Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Min Wang | -- | 1612 | 2024-03-14 09:44:47 | | | |
| 2 | Peter Tang | Meta information modification | 1612 | 2024-03-14 09:51:08 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Wang, G.; Zhao, W.; Mansoor, M.; Liu, Y.; Wang, X.; Zhang, K.; Xiao, C.; Liu, Q.; Mao, L.; Wang, M.; et al. Mesoporous Carbon in PEMFC Catalysts. Encyclopedia. Available online: https://encyclopedia.pub/entry/56247 (accessed on 12 January 2026).
Wang G, Zhao W, Mansoor M, Liu Y, Wang X, Zhang K, et al. Mesoporous Carbon in PEMFC Catalysts. Encyclopedia. Available at: https://encyclopedia.pub/entry/56247. Accessed January 12, 2026.
Wang, Guanxiong, Wei Zhao, Majid Mansoor, Yinan Liu, Xiuyue Wang, Kunye Zhang, Cailin Xiao, Quansheng Liu, Lingling Mao, Min Wang, et al. "Mesoporous Carbon in PEMFC Catalysts" Encyclopedia, https://encyclopedia.pub/entry/56247 (accessed January 12, 2026).
Wang, G., Zhao, W., Mansoor, M., Liu, Y., Wang, X., Zhang, K., Xiao, C., Liu, Q., Mao, L., Wang, M., & Lv, H. (2024, March 14). Mesoporous Carbon in PEMFC Catalysts. In Encyclopedia. https://encyclopedia.pub/entry/56247
Wang, Guanxiong, et al. "Mesoporous Carbon in PEMFC Catalysts." Encyclopedia. Web. 14 March, 2024.
Copy Citation
Developing durable oxygen reduction reaction (ORR) electrocatalysts is essential to step up the large-scale applications of proton exchange membrane fuel cells (PEMFCs). Traditional ORR electrocatalysts provide satisfactory activity, yet their poor durability limits the long-term applications of PEMFCs. Porous carbon used as catalyst support in Pt/C is vulnerable to oxidation under high potential conditions, leading to Pt nanoparticle dissolution and carbon corrosion.
mesoporous carbon
intermetallic
oxygen reduction reaction
mass transport resistance
1. Introduction
Recently, large-scale applications of proton exchange membrane fuel cells (PEMFCs) have sparked a meteoric rise in fuel cell technology. Among the forerunners in this field is Toyota’s Mirai fuel cell vehicle, which features a stunning improvement in the volumetric power density of its stack, from 3.5 kW/L in its first iteration to 5.4 kW/L in its second [1]. Because of this 41.9% improvement in performance measures, the Mirai is now among the top products in terms of volumetric power density in the world [2]. However, the catalyst system based on mesoporous carbon (MPC) embedded with PtCo alloy nanoparticles is what makes this advancement genuinely ground-breaking (Figure 1). In contrast to the first-generation Mirai’s use of Pt distributed on conventional solid carbon supports, the second-generation system uses a novel combination of PtCo alloy nanoparticles with MPC scaffolds [3].
