Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sabri Saeed Sanabani | -- | 2227 | 2024-03-13 13:57:22 | | | |
| 2 | Rita Xu | Meta information modification | 2227 | 2024-03-14 02:46:56 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Clissa, P.B.; Della-Casa, M.S.; Zychar, B.C.; Sanabani, S.S. Snake Venom Disintegrins in Angiogenesis. Encyclopedia. Available online: https://encyclopedia.pub/entry/56213 (accessed on 07 February 2026).
Clissa PB, Della-Casa MS, Zychar BC, Sanabani SS. Snake Venom Disintegrins in Angiogenesis. Encyclopedia. Available at: https://encyclopedia.pub/entry/56213. Accessed February 07, 2026.
Clissa, Patricia Bianca, Maisa Splendore Della-Casa, Bianca Cestari Zychar, Sabri Saeed Sanabani. "Snake Venom Disintegrins in Angiogenesis" Encyclopedia, https://encyclopedia.pub/entry/56213 (accessed February 07, 2026).
Clissa, P.B., Della-Casa, M.S., Zychar, B.C., & Sanabani, S.S. (2024, March 13). Snake Venom Disintegrins in Angiogenesis. In Encyclopedia. https://encyclopedia.pub/entry/56213
Clissa, Patricia Bianca, et al. "Snake Venom Disintegrins in Angiogenesis." Encyclopedia. Web. 13 March, 2024.
Copy Citation
Angiogenesis, the formation of new blood vessels, plays a critical role in various physiological and pathological conditions. Snake venom disintegrins (SVDs) have been identified as significant regulators of this process.
snake venom
angiogenesis
integrins
1. Introduction
1.1. Angiogenesis
Angiogenesis is a complex biological process involving the formation of new blood vessels from preexisting vessels [1]. It plays a crucial role in various physiological and pathological conditions, including embryogenesis, wound healing, and tumor growth [2]. The process of angiogenesis is tightly regulated by multiple signaling pathways and factors. Matrix metalloproteinases are primarily responsible for degrading the basement membrane surrounding existing blood vessels, allowing endothelial cells to migrate and proliferate toward the angiogenic stimulus [3]. One of the key proangiogenic factors is vascular endothelial growth factor (VEGF), which is essential for the formation of new blood vessels during embryonic development and is produced by various cell types, including tumors [4]. VEGF binds to specific receptors on endothelial cells, promoting their survival, migration, and differentiation [5][6]. Other factors involved in angiogenesis regulation include fibroblast growth factors (FGFs), platelet-derived growth factor (PDGF), and angiopoietins [3]. These factors act synergistically to ensure the proper formation and remodeling of blood vessels. The regulation of angiogenesis is maintained through a dynamic balance between proangiogenic and antiangiogenic factors. This balance can be disrupted in various diseases, leading to either excessive or insufficient blood vessel formation [1]. Insufficient angiogenesis, on the other hand, can lead to tissue ischemia, impaired wound healing, and various cardiovascular diseases [2]. Peripheral artery disease (PAD) is an example where the narrowing or blockage of blood vessels reduces blood flow to the legs or arms, resulting in pain, skin ulcers, and an increased risk of amputation [2]. Therapeutic angiogenesis, which aims to promote blood vessel formation, has been investigated as a potential treatment approach for PAD, with the administration of VEGF or the use of gene therapy [1].
It is important to emphasize that integrins, a group of transmembrane proteins that play a crucial role in cell adhesion and communication between cells and the extracellular environment, are the focus of interest, as they play an important role in angiogenesis [7].
1.2. Integrins
Integrins play an essential role in the regulation of biological processes such as cell migration, adhesion, proliferation, differentiation and signaling [8]. Integrins are heterodimeric glycoproteins consisting of alpha and beta subunits, which together form a complex transmembrane receptor [9]. A total of 18 alpha subunits and eight beta subunits have been identified in mammalian cells, allowing the formation of 24 different heterodimers. The α subunit determines affinity to the extracellular matrix component (ECM), while the β subunit associates with cytoplasmic structural and regulatory proteins. These subunits have a long transmembrane domain and a short cytoplasmic domain associated with cytoskeletal proteins [10]. Although integrins are constitutively expressed on the cell surface, they need to be activated to interact with their ligands [11]. Such activation can occur in the presence of chemokines and cytokines and is characterized by a conformational change at the extracellular integrin domain that exposes their binding sites on the α and β subunits, allowing them to interact with their ligands on the ECM or with proteins on the membranes of neighboring cells. This interaction is primarily controlled by a conserved tripeptide pattern of arginine, glycine, and aspartate, commonly known as the Arg-Gly-Asp motif (RGD) [12][13][14]. They are crucial for cell adhesion, migration, and signal transduction by mediating interactions between cells and ECM proteins. The main role of these molecules is to provide a link between the cytoskeleton of the cell and certain ECM components, such as fibronectin, vitronectin, laminin, and collagen. In addition, they are responsible for triggering intracellular signal transduction pathways upon interaction with the ECM. Integrins can undergo conformational changes that influence their ligand binding properties and downstream signaling events [15]. They possess a unique arrangement of cysteine residues that enables them to adopt a compact and stable structure consisting of a well-defined loop disulfide array [16]. This structural motif is critical for their high-affinity binding to integrins as it allows them to bind to specific integrin subunits and block their ligand binding sites. In addition, integrins also interact with growth factor receptors to regulate cell migration, blood vessel development, and angiogenesis. Importantly, the understanding of the mechanism of action of integrins paralleled the discovery of proteins from snake venoms, called disintegrins, which act as potent inhibitors of platelet aggregation and integrin receptor-dependent cell adhesion [17][18].
