Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marcelino Pérez-Bermejo | -- | 1792 | 2024-03-13 13:39:02 | | | |
| 2 | Wendy Huang | Meta information modification | 1792 | 2024-03-14 05:36:43 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ventura, I.; Meira-Blanco, G.C.; Legidos-García, M.E.; Pérez-Bermejo, M.; Murillo-Llorente, M.T. Immune Bases of Juvenile Idiopathic Arthritis. Encyclopedia. Available online: https://encyclopedia.pub/entry/56212 (accessed on 07 February 2026).
Ventura I, Meira-Blanco GC, Legidos-García ME, Pérez-Bermejo M, Murillo-Llorente MT. Immune Bases of Juvenile Idiopathic Arthritis. Encyclopedia. Available at: https://encyclopedia.pub/entry/56212. Accessed February 07, 2026.
Ventura, Ignacio, Gemma Clara Meira-Blanco, María Ester Legidos-García, Marcelino Pérez-Bermejo, María Teresa Murillo-Llorente. "Immune Bases of Juvenile Idiopathic Arthritis" Encyclopedia, https://encyclopedia.pub/entry/56212 (accessed February 07, 2026).
Ventura, I., Meira-Blanco, G.C., Legidos-García, M.E., Pérez-Bermejo, M., & Murillo-Llorente, M.T. (2024, March 13). Immune Bases of Juvenile Idiopathic Arthritis. In Encyclopedia. https://encyclopedia.pub/entry/56212
Ventura, Ignacio, et al. "Immune Bases of Juvenile Idiopathic Arthritis." Encyclopedia. Web. 13 March, 2024.
Copy Citation
Juvenile Idiopathic Arthritis (JIA) is an immune-mediated disease affecting children under sixteen for at least six weeks. It manifests with joint inflammation, stiffness, and restricted movement. Unlike adult rheumatoid arthritis, JIA is often outgrown, but it may impact bone development in those still growing. Juvenile Idiopathic Arthritis (JIA) is currently the most common chronic rheumatic disease in children. It is known to have no single identity, but a variety of diagnoses.
Juvenile Idiopathic Arthritis (JIA)
autoimmune disease
ANA
MHC
TNF-α
S100 proteins
IL
1. Introduction
The ILAR (International League of Associations for Rheumatology) classifies JIA into seven categories that often manifest with distinctive symptoms and help guide treatment. These are systemic juvenile arthritis, oligoarticular JIA, polyarticular JIA, psoriatic arthritis, enthesitis-related arthritis (ERA), and undifferentiated arthritis, the diagnosis of which requires the individual to have no condition or to meet criteria for more than one category [1][2][3][4].
JIA is currently the most common chronic rheumatological disorder in childhood. Females tend to be more affected, although no gender differences are found in systemic JIA and males are more affected in JIA enthesitis. Incidence and prevalence can vary, possibly attributed to under-diagnosis. The estimated overall incidence ranges from 1.6 to 23 per 100,000 children under sixteen years annually. The prevalence is approximately 3.8 to 400 per 100,000 [5]. Oligoarticular JIA is the most prevalent, followed by the polyarticular form, with the psoriatic type being the least common diagnosis. Efforts have been made to link environmental factors, including vaccination, breastfeeding, or trauma, to various JIA categories. However, limited correlation has been established between the environment and JIA, with genetic predisposition being a more significant factor [5]. Studies indicate a higher susceptibility for boys compared to girls, and the peak age for JIA development is reported to be 2–3 years [6]. In addition, other underlying risk factors for many diseases, such as prenatal maternal smoking, hospitalisation during the first year of life or day care during the first six years of life, have not been found to be associated with the development of JIA [7].
The treatment objective is not curative but aims to support children with JIA in sustaining a dignified, socially active, and physically engaged quality of life. This involves employing a combination of strategies to alleviate inflammation and pain while preserving joint movement and strength. Early initiation of treatment is essential to optimize individualized responses based on factors such as age, JIA type, onset, and severity, striving for the most effective outcomes [8].
As a first step, non-steroidal anti-inflammatory drugs are prescribed as a symptomatic treatment, inhibiting cyclooxygenase (COX) and interfering with prostaglandin synthesis [9]. The second step will be to add corticosteroids, which are anti-inflammatory drugs with immunosuppressive capacities, which may be the therapy of choice in severe cases with systemic effects [9][10]. The prescription of disease-modifying antirheumatic drugs is the third step in the treatment of JIA. The drug of choice is methotrexate, acting as a remission inducer and establishing the primary therapeutic axis in JIA patients. The fourth and final step on the therapeutic ladder involves biologic agents, which have specific actions against cells of the inflammatory response and are intended for hospital use only [11].