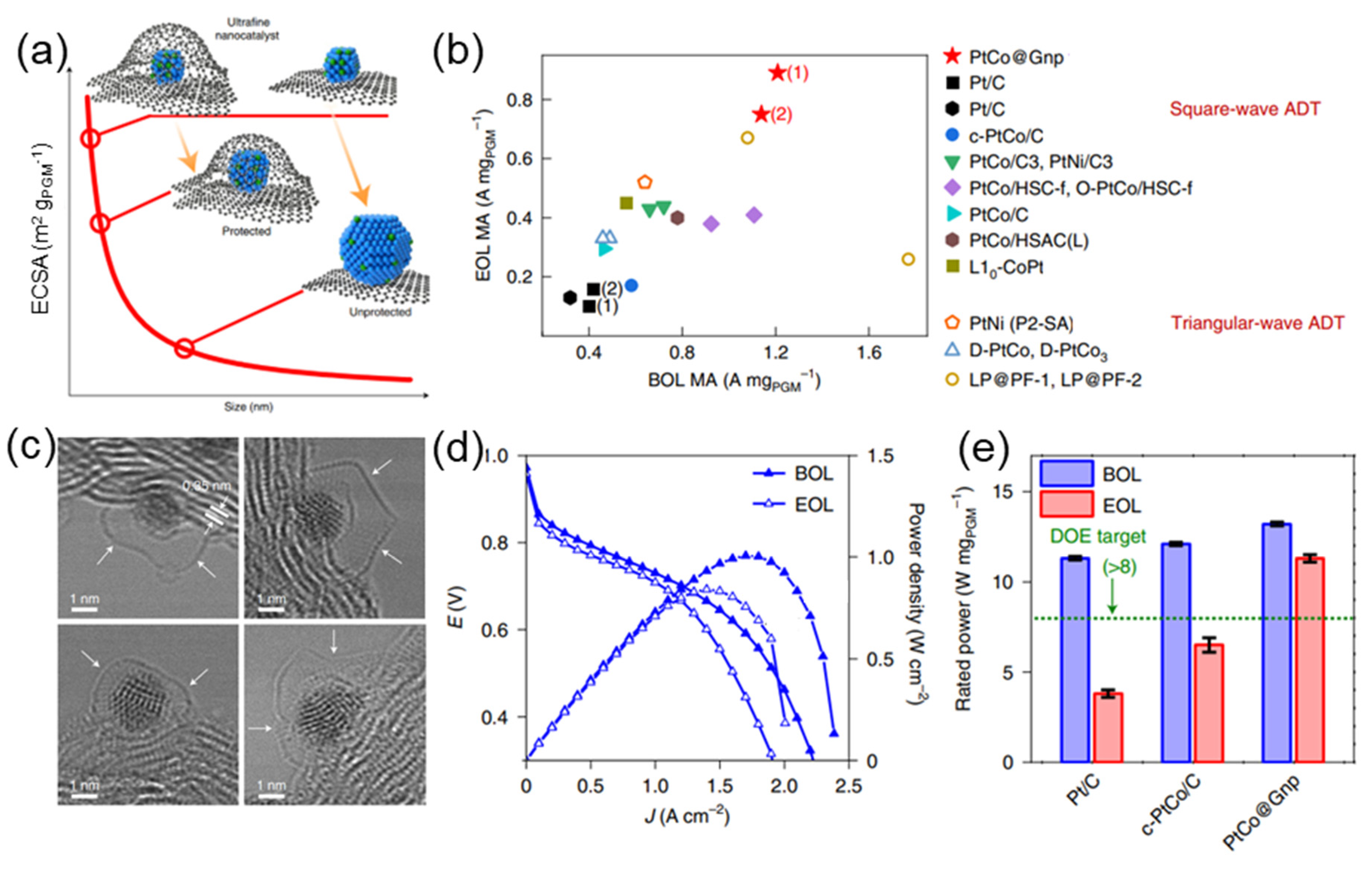
Figure 1. (a) Schematic illustration of ultrafine nanocatalysts encaged in graphene pockets and their impact on ECSA retention after an ADT. (b) Comparison of mass activity between PtCo catalysts with the state of the art in the literature. (c) The enclosure of ultrafine nanoparticle by graphene nanopockets (indicated by white arrows). (d) Comparison of PtCo@Gnp at BOL and EOL. (e) Rated power of MEAs normalized by the total PGM loading [3].
The novel feature resides in the MPC complex design, allowing for the uniform distribution of PtCo nanoparticles and neatly accommodating them within its mesoporous channels. This configuration’s unique benefits include reducing the ionomer’s interaction with the Pt surface and avoiding the poisoning impact of the sulfonic acid group. Additionally, it improves the mass-specific activity of the Pt catalysts, ushering in a new era of fuel cell efficiency while simultaneously decreasing Pt consumption [4]. The PEMFC ecosystem has been rethought from the ground up, providing a model for the future of environmentally friendly, fuel-efficient transportation [5]. The new Mirai is more than just an improvement; it is a technological leap that, thanks to advances in materials science, ushers in a new era for hydrogen fuel cell automobiles [6]. Though the overarching goal on both sides of the Pacific is to improve fuel cell technology, a new story is emerging about the significance of MPC supports and Pt nanowires in redefining ORR kinetics and catalyst longevity, particularly at low Pt loadings [7][8].