1.3. Snake Venom Metalloproteinases and Snake Venom Disintegrins
Snake venom metalloproteinases (SVMPs) are a major component of most crotalid and viperid venoms [19]. SVMPs are known to be a class of key toxins involved in the pathophysiology associated with viperid venoms and are classified into classes and subclasses from P-I to P-III according to the organization of their domains, as shown in Figure 1 [20]. In general, class PI includes metalloproteinases that have only a catalytic domain containing zinc; class PII, metalloproteinases that have a catalytic domain followed by a disintegrin domain containing the tripeptide RGD (arginine-glycine-aspartic acid); and class PIII, metalloproteinases that have a catalytic domain, a disintegrin-like domain, and a cysteine-rich domain [21].
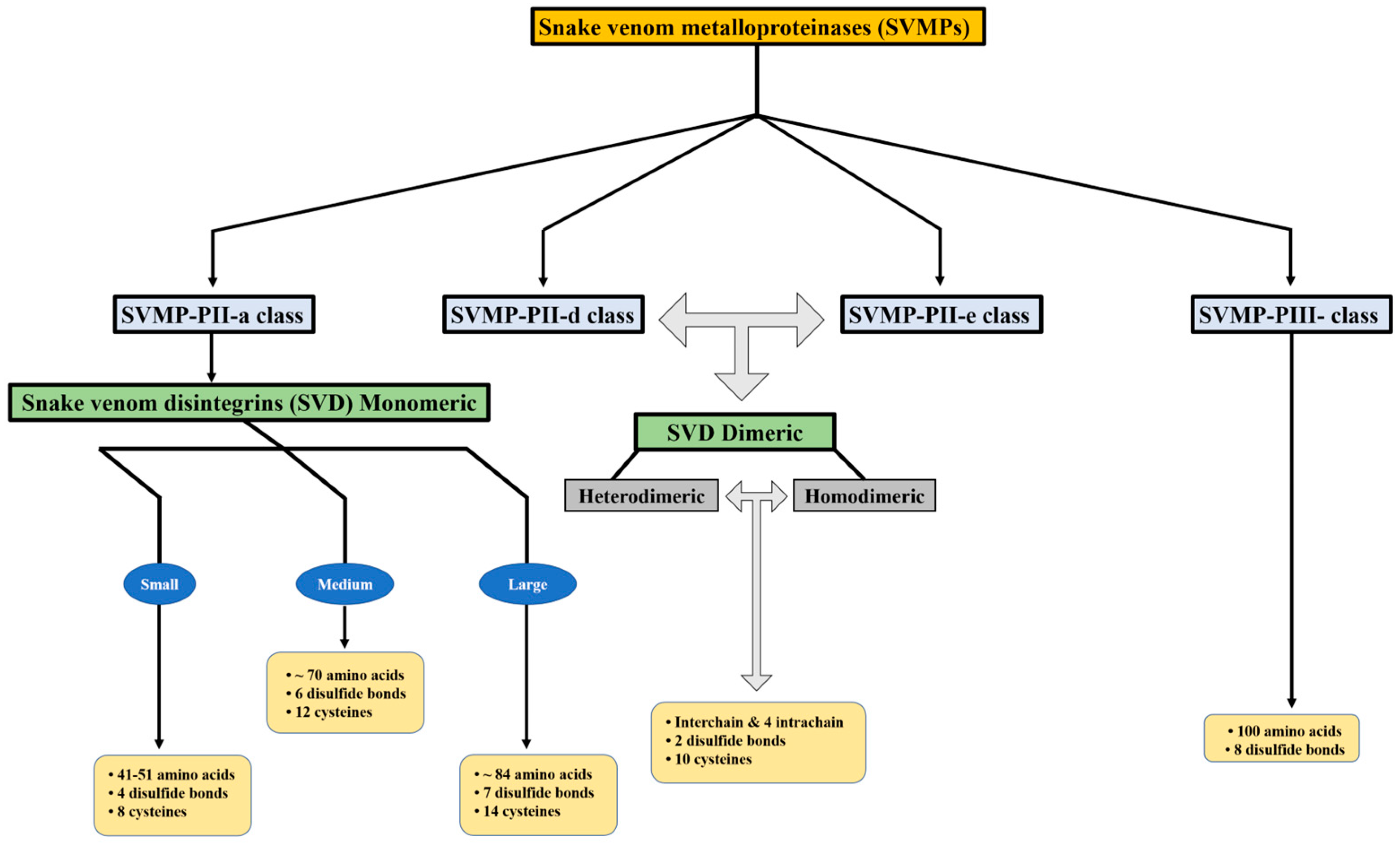
Figure 1. Disintegrins are categorized according to their structural composition. The number of disulfide bonds and length of the polypeptide chain determine this categorization.
