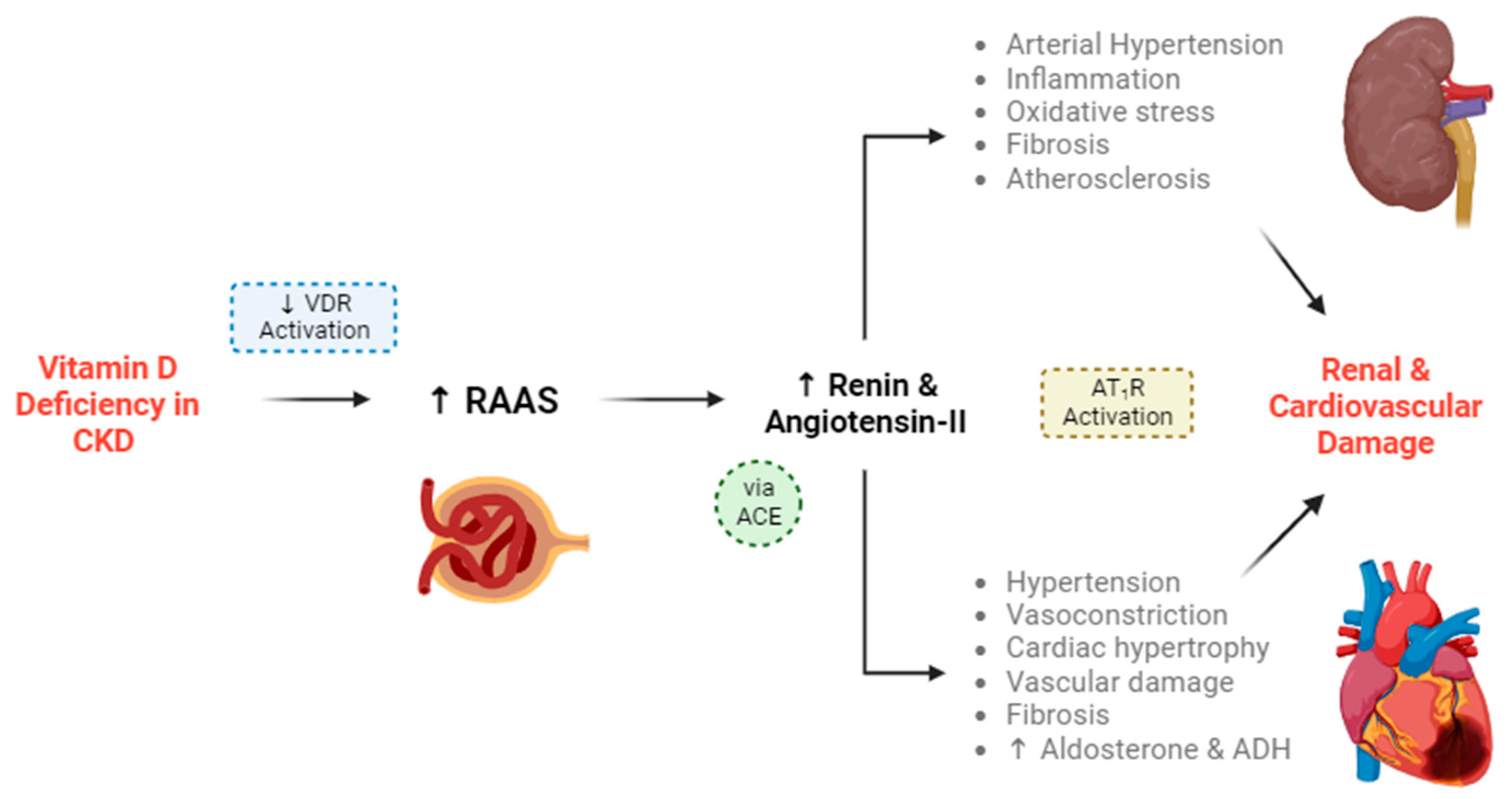
Figure 2. Vitamin D deficiency leads to the progression of chronic kidney disease (CKD) and cardiovascular disease (CVD). Vitamin D deficiency leads to a decrease in VDR-mediated functions. VDR activation is responsible for stimulation of the juxtaglomerular apparatus, which usually suppresses the renin–angiotensin–aldosterone system (RAAS) and the secretion of renin. In the absence of VDR activation (created by vitamin D deficiency), intracellular calcium is not stimulated within the juxtaglomerular apparatus, and RAAS activity increases. Renin and subsequent angiotensin-II production will increase in turn. Renin cleaves angiotensinogen into angiotensin I, which is then converted into angiotensin II by the angiotensin-converting enzyme (ACE). Angiotensin-II can produce more downstream angiotensin sub-types.