2. MPC in PEMFC Catalysts
In fuel cell technology, navigating complex carbon support systems is analogous to trying to thread a needle. MPC has been identified as a significant advancement in optimizing catalytic activity and long-term stability [9]. This substance exhibits a composition extending beyond pure carbon, encompassing intricate structures characterized by disordered and ordered networks. Additionally, it possesses a diverse variety of pore sizes, ranging from 2 to 50 nm [10]. The distinctive structural peculiarity of MPC confers it with a notable advantage in addressing the inherent restrictions that have hitherto hindered fuel cell catalysts [11]. However, why limit ourselves to solely enhancing catalyst activity? The primary difficulty involves protecting the Pt catalyst from the deleterious effects of sulfonate groups found in perfluoro sulfonic acid ionomers (PFSAs), which have been seen to reduce the activity of Pt by a significant factor of 2–4 [12].
Catalysts used in PEMFCs are profoundly affected by the structure and morphology of the carbon supports used in their design and performance [13]. One must be aware of the potential “poisoning” effects of sulfonic acid groups, especially from perfluoro sulfonic acid ionomers, on Pt activity, even though there are many methods for tuning the intrinsic activity of catalysts [14]. Standard porous carbon supports enable the deposition of Pt nanoparticles within their pore structures, reducing their interaction with sulfonic acid groups. However, these supports present difficulties in the mass transfer of reaction gas (O2) and conduction of protons (H+), which limit their usefulness [15].
The aforementioned constraints can be a significant impediment, particularly in areas with high current density, which is important in heavy-duty vehicles [16]. The unique contributions of MPC are significant in providing an optimal compromise [17]. The distinctive architectural and electronic characteristics of MPC render it an exceedingly auspicious substrate for PtCo intermetallics [18]. MPC is a supplementary material that effectively integrates the catalytic function and electrochemical kinetics within PEMFCs. The utilization of this technique leads to an increase in the stability and visibility of active sites responsible for the ORR [19]. Additionally, it greatly enhances the transportation of oxygen inside the catalyst layer. As a result, this approach effectively tackles the drawbacks associated with conventional carbon supports. The anti-corrosion and anti-oxidation capabilities of MPC supports have been significantly improved by the progress made in surface functionalization, graphitization, and heteroatom doping [20]. Furthermore, novel techniques, such as high-temperature annealing, have been devised to enhance the durability and effectiveness of these MPC supports, especially when subjected to rapid stress testing [21].
By merging the inherent benefits of MPC with PtCo intermetallics, it becomes possible to address the difficulties related to Pt poisoning, mass transfer resistance, and proton conduction. The collaboration between MPC and PtCo intermetallics demonstrates a possible avenue for achieving the desired long-term performance and durability objectives of PEMFCs, specifically in the challenging and dynamic domain of heavy-duty vehicles (Figure 2a,b) [22][23][24][25][26]. Hence, the significance of employing MPC support in augmenting the efficiency of PEMFCs becomes apparent when considering the broader framework encompassing catalyst durability, reactivity, and operational complexities. The scaffold is not solely a passive structure but rather an active entity that plays a vital role in influencing both catalyst kinetics and mass transport phenomena (Figure 2c–e) [15].