Snake venom disintegrins (SVDs) are a class of proteins derived from SVMPs. The disintegrins present in snake venoms can be formed in the venom gland in two distinct ways: (1) By proteolysis of SVMPs of class P-II, where cleavage occurs between the catalytic domain and the disintegrin domain, leaving only the disintegrin domain. These disintegrins are known as RGD-dependent disintegrins, which are abundant in viperid venoms and contain the sequence XGD (X-Gly-Asp), MLD (Met-Leu-Asp), or K/RTS (Lys/Arg-Thr-Ser) on the exposed surface of the loop that specifically binds to integrins on the surface of different cell types [22][23]. The amino acid sequences immediately adjacent to the RGD site of disintegrins could form an extended RGD locus that, in conjunction with the conformational representation of the RGD sequence, could be involved in determining integrin selectivity and affinity [13]. The family of disintegrins containing the RGD motif is widely recognized as the most extensive and well-studied group. Most of these units function as monomers (small, medium, or large), but a subgroup has the ability to combine into dimers and form homo- or heterodimers [24]. (2) Proteolysis of class P-III SVMPs results in fragments that covalently link the disintegrin-like and cysteine-rich domains and are referred to as ECD-disintegrin-like/cysteine-rich domains (Figure 2). This disintegrin-like domain has a sequence of non-RGD tripeptides in its binding site [21][25].
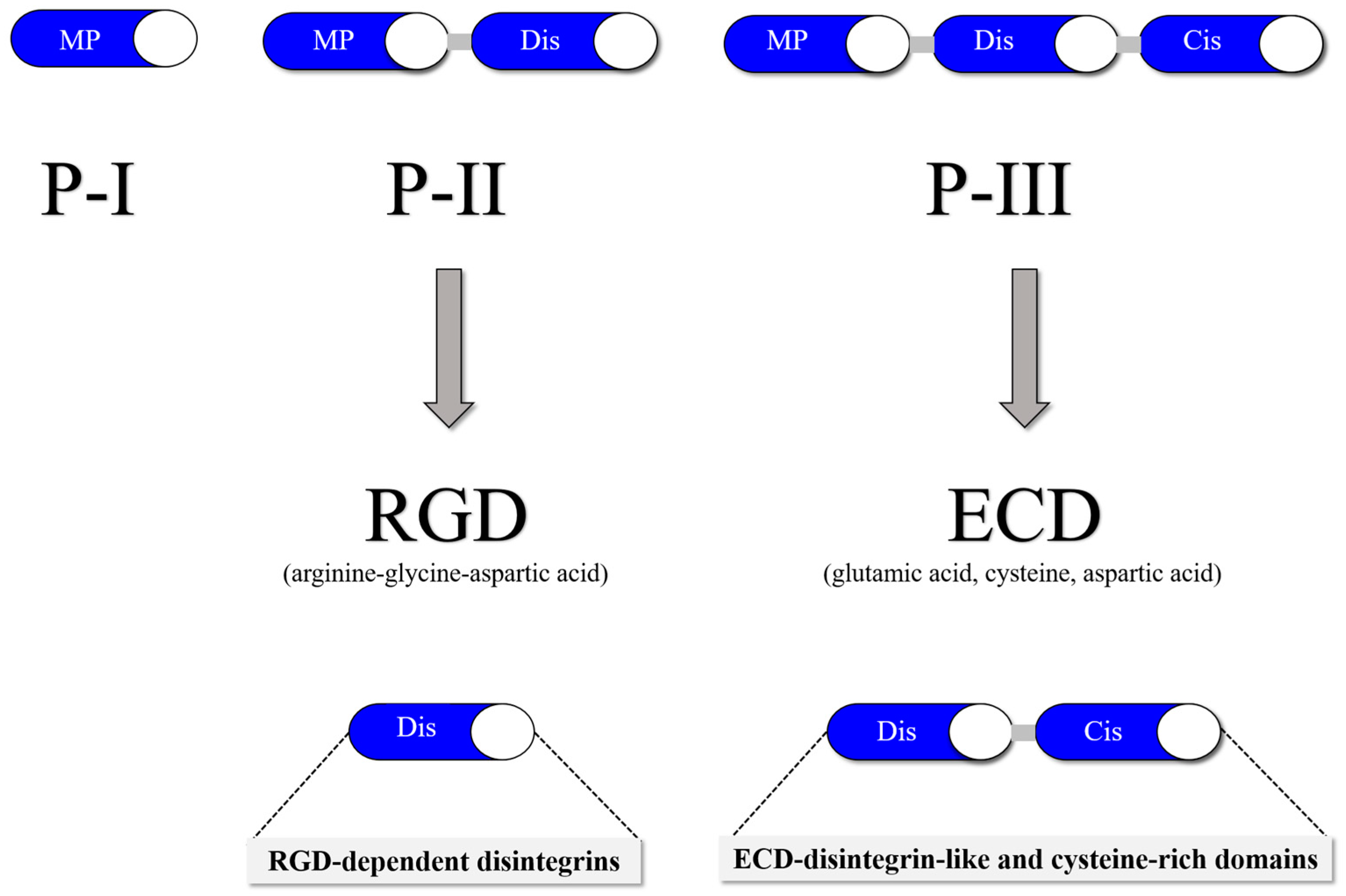
Figure 2. SVMP classification is divided into three classes: P-I (20–30 kDa), which in its mature form contains a metalloproteinase domain; P-II (30–60 kDa), which contains a disintegrin domain linked to the C-terminus of the metalloproteinase domain; and P-III (60–100 kDa), which consists of a metalloproteinase domain, a disintegrin-like domain and a cysteine-rich domain.
2. Utilization of SVD as Pro-Angiogenic Agents
While disintegrins are usually known for their antiangiogenic properties, recent research has also revealed their potential as proangiogenic agents. Alternagin-C (ALT-C) is an ECD-containing disintegrin-like/cysteine-rich disintegrin isolated from the venom of the snake Rhinocerophis alternatus that induces endothelial cell proliferation and angiogenesis both in vitro and in vivo by upregulating the expression of VEGF and its receptors [26]. ALT-C binds to the major collagen receptor α2β1 integrin, inhibiting cell adhesion to collagen, triggering downstream signaling molecules, and inducing a significant increase in several genes related to cell cycle control (VEGF, inducible early growth response, interleukin 11, early growth