Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Stefano Raffaele Giannubilo | -- | 4310 | 2024-03-13 10:21:51 | | | |
| 2 | Camila Xu | Meta information modification | 4310 | 2024-03-14 02:10:19 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Giannubilo, S.R.; Marzioni, D.; Tossetta, G.; Montironi, R.; Meccariello, M.L.; Ciavattini, A. Paternal Role in Pregnancy and in Birth Outcome. Encyclopedia. Available online: https://encyclopedia.pub/entry/56197 (accessed on 08 February 2026).
Giannubilo SR, Marzioni D, Tossetta G, Montironi R, Meccariello ML, Ciavattini A. Paternal Role in Pregnancy and in Birth Outcome. Encyclopedia. Available at: https://encyclopedia.pub/entry/56197. Accessed February 08, 2026.
Giannubilo, Stefano Raffaele, Daniela Marzioni, Giovanni Tossetta, Ramona Montironi, Maria Liberata Meccariello, Andrea Ciavattini. "Paternal Role in Pregnancy and in Birth Outcome" Encyclopedia, https://encyclopedia.pub/entry/56197 (accessed February 08, 2026).
Giannubilo, S.R., Marzioni, D., Tossetta, G., Montironi, R., Meccariello, M.L., & Ciavattini, A. (2024, March 13). Paternal Role in Pregnancy and in Birth Outcome. In Encyclopedia. https://encyclopedia.pub/entry/56197
Giannubilo, Stefano Raffaele, et al. "Paternal Role in Pregnancy and in Birth Outcome." Encyclopedia. Web. 13 March, 2024.
Copy Citation
Recurrent pregnancy loss (RPL) is defined as three or more consecutive abortions and constitutes about 1% of all cases of pregnancy loss. RPL is a multifactorial disease and recognizes several causes, including anatomic (uterine malformations), endocrine, infectious, immunologic, genetic, and idiopathic.
father
paternal
placenta
pre-eclampsia
pregnancy
preterm
1. The Father and Pregnancy Loss
Recurrent pregnancy loss (RPL) is defined as three or more consecutive abortions and constitutes about 1% of all cases of pregnancy loss [1]. RPL is a multifactorial disease and recognizes several causes, including anatomic (uterine malformations), endocrine, infectious, immunologic, genetic, and idiopathic. The genetic causes are estimated to affect 50% of abortions, and there are still not enough studies on the role of paternal factors in RPL [2]. The most common structural abnormality which can impact reproductive success is balanced translocation, a translocation or reciprocal inversion (or Robertsonian), with a prevalence of 0.1% in the population [3]. However, in couples with repeat abortions, the prevalence reaches the 8% [4]. For example, if a dad is a carrier of a 21q21q translocation, the risk of generating trisomy 21 will be 100%, which can lead to an early abortion in 50–80% of cases [5]. When one of the parents is a carrier of a balanced translocation, the risk of Down syndrome increases; specifically, the risk would be 10–15% when the carrier is the mother and 2–3% when the carrier is the father [6]. In this regard, a higher incidence of disomy of the sex chromosomes [7] and a higher susceptibility of chromatin to acid denaturation [8] was detected in the spermatozoa of couples with repeated miscarriage and infertility. Regarding point mutations, the role of mutation of the gene for Human leukocyte antigen-G (HLA-G), a leukocyte antigen expressed from fetal tissues and the maternal–fetal interface was considered. A variant in the Alpha-2 domain of this gene is associated with repeat abortions (up to five abortions) [9]. Although there are contradictory reports available, with regard to the association of maternal thrombophilia with adverse pregnancy outcomes, there is extensive literature about the role of certain maternal thrombophilic mutations, including Leiden Factor V (rs6025), of the gene for prothrombin (20210G > A) (rs1799963), of methylenetetrahydrofolic reductase (MTHFR 677C > T) (rs1801133) in recurrent abortion [10][11]. This appears to be little studied on the paternal side, although extraembryonic tissues are composed of proteins encoded by genes from both parents. There is evidence that paternal hyperhomocysteinemia (from the mutation of the MTHFR gene) would increase the risk of miscarriage 6.92-fold [12]. The exact contribution of possible “fetal thrombophilia” inherited from the father has not been established [13][14]. Another paternal factor that may affect reproductive success is the microdeletion of the chromosome Y. Men with this defect and severe oligozoospermia or azoospermia are able to reproduce via assisted reproductive technologies. Although inheritance of the Y microdeletion apparently has no somatic effect on male offspring [15], there remains a risk that transmission of the microdeletions from father to son confers adverse effects on male fertility. Paternal age affects reproductive success on several fronts, from the increased prevalence of aneuploidies to reduced quality of spermatozoa (particularly motility), reduced reproductive rate, and the increased incidence of at least 20 autosomal dominant diseases (Apert syndrome, achondroplasia, Marfan syndrome, etc.). Paternal age is a known risk factor in cases of miscarriage, i.e., advanced age in men can increase the risk of spontaneous abortion [16]. The fact that seminal fluid can modulate both sperm–egg interaction and maternal reproductive apparatus responses [17] indicates the important role of seminal fluid in regulating reproduction. In general, events of chromosome non-disjunction with aging are more likely to occur, particularly in sex chromosomes, and, although most constitutional aneuploidies come from the female germ line, all men produce about 3–5% aneuploid sperm [18][19][20]. In addition, sperm quality and paternal obesity are related to fertilization rate and embryo development. Male obesity has been shown to compromise time to conception, fertilization rates, sperm capacitation, and the ability for the sperm to bind to the oocyte [21].