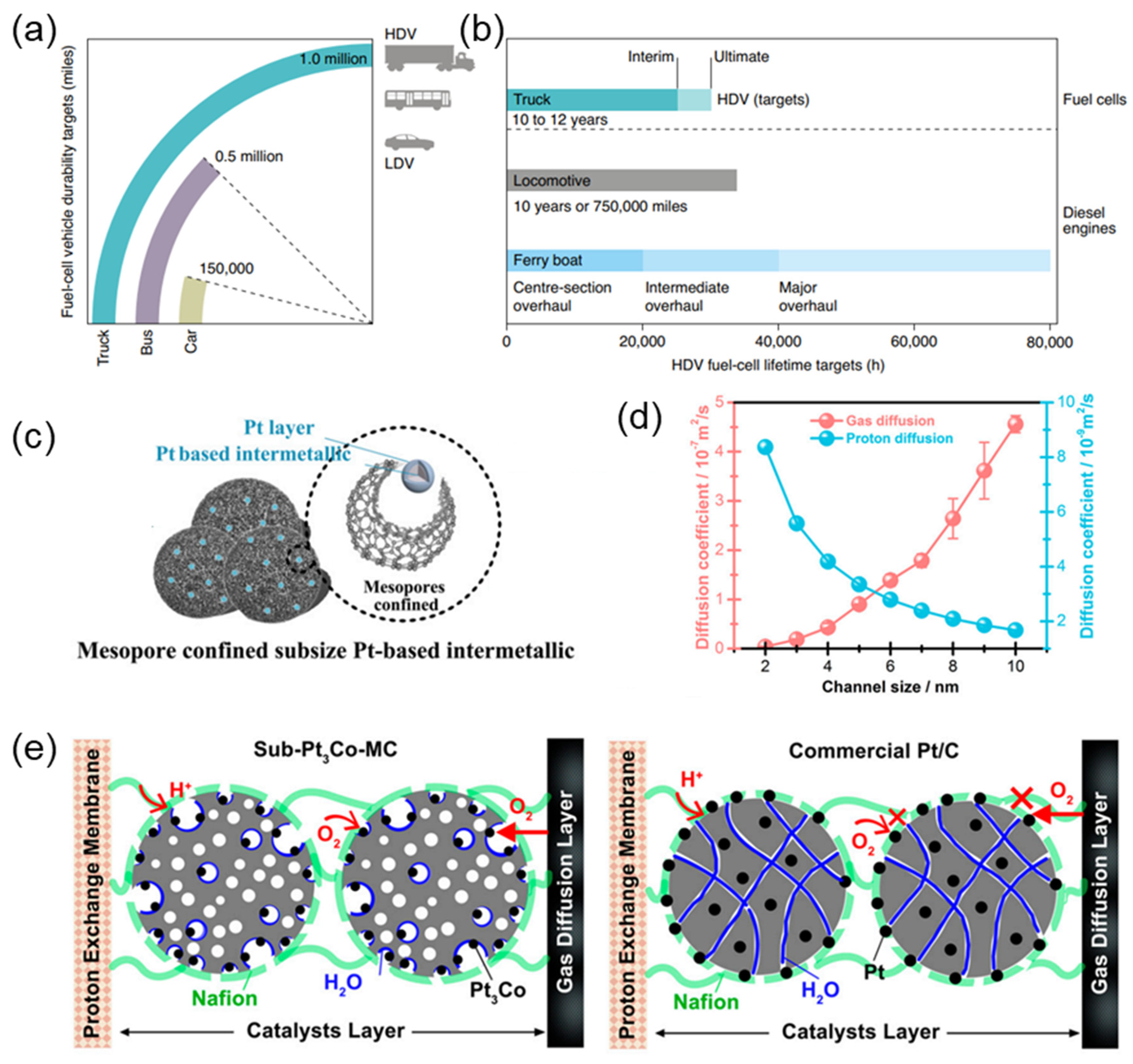
Figure 2. (a) Fuel cell durability targets for LDVs and HDVs expressed in terms of miles. (b) Comparison of HDV fuel cell lifetime targets with the useful service lifetime of current diesel engines for rail [25]. (c) Structure of Pt-M intermetallic–Pt skin with mesopore confined. (d) The proton and gas diffusion capacity. (e) The anti-poisoning effect with three-phase interface in sub-Pt3Co-MC and the poison effect by Nafion ionomer in commercial Pt/C [15].
To make breakthroughs in fuel cell technology, researchers need a deep familiarity with the intricate relationships between catalysts, supports, and operating parameters. Protecting Pt nanoparticles from sulfonic acid groups with conventional MPC catalysts can maintain their catalytic activity. This benefit, however, comes at the expense of stifling O2 and H+ mass transfer efficiency, especially in high-current-density regions above 2.0 A/cm2 [27]. The appropriate carbon support should be more than merely a safe habitat for Pt if high performance is to be achieved, especially at a modest platinum loading (10 g/vehicle or 0.1 mgPt/cm2 MEA).