response 2 and 3, and the insulin-induced gene) [27]. ALT-C also induced significant cytoskeleton dynamic changes with the polymerization of F-actin, focal adhesion kinase (FAK), and phosphoinositol 3-kinase (PI3K) activation, as well as erk-2 translocation [28]. ALT-C induced the formation of new vessels, and the expression of VEGF in the injured tissue indicated the usefulness and effectiveness of ALT-C as a proangiogenic disintegrin-like protein [29].
2.1. Tissue Regeneration and Wound Healing
Wound healing and regeneration are multifaceted biological phenomena that occur throughout the human lifespan. After an injury, various cellular processes are immediately initiated and coordinated to initiate a response [30]. Improper repair procedures can cause this process to be delayed, with immediate consequences for the individual, including physical discomfort, impaired rehabilitation progress, limb amputation, and in the most severe cases, death from septicemia [31]. Like other natural healing processes, the repair mechanism also relies on the coordination of different cell activities vital for recovery. These activities encompass cell survival, growth, movement, and the creation of new cells. They are orchestrated through cellular interactions with both the extracellular matrix (ECM) that surrounds them and with other neighboring cells. These interactions are made possible by specialized receptors found on cell membranes, which belong to the integrin family [32]. A recent study led by Ferreira and colleagues [33] used an in vivo model with subcutaneous sponge implants to investigate the potential of jararhagin-C (Jar-C). Their goal was to understand how Jar-C might stimulate collagen deposition and the production of important soluble substances, including VEGF and transforming growth factor beta-1 (TGFβ-1). These substances play important roles in processes such as angiogenesis and fibrogenesis, which are closely associated with tissue repair. The results of the study suggest that Jar-C may have positive effects on tissue repair by promoting these natural responses in the body. Moreover, Alternagin-C is also the subject of research. Within 7 days, this cysteine-rich, disintegrin-like protein accelerates wound healing in rats by increasing type I collagen deposition and fibroblast density and reducing inflammation [34]. In another study, Rabelo et al. [35] revealed that ALT-C increased collagen synthesis in mouse fibrovascular tissue. From the above results, it can be concluded that SVD has the ability to promote angiogenesis by modulating endothelial cell behavior, facilitating cell migration, and upregulating proangiogenic factors.