There is also evidence that VDR activation by calcitriol may also downregulate other RAAS components aside from renin, including the Ang II type one receptor, renin receptor and transforming growth factor beta [24]. This can aid in the reduction of renal blood pressure and fibrogenesis. VDR activation by calcitriol could also have anti-inflammatory effects, via the suppression of nuclear factor-kB (NF-kB) activation. It also appears that VDR can form complexes with various transcription factors and engage in crosstalk with a wide range of cellular signals [25], thus illustrating the depth of the relationship between VDR activation and RAAS components.
The suppression of the NF-kB pathway by calcitriol has extra-renal consequences too. Its suppression of the pathway is twofold: calcitriol suppresses NF-kB nuclear migration and phosphorylation, and it downregulates IkB phosphorylation (a protein involved in NF-kB signalling) by suppressing ROS activity [26]. The NF-kB pathway promotes pro-inflammatory cytokine expression, and thus a reduction in NF-kB activity results in a reduction in inflammatory markers. Thus, the suppression of the NF-kB pathway in turn can prevent insulin resistance [27], have neuroprotective effects against ischaemic strokes [26], and protect against other inflammatory disorders. Therefore, both the inhibitions of the RAAS and the NF-kB pathway are responsible for the “reno-protective” effects of vitamin D [28].
It is also worth noting the role of vitamin D in muscle health, perhaps best characterised by the link between vitamin D deficiency and sarcopenia, a generalised degenerative skeletal muscle disorder [29][30]. Low serum 25(OH)D levels are associated with sarcopenia in haemodialysis patients [29]. This is explained by the fact that calcitriol binds to VDRs in skeletal myocytes, stimulating protein synthesis [31][32]. Patients suffering from renal failure have an increased risk for the development of sarcopenia, due to their accelerated protein catabolism, the dialysis procedure itself, as well as their low energy and protein intakes [33]. Therefore, replenishing vitamin D levels would facilitate the restoration of muscle health [33].
3. Impact of Vitamin D Deficiency on CKD
Vitamin D deficiency is associated with a higher risk of mortality, secondary and tertiary hyperparathyroidism [34]. Due to impaired renal function, the eGFR is reduced, and there is therefore a decline in the conversion of 25(OH)D to calcitriol, the active form of vitamin D. The latter reduces intestinal calcium absorption and, together with phosphate retention, contributes to the onset of secondary and tertiary hyperparathyroidism.
CKD Patients who are vitamin D deficient have high mortality rates [35] and an increased cardiovascular risk [36]. In addition, the elevation of FGF23, which is linked with vitamin D deficiency, is associated with the progression of CKD towards end-stage renal disease (ESRD), the occurrence of cardiovascular (CVS) events and increased mortality rates in patients with CKD [37][38].
The serum total 25(OH)D concentrations were predictive of renal outcomes, such as the doubling of serum creatinine in ESRD, and associated with disease progression and morality [39][40]. A meta-analysis of prospective studies demonstrated an increased relationship between all-cause mortality in patients with CKD and serum total 25(OH)D concentrations [41]. Conversely, a recent systematic review and meta-analysis concluded that higher levels of serum 25(OH)D were associated with lower risks of all-cause mortality [42].