Currently, under-diagnosis of JIA is a barrier to treatment and research. The fact that it is a childhood disease is relevant when thinking about treatment, due to its characteristics to be considered [5]. The prognosis is not as optimistic as initially perceived. In numerous cases, patients do not achieve remission, and the disease persists for years, significantly impacting the quality of life of affected children. Effective treatment necessitates multidisciplinary collaboration to stabilise and enhance clinical manifestations [12]. Children are rarely affected by immune disorders, but when they are, early treatment can improve quality of life and prognosis and prevent irreversible damage in adulthood. Early diagnosis can therefore benefit patients with JIA, which in most cases goes undetected, leading to under-diagnosis, which can have a negative impact on children affected by the disease as they grow up. Understanding the immunopathogenesis of Juvenile Idiopathic Arthritis (JIA) necessitates a comprehensive examination of key immunological parameters. Antinuclear Antibodies (ANA) have been identified as potential markers, signifying their association with autoimmune responses in JIA. Concurrently, investigations into the Major Histocompatibility Complex (MHC) reveal its role in influencing the genetic predisposition to JIA. Tumour Necrosis Factor Alpha (TNF-α), an inflammatory mediator, is implicated in the pathogenesis of JIA, elucidating its involvement in the inflammatory cascade. Elevated levels of S100 proteins, correlated with inflammation, further emphasize the intricate immune response in JIA. Additionally, the exploration of various interleukins, including IL-1 and IL-6, underscores their potential significance in the disease process. In summary, the multifaceted nature of JIA is unravelled by scrutinizing these immunological parameters, offering valuable insights into the disease’s aetiology and paving the way for targeted therapeutic interventions.
2. ANA
ANAs are autologous immunoglobulins that target cells and cytoplasmic components linked to rheumatic diseases. Despite their heightened sensitivity, ANAs lack specificity for any particular rheumatic disease and are detected in roughly 100% of rheumatic patients and 3–15% of the healthy population. Due to this lack of specificity, ANAs are not a valuable diagnostic parameter but serve a limited role in confirming the presence of an autoimmune disease [13].
3. Major Histocompatibility Complex (MHC)
The human leukocyte antigen (HLA) system plays a crucial role in the immune system. Governed by genes on chromosome six, this system encodes surface molecules responsible for presenting antigenic peptides to T-cell receptors. It consists of two main classes: class I and class II. In MHC I, transmembrane glycoprotein molecules are located on the surface of nucleated cells. They comprise an alpha heavy chain linked to a beta2 microglobulin through two binding domains, with the heavy chain encoded by the HLA-A, HLA-B, and HLA-C genes. TCD8+ cells, often possessing a cytotoxic function, interact with this type of major histocompatibility complex and are capable of recognizing infected cells [13]. MHC II molecules are located on antigen-presenting cells, activated T cells, or cells induced by interferon gamma. These molecules consist of two polypeptide chains, alpha and beta, each with two domains: one peptide-binding and one Ig-like. These chains are encoded by genes such as HLA-DP, -DQ, or -DR. T cells responding to MHC II molecules typically express CD4 and function as helper cells. The HLA-B27 allele, found in MHC type I, is linked to numerous autoimmune diseases. In JIA, it may be present in patients with enthesitis-type JIA and is strongly associated with ankylosing spondylitis. Therefore, it is plausible that patients with this JIA subtype may develop spondylitis over time. However, HLA-B27 is also present in 5–15% of the general population, limiting its specificity as a diagnostic test [14].
4. Tumour Necrosis Factor (TNF-α)
Tumour necrosis factor (TNF) is an inflammatory cytokine produced by macrophages and monocytes during acute inflammation. It plays a crucial role in signalling events that lead to necrosis or apoptosis, making it significant in infection and cancer resistance. TNF-α primarily exerts its effects by binding to cell membrane receptors with a molecular weight of 55 kDa or 75 kDa. A distinctive feature of TNF is its extracellular domain, comprising 2–6 cysteine-rich repeats. Additionally, structurally related “decoy receptors” complementarily bind to TNF molecules, rescuing cells from apoptosis [15]. TNF-α is implicated in both the psoriatic and enthesitic forms of JIA, where an abnormal synthesis of inflammatory cytokines occurs in the synovial membrane. It plays a crucial role in initiating, maintaining, and destroying synovial tissue by expressing pro-inflammatory genes like IL-6, IL-1, and IL-18. These cytokines activate fibroblasts, leading to the synthesis of matrix metalloproteases that degrade cartilage, and osteoclasts, causing complete destruction of joint architecture. In systemic lupus erythematosus (SLE), TNF-α’s role remains controversial, yet it is associated with higher levels of autoantibodies and kidney damage. In type 1 diabetes mellitus (DM1), TNF-α acts cytotoxically in pancreatic islets, inducing β-cell apoptosis and inhibiting insulin secretion. Additionally, the presence of dendritic cells and macrophages in the early stages of diabetes has been linked to β-cell inflammation [15].