2.2. Cardiovascular Diseases
Integrins have a considerable influence on the development of cardiac fibrosis. The diseased heart exhibits altered expression and integrin functions [36]. Targeting integrins and their associated proteins can be a potential therapeutic target for myocardial fibrosis. Certain disintegrins have been extensively researched and subsequently approved by the Food and Drug Administration (FDA), making them viable pharmaceutical agents in modern medicine. Tirofiban, marketed as Aggrastat®, is a synthetic pharmaceutical agent developed by Medicure International, Inc. of Winnipeg, MB, Canada. This drug is derived from the RGD domain found in Echistatin [37]. In addition, this compound undergoes a chemical change that increases its affinity for platelet glycoproteins, especially GPIIb/IIIa receptors [38]. Therefore, this drug is able to prevent platelet aggregation and other thrombotic activities by competing with fibrinogen for the RGD domain recognition site in the GPIIb/IIIa receptor [37][39][40]. In 1998, the Food and Drug Administration (FDA) approved tirofiban as a therapeutic intervention for acute coronary syndrome [41]. Additionally, in 1998, the FDA approved eptifibatide (Integrilin®, Millennium Pharmaceuticals, Inc., Cambridge, MA, USA), an alternative molecule to inhibit platelet aggregation. Schering-Plough subsequently acquired license rights to this drug in 2005 [42]. The development of this active substance took place in parallel with research into synthetic peptide analogs of barbourin, a disintegrin from Sistrurus miliarius barbouri [43].
Cardiovascular diseases, including acute myocardial infarction, coronary artery disease, endothelial dysfunction, and chronic ischemia, are considered one of the leading causes of death worldwide [44]. Therefore, there is great interest in pharmaceutical agents that offer a new and effective therapeutic approach to improve the functionality of the myocardium and/or facilitate its regeneration after injury. Platelets have been studied extensively because of their crucial role in primary hemostasis, the body’s initial response to arterial injury. Only recently, however, have researchers begun to study their contribution to immunological processes, cardiovascular disease, cancer, and various other pathological conditions [45][46][47]. The glycoprotein receptor GPIIbIIIa has been extensively studied as a primary receptor on platelets for the functional effects of snake venom-derived disintegrins. Each individual platelet is equipped with approximately 80,000 GPIIbIIIa receptors located both on the plasma membrane and in the α-granules [48]. After activation, the number of these receptors increases significantly, facilitating the formation of a permanent hemostatic plug [49]. Certain SVDs, including those of Agkistrodon piscivorus piscivorus and Echis carinatus sochureki, namely, Applagin and Echistatin, show remarkable affinity for the RGD motif on resting platelets. This specific binding results in the potent inhibition of platelet aggregation. Consequently, SVDs have emerged as promising candidates for drug development to antagonize platelet integrins, demonstrating their pharmacological potential as both platelet aggregation inhibitors and antithrombotic agents. From a clinical perspective, these drugs can effectively reduce the likelihood of acute ischemic episodes and serve as preventive measures against thrombotic sequelae. RGD disintegrins have been extensively studied and are considered the largest family within this category [17][50]. A number of disintegrins have been extracted from snake venoms, particularly viper venom, and characterized as agents with antithrombotic properties [51]. For example, trigramin inhibits platelet aggregation in platelet-rich plasma triggered by adenosine diphosphate (ADP), collagen, or epinephrine [52]. The same disintegrin has been shown to inhibit platelet aggregation both in vitro and in vivo by preventing the binding of fibrinogen to platelets induced by agonists of aggregation, such as ADP-activated platelets [53].
By promoting angiogenesis, SVDs have shown promise in preclinical models of cardiac ischemia. Previous studies have shown that administration of ALT-C in a single dose after a period of 7–9 days resulted in an increase in cardiac muscle contractile force in fish [29][54]. This intervention also led to an upregulation of the expression of important proteins involved in calcium processing and an increase in the level of VEGF in the myocardium. In addition, administration of ALT-C stimulated angiogenesis and thus protected cardiomyocytes from the deleterious effects of negative tropism caused by hypoxia/reoxygenation. Evaluation of the safety and efficacy of disintegrins in promoting angiogenesis and improving cardiac function in patients with cardiovascular disease requires the conduct of clinical trials.