Therefore, to ensure that patients with CKD avoid vitamin D deficiency and prevent complications such as secondary and tertiary hyperparathyroidism and other co-morbidities [43][44][45], the Kidney Disease Outcomes Quality Initiative (KDOQI) and Kidney Disease Improving Global Outcomes (KDIGO) group have suggested the use of vitamin D supplementation [44][45].
Vitamin D Supplementation in CKD Patients
Vitamin D supplementation is associated with the reduced risk of all-cause mortality [46] and cardiovascular mortality in patients in CKD, including those with ESRD [45][47]. In particular, therapies with calcitriol and analogues are associated with reduced mortality in CKD patients, particularly those suffering from SHPT [48].
CKD patients are deficient in vitamin D, even in the early stages of the disease [49]. Vitamin D plays a vital role not only in mineral homeostasis, but also in systemic health. As such, it is advised that vitamin D supplementation in CKD patients begins as soon as possible, to ensure a pool of vitamin D can be turned into calcitriol.
During the early stages of CKD, where there is still evidence of residual renal function, supplementation can be achieved for CKD patients with oral forms of inactive vitamin D3 or D2. Vitamin D3 (cholecalciferol) is the natural form synthesised in the dermis, whilst vitamin D2 (ergocalciferol) is a synthetic product made using fungi [50]. Another reason is that vitamin D2 is associated with higher catabolic processes, and therefore, the overall improvement in serum vitamin D levels is not as sustainable as that seen with D3. Therefore, vitamin D3 is superior to vitamin D2 in raising the total 25(OH)D and thus ideally used for supplementation [51].
It is important to appreciate that the renal production of calcitriol becomes suppressed during Stages 3–4 of CKD [52][53] that are characterised by the significant loss of renal 1-𝛼-hydroxylase, and the development of SHPT with declining kidney function. The current KDIGO guidelines recommend that calcitriol and vitamin D analogue supplementation should be reserved for predialysis CKD patients with severe and progressive hyperparathyroidism [2]. However, in line with the above points and existing clinical research [52][53][54], vitamin D supplementation (ideally with D3) should begin immediately after diagnosis to facilitate endogenous conversion into calcitriol and its positive downstream effects.
References
- Lu, E.M.C. The role of vitamin D in periodontal health and disease. J. Periodontal Res. 2023, 58, 213–224.
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int. Suppl. 2017, 7, 1–59.
- Schaeffner, E.S.; Ebert, N.; Delanaye, P.; Frei, U.; Gaedeke, J.; Jakob, O.; Kuhlmann, M.K.; Schuchardt, M.; Tölle, M.; Ziebig, R.; et al. Two novel equations to estimate kidney function in persons aged 70 years or older. Ann. Intern. Med. 2012, 157, 471–481.
- Stevens, P.E.; Levin, A. Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Evaluation and management of chronic kidney disease: Synopsis of the kidney disease: Improving global outcomes 2012 clinical practice guideline. Ann. Intern. Med. 2013, 158, 825–830.
- Tonetti, M.S.; Jepsen, S.; Jin, L.; Otomo-Corgel, J. Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: A call for global action. J. Clin. Periodontol. 2017, 44, 456–462.
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. S1), S173–S182.
- Parsegian, K.; Randall, D.; Curtis, M.; Ioannidou, E. Association between periodontitis and chronic kidney disease. Periodontol. 2000 2022, 89, 114–124.
- Tonetti, M.S.; Van Dyke, T.E.; on behalf of Working Group 1 of the Joint EFP/AAP Workshop. Periodontitis and atherosclerotic cardiovascular disease: Consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J. Periodontol. 2013, 84 (Suppl. S4), S24–S29.
- Deschamps-Lenhardt, S.; Martin-Cabezas, R.; Hannedouche, T.; Huck, O. Association between periodontitis and chronic kidney disease: Systematic review and meta-analysis. Oral Dis. 2019, 25, 385–402.