Combining nano-architecture channels for the unrestricted flux of H+ and O2 with micro-scale isolation of Pt nanoparticles from ionomers should be a feat of engineering. Using MPC supports that are functionally graded, with different pore shapes at different depths, is a novel method. Such hierarchical arrangements would provide the optimal compromise between preserving mass transfer efficiency and isolating Pt nanoparticles. These graded supports can potentially optimize pore depth and tortuosity for each application, whereas in conventional MPC, these factors may limit performance. Imagine an MPC architecture with multiple layers, each serving a different purpose, such as one layer for optimal Pt nanoparticle implantation, another for quick proton transfer, and still another tuned for maximum O2 diffusion. By strategically engineering these functionally graded layers, we can overcome obstacles like catalyst poisoning and mass transfer limits, satisfying demands for increased activity and longer lifetimes [28][29].
Although the concept is rather simple, the severe inhomogeneous nature and the size of the carbon black particles make visualization and development of the carbon difficult. Several methods have been developed for the synthesis of MPC, including (1) activation methods (physical activation and chemical activation), (2) template methods (hard template and soft template) [30], (3) carbonized method (the carbonization of polymer/polymer blends, organic aerogels, etc.), and (4) catalytic activation using metal ions. Currently, the “template method” is mainly used to prepare MPC. The carbon precursor is attached to the template material, such as silica, magnesium oxide (MgO), etc., and after high-temperature heat treatment, the templating agent removed via etching, forming a carbon support with a mesoporous pore structure [31][32][33]. Recently, the synthesis procedure for mesoporous carbons using magnesium oxide as a template has been industrialized [34].
For catalysis, new studies highlight the synergistic potential of mesoporous carbon supports with PtCo nanoparticles. Three prominent advantages are as follows: (1) The utilization of advanced technology enables the efficient and uniform distribution of PtCo nanoparticles within the mesoporous carbon matrix. (2) The placement of PtCo nanoparticles within the mesopores effectively isolates them from sulfonic acid groups, resulting in improved kinetics for catalyst reactions involving H+ and O2. (3) Integrating these state-of-the-art MPC-supported catalysts into electrode assemblies leads to reduced resistance in O2 mass transfer and exceptional performance in high-current-density conditions. Consequently, the power density of PEMFCs is significantly enhanced.
Hydrogen has a high gravimetric energy density, making it ideal for heavy-duty applications such as ships and trains. This is changing the economic dynamics around hydrogen-based technologies, which were previously focused on light-duty vehicles. The cost of ownership has become a significant concern due to trucks needing more durable and cost-efficient fuel cells that fulfill higher criteria than those used in light-duty vehicles. The game-changing potential resides in integrating MPC substrates with advanced Pt alloy production processes, enabling the engineering of catalysts with unparalleled reaction efficiency. The use of this synergistic strategy not only enhances the performance metrics but also presents a persuasive argument for the cost-effective implementation of robust hydrogen fuel cell systems [25].
References
- Jiao, K.; Xuan, J.; Du, Q.; Bao, Z.; Xie, B.; Wang, B.; Zhao, Y.; Fan, L.; Wang, H.; Hou, Z.; et al. Designing the next generation of proton-exchange membrane fuel cells. Nature 2021, 595, 361–369.
- Kodama, K.; Nagai, T.; Kuwaki, A.; Jinnouchi, R.; Morimoto, Y. Challenges in applying highly active Pt-based nanostructured catalysts for oxygen reduction reactions to fuel cell vehicles. Nat. Nanotechnol. 2021, 16, 140–147.
- Zhao, Z.; Liu, Z.; Zhang, A.; Yan, X.; Xue, W.; Peng, B.; Xin, H.L.; Pan, X.; Duan, X.; Huang, Y. Graphene-nanopocket-encaged PtCo nanocatalysts for highly durable fuel cell operation under demanding ultralow-Pt-loading conditions. Nat. Nanotechnol. 2022, 17, 968–975.
- Li, F.-M.; Huang, L.; Zaman, S.; Guo, W.; Liu, H.; Guo, X.; Xia, B.Y. Corrosion Chemistry of Electrocatalysts. Adv. Mater. 2022, 34, 2200840.
- Wang, X.X.; Swihart, M.T.; Wu, G. Achievements, challenges and perspectives on cathode catalysts in proton exchange membrane fuel cells for transportation. Nat. Catal. 2019, 2, 578–589.