References
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases. Nature 2000, 407, 249–257.
- Folkman, J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1995, 1, 27–31.
- Potente, M.; Gerhardt, H.; Carmeliet, P. Basic and therapeutic aspects of angiogenesis. Cell 2011, 146, 873–887.
- Matsumoto, T.; Claesson-Welsh, L. VEGF receptor signal transduction. Sci. STKE 2001, 2001, re21.
- Zhang, F.; Tang, Z.; Hou, X.; Lennartsson, J.; Li, Y.; Koch, A.W.; Scotney, P.; Lee, C.; Arjunan, P.; Dong, L.; et al. VEGF-B is dispensable for blood vessel growth but critical for their survival, and VEGF-B targeting inhibits pathological angiogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 6152–6157.
- Dvorak, H.F. Vascular permeability factor/vascular endothelial growth factor: A critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J. Clin. Oncol. 2002, 20, 4368–4380.
- Swenson, S.; Ramu, S.; Markland, F.S. Anti-angiogenesis and RGD-containing snake venom disintegrins. Curr. Pharm. Des. 2007, 13, 2860–2871.
- Usami, Y.; Fujimura, Y.; Miura, S.; Shima, H.; Yoshida, E.; Yoshioka, A.; Hirano, K.; Suzuki, M.; Titani, K. A 28 kDa-protein with disintegrin-like structure (jararhagin-C) purified from Bothrops jararaca venom inhibits collagen- and ADP-induced platelet aggregation. Biochem. Biophys. Res. Commun. 1994, 201, 331–339.
- Takada, Y.; Ye, X.; Simon, S. The integrins. Genome Biol. 2007, 8, 215.
- Rose, D.M.; Alon, R.; Ginsberg, M.H. Integrin modulation and signaling in leukocyte adhesion and migration. Immunol. Rev. 2007, 218, 126–134.
- Hynes, R.O. Integrins: Bidirectional, allosteric signaling machines. Cell 2002, 110, 673–687.
- Ruoslahti, E.; Pierschbacher, M.D. New perspectives in cell adhesion: RGD and integrins. Science 1987, 238, 491–497.
- Lu, X.; Lu, D.; Scully, M.F.; Kakkar, V.V. Integrins in drug targeting-RGD templates in toxins. Curr. Pharm. Des. 2006, 12, 2749–2769.
- Marcinkiewicz, C.; Vijay-Kumar, S.; McLane, M.A.; Niewiarowski, S. Significance of RGD loop and C-terminal domain of echistatin for recognition of αIIbβ3 and αvβ3 integrins and expression of ligand-induced binding site. Blood 1997, 90, 1565–1575.
- Kamata, T.; Handa, M.; Sato, Y.; Ikeda, Y.; Aiso, S. Membrane-proximal α/βstalk interactions differentially regulate integrin activation. J. Biol. Chem. 2005, 280, 24775–24783.
- Calvete, J.J. Snake venomics: From the inventory of toxins to biology. Toxicon 2013, 75, 44–62.
- Arruda Macedo, J.K.; Fox, J.W.; de Souza Castro, M. Disintegrins from snake venoms and their applications in cancer research and therapy. Curr. Protein Pept. Sci. 2015, 16, 532–548.
- Marcinkiewicz, C. Applications of snake venom components to modulate integrin activities in cell-matrix interactions. Int. J. Biochem. Cell Biol. 2013, 45, 1974–1986.
- Tasoulis, T.; Isbister, G.K. A Review and Database of Snake Venom Proteomes. Toxins 2017, 9, 290.
- Fox, J.W.; Serrano, S.M. Timeline of key events in snake venom metalloproteinase research. J. Proteom. 2009, 72, 200–209.
- Fox, J.W.; Serrano, S.M. Insights into and speculations about snake venom metalloproteinase (SVMP) synthesis, folding and disulfide bond formation and their contribution to venom complexity. FEBS J. 2008, 275, 3016–3030.
- Calvete, J.J.; Marcinkiewicz, C.; Monleon, D.; Esteve, V.; Celda, B.; Juarez, P.; Sanz, L. Snake venom disintegrins: Evolution of structure and function. Toxicon 2005, 45, 1063–1074.
- Schonthal, A.H.; Swenson, S.D.; Chen, T.C.; Markland, F.S. Preclinical studies of a novel snake venom-derived recombinant disintegrin with antitumor activity: A review. Biochem. Pharmacol. 2020, 181, 114149.
- Calvete, J.J.; Moreno-Murciano, M.P.; Theakston, R.D.; Kisiel, D.G.; Marcinkiewicz, C. Snake venom disintegrins: Novel dimeric disintegrins and structural diversification by disulphide bond engineering. Biochem. J. 2003, 372, 725–734.