- Sharma, P.; Fenton, A.; Dias, I.H.K.; Heaton, B.; Brown, C.L.; Sidhu, A.; Rahman, M.; Griffiths, H.R.; Cockwell, P.; Ferro, C.J.; et al. Oxidative stress links periodontal inflammation and renal function. J. Clin. Periodontol. 2021, 48, 357–367.
- Benedik, E. Sources of vitamin D for humans. Int. J. Vitam. Nutr. Res. 2022, 92, 118–125.
- Lehmann, B.; Genehr, T.; Knuschke, P.; Pietzsch, J.; Meurer, M. UVB-Induced Conversion of 7-Dehydrocholesterol to 1α,25-Dihydroxyvitamin D3 in an In Vitro Human Skin Equivalent Model. J. Investig. Dermatol. 2001, 117, 1179–1185.
- Chang, S.W.; Lee, H.C. Vitamin D and health—The missing vitamin in humans. Pediatr. Neonatol. 2019, 60, 237–244.
- Bikle, D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329.
- Christakos, S.; Ajibade, D.V.; Dhawan, P.; Fechner, A.J.; Mady, L.J. Vitamin D: Metabolism. Endocrinol. Metab. Clin. N. Am. 2010, 39, 243–253.
- Rochel, N.; Wurtz, J.M.; Mitschler, A.; Klaholz, B.; Moras, D. The crystal structure of the nuclear receptor for vitamin D bound to its natural ligand. Mol. Cell. 2000, 5, 173–179.
- Silver, J.; Naveh-Many, T. FGF-23 and secondary hyperparathyroidism in chronic kidney disease. Nat. Rev. Nephrol. 2013, 9, 641–649.
- Nakashima, A.; Yokoyama, K.; Yokoo, T.; Urashima, M. Akio Nakashima, Keitaro Yokoyama, Takashi Yokoo, Mitsuyoshi Urashima. World J. Diabetes 2016, 7, 89–100.
- Hewison, M. Vitamin D and the immune system: New perspectives on an old theme. Endocrinol. Metab. Clin. N. Am. 2010, 39, 365–379.
- Dusso, A.S. Kidney disease and vitamin D levels: 25-hydroxyvitamin D, 1,25-dihydroxyvitamin D, and VDR activation. Kidney Int. Suppl. 2011, 1, 136–141.
- Adams, J.S.; Hewison, M. Update in Vitamin D. J. Clin. Endocrinol. Metab. 2010, 95, 471–478.
- Maranduca, M.A.; Clim, A.; Pinzariu, A.C.; Statescu, C.; Sascau, R.A.; Tanase, D.M.; Serban, D.N.; Branisteanu, D.C.; Branisteanu, D.E.; Huzum, B.; et al. Role of arterial hypertension and angiotensin II in chronic kidney disease (Review). Exp. Ther. Med. 2023, 25, 153.
- Paz Ocaranza, M.; Riquelme, J.A.; García, L.; Jalil, J.E.; Chiong, M.; Santos, R.A.; Lavandero, S. Counter-regulatory renin-angiotensin system in cardiovascular disease. Nat. Rev. Cardiol. 2020, 17, 116–129.
- Freundlich, M.; Quiroz, Y.; Zhang, Z.; Zhang, Y.; Bravo, Y.; Weisinger, J.R.; Li, Y.C.; Rodriguez-Iturbe, B. Suppression of renin–angiotensin gene expression in the kidney by paricalcitol. Kidney Int. 2008, 74, 1394–1402.
- Andress, D.L. Vitamin D in chronic kidney disease: A systemic role for selective vitamin D receptor activation. Kidney Int. 2006, 69, 33–43.
- Tajalli-Nezhad, S.; Karimian, M.; Beyer, C.; Atlasi, M.A.; Tameh, A.A. The regulatory role of Toll-like receptors after ischemic stroke: Neurosteroids as TLR modulators with the focus on TLR2/4. Cell. Mol. Life Sci. 2019, 76, 523–537.