- Liu, D.; Zeng, Q.; Hu, C.; Chen, D.; Liu, H.; Han, Y.; Xu, L.; Zhang, Q.; Yang, J. Light doping of tungsten into copper-platinum nanoalloys for boosting their electrocatalytic performance in methanol oxidation. Nano Res. Energy 2022, 1, 9120017.
- Zaman, S.; Wang, M.; Liu, H.; Sun, F.; Yu, Y.; Shui, J.; Chen, M.; Wang, H. Carbon-based catalyst supports for oxygen reduction in proton-exchange membrane fuel cells. Trends Chem. 2022, 4, 886–906.
- Zaman, S.; Douka, A.I.; Noureen, L.; Tian, X.; Ajmal, Z.; Wang, H. Oxygen reduction performance measurements: Discrepancies against benchmarks. Battery Energy 2023, 2, 20220060.
- Zhang, J.; Chen, G.; Mullen, K.; Feng, X. Carbon-Rich Nanomaterials: Fascinating Hydrogen and Oxygen Electrocatalysts. Adv. Mater. 2018, 40, e1800528.
- Cui, P.; Zhao, L.; Long, Y.; Dai, L.; Hu, C. Carbon-Based Electrocatalysts for Acidic Oxygen Reduction Reaction. Angew. Chem. Int. Ed. Engl. 2023, 62, e202218269.
- Hu, C.; Gao, Y.; Zhao, L.; Dai, L. Carbon-based metal-free electrocatalysts: Recent progress and forward looking. Chem. Catal. 2022.
- Lee, J.K.; Anderson, G.; Tricker, A.W.; Babbe, F.; Madan, A.; Cullen, D.A.; Arregui-Mena, J.D.; Danilovic, N.; Mukundan, R.; Weber, A.Z.; et al. Ionomer-free and recyclable porous-transport electrode for high-performing proton-exchange-membrane water electrolysis. Nat. Commun. 2023, 14, 4592.
- Zhang, Q.; Xia, T.; Huang, H.; Liu, J.; Zhu, M.; Yu, H.; Xu, W.; Huo, Y.; He, C.; Shen, S.; et al. Autocatalytic reduction-assisted synthesis of segmented porous PtTe nanochains for enhancing methanol oxidation reaction. Nano Res. Energy 2023, 2, e9120041.
- Ott, S.; Orfanidi, A.; Schmies, H.; Anke, B.; Nong, H.N.; Hubner, J.; Gernert, U.; Gliech, M.; Lerch, M.; Strasser, P. Ionomer distribution control in porous carbon-supported catalyst layers for high-power and low Pt-loaded proton exchange membrane fuel cells. Nat. Mater. 2020, 19, 77–85.
- Cheng, H.; Gui, R.; Yu, H.; Wang, C.; Liu, S.; Liu, H.; Zhou, T.; Zhang, N.; Zheng, X.; Chu, W.; et al. Subsize Pt-based intermetallic compound enables long-term cyclic mass activity for fuel-cell oxygen reduction. Proc. Natl. Acad. Sci. USA 2021, 118, e2104026118.
- Yan, J.; Ye, F.; Dai, Q.; Ma, X.; Fang, Z.; Dai, L.; Hu, C. Recent progress in carbon-based electrochemical catalysts: From structure design to potential applications. Nano Res. Energy 2023, 2, e9120047.
- Yang, Y.; Tang, M.; Zhang, Z.; Huang, S.; Wu, S. Long-range ordered porous carbon: A new carbon constructed by connecting C60 cages. Nano Res. Energy 2023, 2, e9120065.
- Chong, L.; Wen, J.; Kubal, J.; Sen, F.G.; Zou, J.; Greeley, J.; Chan, M.; Barkholtz, H.; Ding, W.; Liu, D.J. Ultralow-loading platinum-cobalt fuel cell catalysts derived from imidazolate frameworks. Science 2018, 362, 1276–1281.
- Wang, Y.-J.; Fang, B.; Li, H.; Bi, X.T.; Wang, H. Progress in modified carbon support materials for Pt and Pt-alloy cathode catalysts in polymer electrolyte membrane fuel cells. Prog. Mater. Sci. 2016, 82, 445–498.