- Marcinkiewicz, C. Functional characteristic of snake venom disintegrins: Potential therapeutic implication. Curr. Pharm. Des. 2005, 11, 815–827.
- Ramos, O.H.; Terruggi, C.H.; Ribeiro, J.U.; Cominetti, M.R.; Figueiredo, C.C.; Berard, M.; Crepin, M.; Morandi, V.; Selistre-de-Araujo, H.S. Modulation of in vitro and in vivo angiogenesis by alternagin-C, a disintegrin-like protein from Bothrops alternatus snake venom and by a peptide derived from its sequence. Arch. Biochem. Biophys. 2007, 461, 1–6.
- Selistre-de-Araujo, H.S.; Cominetti, M.R.; Terruggi, C.H.; Mariano-Oliveira, A.; De Freitas, M.S.; Crepin, M.; Figueiredo, C.C.; Morandi, V. Alternagin-C, a disintegrin-like protein from the venom of Bothrops alternatus, modulates α2β1 integrin-mediated cell adhesion, migration and proliferation. Braz. J. Med. Biol. Res. 2005, 38, 1505–1511.
- Selistre-de-Araujo, H.S.; Pontes, C.L.; Montenegro, C.F.; Martin, A.C. Snake venom disintegrins and cell migration. Toxins 2010, 2, 2606–2621.
- Monteiro, D.A.; Kalinin, A.L.; Selistre-de-Araujo, H.S.; Nogueira, L.A.N.; Beletti, M.E.; Fernandes, M.N.; Rantin, F.T. Cardioprotective effects of alternagin-C (ALT-C), a disintegrin-like protein from Rhinocerophis alternatus snake venom, on hypoxia-reoxygenation-induced injury in fish. Comp. Biochem. Physiology. Toxicol. Pharmacol. 2019, 215, 67–75.
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321.
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in Chronic Wounds. Int. J. Mol. Sci. 2016, 17, 2085.
- Schnittert, J.; Bansal, R.; Storm, G.; Prakash, J. Integrins in wound healing, fibrosis and tumor stroma: High potential targets for therapeutics and drug delivery. Adv. Drug Deliv. Rev. 2018, 129, 37–53.
- Ferreira, B.A.; Deconte, S.R.; de Moura, F.B.R.; Tomiosso, T.C.; Clissa, P.B.; Andrade, S.P.; Araujo, F.A. Inflammation, angiogenesis and fibrogenesis are differentially modulated by distinct domains of the snake venom metalloproteinase jararhagin. Int. J. Biol. Macromol. 2018, 119, 1179–1187.
- Sant’Ana, E.M.; Gouvea, C.M.; Durigan, J.L.; Cominetti, M.R.; Pimentel, E.R.; Selistre-de-Araujo, H.S. Rat skin wound healing induced by alternagin-C, a disintegrin-like, Cys-rich protein from Bothrops alternatus venom. Int. Wound J. 2011, 8, 245–252.
- Rabelo, L.F.G.; Ferreira, B.A.; Deconte, S.R.; Tomiosso, T.C.; Dos Santos, P.K.; Andrade, S.P.; Selistre de Araujo, H.S.; Araujo, F.A. Alternagin-C, a disintegrin-like protein from Bothrops alternatus venom, attenuates inflammation and angiogenesis and stimulates collagen deposition of sponge-induced fibrovascular tissue in mice. Int. J. Biol. Macromol. 2019, 140, 653–660.
- Turner, C.J.; Badu-Nkansah, K.; Crowley, D.; van der Flier, A.; Hynes, R.O. α5 and αv integrins cooperate to regulate vascular smooth muscle and neural crest functions in vivo. Development 2015, 142, 797–808.
- Topol, E.J.; Byzova, T.V.; Plow, E.F. Platelet GPIIb-IIIa blockers. Lancet 1999, 353, 227–231.
- Gan, Z.R.; Gould, R.J.; Jacobs, J.W.; Friedman, P.A.; Polokoff, M.A. Echistatin. A potent platelet aggregation inhibitor from the venom of the viper, Echis carinatus. J. Biol. Chem. 1988, 263, 19827–19832.
- Hartman, G.D.; Egbertson, M.S.; Halczenko, W.; Laswell, W.L.; Duggan, M.E.; Smith, R.L.; Naylor, A.M.; Manno, P.D.; Lynch, R.J.; Zhang, G.; et al. Non-peptide fibrinogen receptor antagonists. 1. Discovery and design of exosite inhibitors. J. Med. Chem. 1992, 35, 4640–4642.