- Wamberg, L.; Kampmann, U.; Stødkilde-Jørgensen, H.; Rejnmark, L.; Pedersen, S.B.; Richelsen, B. Effects of vitamin D supplementation on body fat accumulation, inflammation, and metabolic risk factors in obese adults with low vitamin D levels-results from a randomized trial. Eur. J. Intern. Med. 2013, 24, 644–649.
- Li, Y.C. Renoprotective effects of vitamin D analogs. Kidney Int. 2010, 78, 134–139.
- Hori, M.; Takahashi, H.; Kondo, C.; Hayashi, F.; Tokoroyama, S.; Mori, Y.; Tsujita, M.; Shirasawa, Y.; Takeda, A.; Morozumi, K.; et al. Association between serum 25-hydroxyvitamin D levels and sarcopenia in patients undergoing chronic haemodialysis. Am. J. Nephrol. 2024. ahead of print.
- Wintermeyer, E.; Ihle, C.; Ehnert, S.; Stöckle, U.; Ochs, G.; De Zwart, P.; Flesch, I.; Bahrs, C.; Nussler, A.K. Crucial Role of Vitamin D in the Musculoskeletal System. Nutrients 2016, 8, 319.
- Simpson, R.U.; Thomas, G.A.; Arnold, A.J. Identification of 1,25-dihydroxyvitamin D3 receptors and activities in muscle. J. Biol. Chem. 1985, 260, 8882–8891.
- Gallieni, M.A.U.R.I.Z.I.O.; Kamimura, S.H.I.G.E.H.I.T.O.; Ahmed, A.D.N.A.N.; Bravo, E.R.I.C.; Delmez, J.A.M.E.S.; Slatopolsky, E.D.U.A.R.D.O.; Dusso, A.D.R.I.A.N.A. Kinetics of monocyte 1 alpha-hydroxylase in renal failure. Am. J. Physiol.-Ren. Physiol. 1995, 268, F746–F753.
- Sabatino, A.; Cuppari, L.; Stenvinkel, P.; Lindholm, B.; Avesani, C.M. Sarcopenia in chronic kidney disease: What have we learned so far? J. Nephrol. 2021, 34, 1347–1372.
- Jean, G.; Terrat, J.C.; Vanel, T.; Hurot, J.M.; Lorriaux, C.; Mayor, B.; Chazot, C. Evidence for persistent vitamin D 1-alpha-hydroxylation in hemodialysis patients: Evolution of serum 1,25-dihydroxycholecalciferol after 6 months of 25-hydroxycholecalciferol treatment. Nephron Clin. Pract. 2008, 110, c58–c65.
- Walker, J.P.; Hiramoto, J.S.; Gasper, W.J.; Auyang, P.; Conte, M.S.; Rapp, J.H.; Lovett, D.H.; Owens, C.D. Vitamin D deficiency is associated with mortality and adverse vascular access outcomes in patients with end-stage renal disease. J. Vasc. Surg. 2014, 60, 176–183.
- Lopez, A.G.; Kerlan, V.; Desailloud, R. Non-classical effects of vitamin D: Non-bone effects of vitamin D. Ann. Endocrinol. 2021, 82, 43–51.
- Nakano, C.; Hamano, T.; Fujii, N.; Matsui, I.; Tomida, K.; Mikami, S.; Inoue, K.; Obi, Y.; Okada, N.; Tsubakihara, Y.; et al. Combined use of vitamin D status and FGF23 for risk stratification of renal outcome. Clin. J. Am. Soc. Nephrol. 2012, 7, 810–819.
- Wolf, M. Update on fibroblast growth factor 23 in chronic kidney disease. Kidney Int. 2012, 82, 737–747.
- Shimada, T.; Kakitani, M.; Yamazaki, Y.; Hasegawa, H.; Takeuchi, Y.; Fujita, T.; Fukumoto, S.; Tomizuka, K.; Yamashita, T. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J. Clin. Investig. 2004, 113, 561–568.
- Melamed, M.L.; Astor, B.; Michos, E.D.; Hostetter, T.H.; Powe, N.R.; Muntner, P. 25-Hydroxyvitamin D levels, race, and the progression of kidney disease. J. Am. Soc. Nephrol. 2009, 20, 2631–2639.