- Zaman, S.; Su, Y.Q.; Dong, C.L.; Qi, R.; Huang, L.; Qin, Y.; Huang, Y.C.; Li, F.M.; You, B.; Guo, W.; et al. Scalable molten salt synthesis of platinum alloys planted in metal-nitrogen-graphene for efficient oxygen reduction. Angew. Chem. Int. Ed. 2022, 61, 202115835–202115843.
- Wei, X.; Wang, R.-Z.; Zhao, W.; Chen, G.; Chai, M.-R.; Zhang, L.; Zhang, J. Recent research progress in PEM fuel cell electrocatalyst degradation and mitigation strategies. EnergyChem 2021, 3, 100061.
- Wood, D.L.; Borup, R.L. Estimation of Mass-Transport Overpotentials during Long-Term PEMFC Operation. J. Electrochem. Soc. 2010, 157, B1251.
- Wu, J.; Yuan, X.Z.; Martin, J.J.; Wang, H.; Zhang, J.; Shen, J.; Wu, S.; Merida, W. A review of PEM fuel cell durability: Degradation mechanisms and mitigation strategies. J. Power Sources 2008, 184, 104–119.
- Zaman, S.; Chen, S. A perspective on inaccurate measurements in oxygen reduction and carbon dioxide reduction reactions. J. Catal. 2023, 421, 221–227.
- Cullen, D.A.; Neyerlin, K.C.; Ahluwalia, R.K.; Mukundan, R.; More, K.L.; Borup, R.L.; Weber, A.Z.; Myers, D.J.; Kusoglu, A. New roads and challenges for fuel cells in heavy-duty transportation. Nat. Energy 2021, 6, 462–474.
- Chung, H.T.; Wu, G.; Li, Q.; Zelenay, P. Role of two carbon phases in oxygen reduction reaction on the Co–PPy–C catalyst. Int. J. Hydrogen Energy 2014, 39, 15887–15893.
- Kongkanand, A.; Mathias, M.F. The Priority and Challenge of High-Power Performance of Low-Platinum Proton-Exchange Membrane Fuel Cells. J. Phys. Chem. Lett. 2016, 7, 1127–1137.
- Ramaswamy, N.; Gu, W.; Ziegelbauer, J.M.; Kumaraguru, S. Carbon support microstructure impact on high current density transport resistances in PEMFC cathode. J. Electrochem. Soc. 2020, 167, 064515–064527.
- Yarlagadda, V.; Carpenter, M.K.; Moylan, T.E.; Kukreja, R.S.; Koestner, R.; Gu, W.; Thompson, L.; Kongkanand, A. Boosting fuel cell performance with accessible carbon mesopores. ACS Energy Lett. 2018, 3, 618–621.
- Hu, Y.; Guo, X.; Shen, T.; Zhu, Y.; Wang, D. Hollow Porous Carbon-Confined Atomically Ordered PtCo3 Intermetallics for an Efficient Oxygen Reduction Reaction. ACS Catal. 2022, 12, 5380–5387.
- Banham, D.; Feng, F.; Fürstenhaupt, T.; Pei, K.; Ye, S.; Birss, V. Novel Mesoporous Carbon Supports for PEMFC Catalysts. Catalysts 2015, 5, 1046–1067.
- Kamitaka, Y.; Takeshita, T.; Morimoto, Y. MgO-Templated Mesoporous Carbon as a Catalyst Support for Polymer Electrolyte Fuel Cells. Catalysts 2018, 8, 230.
- Li, X.; Forouzandeh, F.; Fürstenhaupt, T.; Banham, D.; Feng, F.; Ye, S.; Kwok, D.Y.; Birss, V. New insights into the surface properties of hard-templated ordered mesoporous carbons. Carbon 2018, 127, 707–717.
- Inagaki, M.; Toyoda, M.; Soneda, Y.; Tsujimura, S.; Morishita, T. Templated mesoporous carbons: Synthesis and applications. Carbon 2016, 107, 448–473.
More
Information
Subjects:
Electrochemistry
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
2 times
(View History)
Update Date:
14 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