- Almeida, G.O.; de Oliveira, I.S.; Arantes, E.C.; Sampaio, S.V. Snake venom disintegrins update: Insights about new findings. J. Venom. Anim. Toxins Incl. Trop. Dis. 2023, 29, e20230039.
- Lang, S.H.; Manning, N.; Armstrong, N.; Misso, K.; Allen, A.; Di Nisio, M.; Kleijnen, J. Treatment with tirofiban for acute coronary syndrome (ACS): A systematic review and network analysis. Curr. Med. Res. Opin. 2012, 28, 351–370.
- Bordon, K.C.F.; Cologna, C.T.; Fornari-Baldo, E.C.; Pinheiro-Junior, E.L.; Cerni, F.A.; Amorim, F.G.; Anjolette, F.A.P.; Cordeiro, F.A.; Wiezel, G.A.; Cardoso, I.A.; et al. From Animal Poisons and Venoms to Medicines: Achievements, Challenges and Perspectives in Drug Discovery. Front. Pharmacol. 2020, 11, 1132.
- Scarborough, R.M.; Rose, J.W.; Hsu, M.A.; Phillips, D.R.; Fried, V.A.; Campbell, A.M.; Nannizzi, L.; Charo, I.F. Barbourin. A GPIIb-IIIa-specific integrin antagonist from the venom of Sistrurus m. barbouri. J. Biol. Chem. 1991, 266, 9359–9362.
- Roth, G.A.; Huffman, M.D.; Moran, A.E.; Feigin, V.; Mensah, G.A.; Naghavi, M.; Murray, C.J. Global and regional patterns in cardiovascular mortality from 1990 to 2013. Circulation 2015, 132, 1667–1678.
- Podrez, E.A.; Byzova, T.V.; Febbraio, M.; Salomon, R.G.; Ma, Y.; Valiyaveettil, M.; Poliakov, E.; Sun, M.; Finton, P.J.; Curtis, B.R.; et al. Platelet CD36 links hyperlipidemia, oxidant stress and a prothrombotic phenotype. Nat. Med. 2007, 13, 1086–1095.
- Venturini, W.; Olate-Briones, A.; Valenzuela, C.; Mendez, D.; Fuentes, E.; Cayo, A.; Mancilla, D.; Segovia, R.; Brown, N.E.; Moore-Carrasco, R. Platelet Activation Is Triggered by Factors Secreted by Senescent Endothelial HMEC-1 Cells In Vitro. Int. J. Mol. Sci. 2020, 21, 3287.
- Huilcaman, R.; Venturini, W.; Fuenzalida, L.; Cayo, A.; Segovia, R.; Valenzuela, C.; Brown, N.; Moore-Carrasco, R. Platelets, a Key Cell in Inflammation and Atherosclerosis Progression. Cells 2022, 11, 1014.
- Bledzka, K.; Smyth, S.S.; Plow, E.F. Integrin αIIbβ3: From discovery to efficacious therapeutic target. Circ. Res. 2013, 112, 1189–1200.
- Kolvekar, N.; Bhattacharya, N.; Sarkar, A.; Chakrabarty, D. How snake venom disintegrins affect platelet aggregation and cancer proliferation. Toxicon 2023, 221, 106982.
- Rivas-Mercado, E.A.; Garza-Ocañas, L. Disintegrins obtained from snake venom and their pharmacological potential. Med. Univ. 2017, 19, 32–37.
- Canas, C.A.; Castano-Valencia, S.; Castro-Herrera, F.; Canas, F.; Tobon, G.J. Biomedical applications of snake venom: From basic science to autoimmunity and rheumatology. J. Transl. Autoimmun. 2021, 4, 100076.
- Huang, T.F.; Ouyang, C. Action mechanism of the potent platelet aggregation inhibitor from Trimeresurus gramineus snake venom. Thromb. Res. 1984, 33, 125–138.
- Cook, J.J.; Huang, T.F.; Rucinski, B.; Strzyzewski, M.; Tuma, R.F.; Williams, J.A.; Niewiarowski, S. Inhibition of platelet hemostatic plug formation by trigramin, a novel RGD-peptide. Am. J. Physiol. 1989, 256, H1038–H1043.
- Monteiro, D.A.; Kalinin, A.L.; Selistre-de-Araujo, H.S.; Vasconcelos, E.S.; Rantin, F.T. Alternagin-C (ALT-C), a disintegrin-like protein from Rhinocerophis alternatus snake venom promotes positive inotropism and chronotropism in fish heart. Toxicon 2016, 110, 1–11.
More
Information
Subjects:
Medicine, Research & Experimental
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
505
Revisions:
2 times
(View History)
Update Date:
14 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