- Pilz, S.; Tomaschitz, A.; März, W.; Drechsler, C.; Ritz, E.; Zittermann, A.; Cavalier, E.; Pieber, T.R.; Lappe, J.M.; Grant, W.B.; et al. Vitamin D, cardiovascular disease and mortality. Clin. Endocrinol. 2011, 75, 575–584.
- Jayedi, A.; Soltani, S.; Shab-Bidar, S. Vitamin D status and all-cause mortality in patients with chronic kidney disease: A systematic review and dose-response meta-analysis. J. Clin. Endocrinol. Metab. 2017, 102, 2136–2145.
- Al-Aly, Z.; Qazi, R.A.; González, E.A.; Zeringue, A.; Martin, K.J. Changes in serum 25-hydroxyvitamin D and plasma intact PTH levels following treatment with ergocalciferol in patients with CKD. Am. J. Kidney Dis. 2007, 50, 59–68.
- DeVille, J.; Thorp, M.L.; Tobin, L.; Gray, E.; Johnson, E.S.; Smith, D.H. Effect of ergocalciferol supplementation on serum parathyroid hormone and serum 25-hydroxyvitamin D in chronic kidney disease. Nephrology 2006, 11, 555–559.
- Ikizler, T.A.; Cuppari, L. The 2020 Updated KDOQI Clinical Practice Guidelines for Nutrition in Chronic Kidney Disease. Blood Purif. 2021, 50, 667–671.
- Chowdhury, R.; Kunutsor, S.; Vitezova, A.; Oliver-Williams, C.; Chowdhury, S.; Kiefte-de-Jong, J.C.; Khan, H.; Baena, C.P.; Prabhakaran, D.; Hoshen, M.B.; et al. Vitamin D and risk of cause specific death: Systematic review and meta-analysis of observational cohort and randomised intervention studies. BMJ 2014, 348, g1903.
- Skrobot, A.; Demkow, U.; Wachowska, M. Immunomodulatory Role of Vitamin D: A Review. Adv. Exp. Med. Biol. 2018, 1108, 13–23.
- Duranton, F.; Rodriguez-Ortiz, M.E.; Duny, Y.; Rodriguez, M.; Daurès, J.P.; Argilés, A. Vitamin D treatment and mortality in chronic kidney disease: A systematic review and meta-analysis. Am. J. Nephrol. 2013, 37, 239–248.
- Mehrotra, R.; Kermah, D.A.; Salusky, I.B.; Wolf, M.S.; Thadhani, R.I.; Chiu, Y.W.; Martins, D.; Adler, S.G.; Norris, K.C. Chronic kidney disease, hypovitaminosis D, and mortality in the United States. Kidney Int. 2009, 76, 977–983.
- Cheng, S.; Coyne, D. Vitamin D and outcomes in chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2007, 16, 77–82.
- Armas, L.A.G.; Hollis, B.W.; Heaney, R.P. Vitamin D2 Is Much Less Effective than Vitamin D3 in Humans. J. Clin. Endocrinol. Metab. 2004, 89, 5387–5391.
- Jones, G. Expanding role for vitamin D in chronic kidney disease: Importance of blood 25-OH-D levels and extra-renal 1alpha-hydroxylase in the classical and nonclassical actions of 1alpha,25-dihydroxyvitamin D(3). Semin. Dial. 2007, 20, 316–324.
- Heaney, R.P. Vitamin D in Health and Disease. Clin. J. Am. Soc. Nephrol. 2008, 3, 1535–1541.
- Christodoulou, M.; Aspray, T.J.; Schoenmakers, I. Vitamin D Supplementation for Patients with Chronic Kidney Disease: A Systematic Review and Meta-analyses of Trials Investigating the Response to Supplementation and an Overview of Guidelines. Calcif. Tissue Int. 2021, 109, 157–178.
More
Information
Subjects:
Pathology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
799
Revisions:
2 times
(View History)
Update Date:
14 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