5. S100 Proteins
The S100 proteins, a family of twenty-four cytosolic calcium-binding proteins, are distributed across intracellular, extracellular, and regulatory domains. Apart from their roles in adaptive immunity, tissue development, and repair, S100 proteins play a crucial part in regulating proliferation, differentiation, Ca2+ homeostasis, and inflammation [16]. Specifically implicated in inflammation-mediated responses, these proteins are released into an acellular compartment during cellular stress or inflammation. They then bind to surface receptors, activating intracellular signalling pathways associated with cell migration, proliferation, apoptosis, or inflammation [17]. Activation of S100 proteins is observed in systemic JIA and LSE, making their determination valuable in differentiating other febrile and autoinflammatory syndromes.
6. Interleukins (IL)
Interleukins (ILs) are cytokines expressed not only by leukocytes, as initially believed, but also by various other cell types. Their significance lies in the differentiation and activation of immune cells, encompassing both pro-inflammatory and anti-inflammatory properties. Interleukins play pivotal roles in the activation of inflammatory or immune processes, exhibiting both paracrine and autocrine functions. Additionally, they frequently impact the synthesis and actions of other interleukins [18][19].
Figure 1 discusses the aetiopathogenesis and key molecules involved in JIA. It also indicates potentially interesting pathways to block, highlighted with a red circle. In the specific context of JIA, it is highlighted that the receptor for IL-17 is found on various cells of the immune system and that its activation by IL-17 promotes inflammation and the production of inflammatory cells, thus contributing to the development and progression of JIA. In the inflamed joints of children with JIA, S100 proteins secreted by inflammatory cells such as macrophages and neutrophils are deposited. These deposits contribute to cartilage loss and joint pain and are found in the articular cartilage, synovial membrane, and synovial fluid.
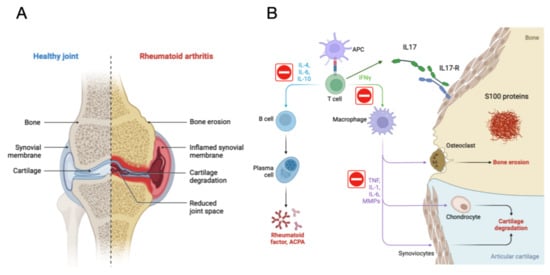
Figure 1. Aetiopathogenesis and Molecules Involved in JIA. Juvenile Idiopathic Arthritis (JIA) stands out as the most prevalent chronic arthritis in children, characterised by joint inflammation and pain. Key molecules in the pathogenesis of JIA are identified: Tumour Necrosis Factor-alpha (TNFα), Interleukin 17 (IL-17), S100 Proteins in serum. Medications for the treatment of JIA and other autoimmune diseases are described, highlighting TNF-α Inhibitors. In the figure, potentially interesting pathways to block are indicated with a red circle. In the specific context of Juvenile Idiopathic Arthritis (JIA), the receptor for IL-17 is found in various immune system cells, including T cells, macrophages, neutrophils, and fibroblasts. Activation of this receptor by IL-17 promotes inflammation and the production of inflammatory cells, contributing to the development and progression of JIA. In the inflamed joints of children with JIA, S100 proteins are deposited, secreted by inflammatory cells such as macrophages and neutrophils. These deposits occur in the articular cartilage, synovial membrane, and synovial fluid, contributing to cartilage loss and joint pain. Elevated levels of S100 proteins in blood and joints are used for the diagnosis and monitoring of JIA and other inflammatory diseases. (A) show the joint of a healthy individual compared to a person with JIA, and (B) show the cellular mechanism involved in JIA. Created by researchers.
References
- Ombrello, M.J.; Arthur, V.L.; Remmers, E.F.; Hinks, A.; Tachmazidou, I.; Grom, A.A.; Foell, D.; Martini, A.; Gattorno, M.; Özen, S.; et al. Genetic architecture distinguishes systemic juvenile idiopathic arthritis from other forms of juvenile idiopathic arthritis: Clinical and therapeutic implications. Ann. Rheum. Dis. 2017, 76, 906–913.
- Onel, K.B.; Horton, D.B.; Lovell, D.J.; Shenoi, S.; Cuello, C.A.; Angeles-Han, S.T.; Becker, M.L.; Cron, R.Q.; Feldman, B.M.; Ferguson, P.J.; et al. 2021 American College of Rheumatology Guideline for the Treatment of Juvenile Idiopathic Arthritis: Therapeutic Approaches for Oligoarthritis, Temporomandibular Joint Arthritis, and Systemic Juvenile Idiopathic Arthritis. Arthritis Care Res. 2022, 74, 521–537.