2. The Father and the Duration of Pregnancy
According to a Swedish study, it is the paternal genes that determine the duration of pregnancy. Olesen et al. [22], from the University of Aarhus, studied two groups of women who have had at least two children. The first group consisted of nearly 11,000 women whose first pregnancy had been prolonged (over 42 weeks), and the second group of 3500 women whose first pregnancy had ended within 42 weeks. The data reported in this study indicated that the risk that the second pregnancy would be prolonged was 19.9% in the first group, whereas it was 7.7% in the second group (regular pregnancy). However, if the two children were from different fathers, the probability that the second pregnancy would be prolonged decreased by 15.4%. In addition, compared with children born to the same father, those born from different fathers differed in the duration of pregnancies by more than one week. The study cited has epidemiological features and does not imply genetic significance. It remains that multiple biological, genetic, environmental, and behavioral (such as maternal stress) factors intervene in pregnancy duration; however, the suggestion remains that the phenomenology of pregnancy duration definitely passes through the maternal pathway but is under the influence of the father.
3. The Father and the Thrombophilic Fetus
Several studies have attempted to identify a correlation between adverse obstetric outcomes and maternal thrombophilia, with controversial results; this is particularly true when the expected fetal outcomes are similar to the maternal outcomes [23]. Several obstetrical pathologies have been studied and correlated with alterations in various thrombophilic factors, but some studies report conflicting results. Unexplained fetal death was most associated with heterozygosity of Factor V Leiden, protein C, protein S deficiency, and increased APCR. Intrauterine growth restriction (IUGR), finally, showed a higher prevalence of heterozygosity for Factor II, homozygosity for the MTHFR C677T mutation, and protein S deficiency [24]. Of particular interest is the role of the fetus and its possible thrombophilia even if the exact fetal contribution to placental disfunction is not yet well delineated. The hypothesis of a paternal role in the link between thrombophilia and obstetrical diseases stems from observations that the fetus may have an inherited thrombophilic state, such as the factor V Leiden mutation, not present in the mother [25][26]. The hypothesis contemplated concerns the delicate balance between pro- and anticoagulant factors at the feto-placental interface, therefore the phenomena of fibrin deposition and infarction could arise either from the maternal or fetal side, or from both. The expression of certain paternally derived allelic variants in the trophoblast involved in immune regulation or vascular remodeling has also been linked to adverse pregnancy outcomes associated with abnormal placentation [27][28]. The presence of microthrombi in the chorio-decidual capillaries could, in fact, compromise the physiological anchoring of the villi and lead to the phenomena ranging from activating the coagulatory “cascade” to the thrombosis of vessels in the umbilical cord or multiple placental infarcts. The double homozygosity for thrombophilic mutations necessarily invokes the presence of mutations in both paternal and maternal genetics. The study of placental tissue, in fact, leads to a shift in attention to “fetal thrombophilia” rather than maternal; thus the fetus, with the inherited genetic material, becomes the architect of its own destiny [29]. Hypercoagulable states at this level depend only on the fetal genotype, which is inherited from both parents [30]. This gives rise to the thrombophilic fetus hypothesis [31], since the thrombophilic-related fetal outcomes depend not only on the presence of maternal thrombophilia, but may be an expression of a fetal thrombophilic state inherited by the mother or the father or both. In the scenario of maternal thrombophilia and adverse fetal events, some authors [32] confirm this association for IUGR by asserting that the clinical variability in the severity of IUGR depends on the extent of maternal and/or fetal thrombophilic disorders. The picture instead becomes predictably clear when dealing with thrombotic feto-neonatal outcomes in fetuses (or infants) with thrombophilic factors. This association is confirmed and considered strongly in several case reports with perinatal stroke as their subject [33].