- Schulert, G.S.; Minoia, F.; Bohnsack, J.; Cron, R.Q.; Hashad, S.; KonÉ-Paut, I.; Kostik, M.; Lovell, D.; Maritsi, D.; Nigrovic, P.A.; et al. Effect of Biologic Therapy on Clinical and Laboratory Features of Macrophage Activation Syndrome Associated with Systemic Juvenile Idiopathic Arthritis. Arthritis Care Res. 2018, 70, 409–419.
- Momah, T.; Ray, L. Juvenile idiopathic arthritis: Old disease, new tactics. J. Fam. Pract. 2019, 68, E8–E13.
- Petty, R.E.; Southwood, T.R.; Manners, P.; Baum, J.; Glass, D.N.; Goldenberg, J.; He, X.; Maldonado-Cocco, J.; Orozco-Alcala, J.; Prieur, A.M.; et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: Second revision, Edmonton, 2001. J. Rheumatol. 2004, 31, 390–392.
- Gowdie, P.J.; Tse, S.M. Juvenile idiopathic arthritis. Pediatr. Clin. N. Am. 2012, 59, 301–327.
- Shenoi, S.; Shaffer, M.L.; Wallace, C.A. Environmental Risk Factors and Early-Life Exposures in Juvenile Idiopathic Arthritis: A Case-Control Study. Arthritis Care Res. 2016, 68, 1186–1194.
- Lee, J.J.Y.; Schneider, R. Systemic Juvenile Idiopathic Arthritis. Pediatr. Clin. N. Am. 2018, 65, 691–709.
- Myers, L.K.; Higgins, G.C.; Finkel, T.H.; Reed, A.M.; Thompson, J.W.; Walton, R.C.; Hendrickson, J.; Kerr, N.C.; Pandya-Lipman, R.K.; Shlopov, B.V.; et al. Juvenile arthritis and autoimmunity to type II collagen. Arthritis Rheum. 2001, 44, 1775–1781.
- Hinks, A.; Bowes, J.; Cobb, J.; Ainsworth, H.C.; Marion, M.C.; Comeau, M.E.; Sudman, M.; Han, B.; Juvenile Arthritis Consortium for Immunochip; Becker, M.L.; et al. Fine-mapping the MHC locus in juvenile idiopathic arthritis (JIA) reveals genetic heterogeneity corresponding to distinct adult inflammatory arthritic diseases. Ann. Rheum. Dis. 2017, 76, 765–772.
- Fikri-Benbrahim, O.; Rivera-Hernández, F.; Martínez-Calero, A.; Cazalla-Cadenas, F.; García-Agudo, R.; Mancha-Ramos, J. Treatment with adalimumab in amyloidosis secondary to rheumatoid arthritis: Two case reports. Nefrologia 2013, 33, 404–409, (In English, Spanish).
- Cohen, S.; Hurd, E.; Cush, J.; Schiff, M.; Weinblatt, M.E.; Moreland, L.W.; Kremer, J.; Bear, M.B.; Rich, W.J.; McCabe, D. Treatment of rheumatoid arthritis with anakinra, a recombinant human interleukin-1 receptor antagonist, in combination with methotrexate: Results of a twenty-four-week, multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002, 46, 614–624.
- Malleson, P.; Petty, R.E.; Fung, M.; Candido, E.P. Reactivity of antinuclear antibodies with histones and other antigens in juvenile rheumatoid arthritis. Arthritis Rheumatol. 1989, 32, 919–923.
- Tay, S.H.; Yeo, J.G.; Leong, J.Y.; Albani, S.; Arkachaisri, T. Juvenile Spondyloarthritis: What More Do We Know About HLA-B27, Enthesitis, and New Bone Formation? Front. Med. 2021, 8, 666772.
- Idriss, H.T.; Naismith, J.H. TNF alpha and the TNF receptor superfamily: Structure-function relationship(s). Microsc. Res. Tech. 2000, 50, 184–195.
- Donato, R.; Cannon, B.R.; Sorci, G.; Riuzzi, F.; Hsu, K.; Weber, D.J.; Geczy, C.L. Functions of S100 proteins. Curr. Mol. Med. 2013, 13, 24–57.
- Xia, C.; Braunstein, Z.; Toomey, A.C.; Zhong, J.; Rao, X. S100 Proteins As an Important Regulator of Macrophage Inflammation. Front. Immunol. 2018, 8, 1908.
- Justiz Vaillant, A.A.; Qurie, A. Interleukin. 22 August 2022. In StatPearls ; StatPearls Publishing: Treasure Island, FL, USA, 2023.
- Dinarello, C.A. The IL-1 family and inflammatory diseases. Clin. Exp. Rheumatol. 2002, 20 (Suppl. S27), S1–S13.
More
Information
Subjects:
Rheumatology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
451
Revisions:
2 times
(View History)
Update Date:
14 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