4. The Father and Pre-Eclampsia
Pre-eclampsia is a severe obstetric condition that complicates approximately 3–5% of pregnancies, being the major cause of maternal mortality in developed countries and one of the major causes of iatrogenic prematurity and low-birth-weight infants [34]. The basic pathophysiology of pre-eclampsia involves the incomplete invasion of maternal uterine spiral arteries by extravillous trophoblast cells [35][36]. A previous abortion participates in this regard, while the protective effect of a previous pregnancy is lost in the case of a change of partner [37]. In multiparous women with pre-eclampsia/HELLP syndrome (hemolysis, elevated liver enzyme level, low platelet count), a change of partner is more frequent than in physiological multiparas (25% vs. 3.4%) [38], and in pre-eclamptic women, there is a higher index of cohabitation and sexual intercourse with the partner of the current pregnancy. This, in fact, agrees with the observation of a higher incidence of pre-eclampsia in pregnant adolescents. In a study of a large Norwegian population, pregnancies were studied in all combinations of couples: same or different father and same or different mother in two successive pregnancies. It verified that a father who has “fathered” a pre-eclamptic pregnancy has a double risk of generating a pre-eclamptic pregnancy with another woman as well. This phenomenon has been referred to as “the dangerous father” [39]. The mammalian fetus, after the phase of implantation of the blastocyst, is intimately connected with the uterine tissues and the maternal blood system. From an immunological point of view, this is a real paradox: fetal tissues expressing antigens of paternal inheritance provoke the responses of the immune maternal defenses [40]. A pregnancy being carried to term therefore depends on processes that suppress maternal immunity directed against fetal alloantigens. Maternal exposure to the seminal fluid and the timing of sexual intercourse between the father and the mother before the initiation of pregnancy may have an important role. The deposition of seminal fluid in the female genital tract, in fact, has the ability to initiate, as early as ejaculation, an immune response, predominantly T helper-2 type, to the disadvantage of T helper-1, which instead is associated with insufficient placentation [41]. In another pathway, maternal immune tolerance to fetal HLA-A and HLA-B antigens on the paternal side results from a sort of immunological memory developed during previous exposures to the same antigens contained in seminal fluid. The inverse relationship between the duration of sexual cohabitation and the incidence of pre-eclampsia may make sense in terms of evolution. In fact, human women are among the few mammals who conceive (generally) after multiple exposures to seminal fluid; this might make sense in the evolution of the species considering that those born to a stable couple would be better conceived and better cared for subsequently. Pre-eclampsia, moreover, is a complication typical of humans compared to all other mammals. Considering that the major difference in the human embryo lies in the size of the brain, which requires about 60% of the nutrients during the extraordinary phase of development in the second and third trimesters, and thus, the optimal invasion of the trophoblast into maternal tissues, the immunological compromise between the mother and father surely must be superior. According to Robillard’s theory [42], the low fertility rate of the human female (about 25%) is the price the human species pays for having a brain of large size, which, in fact, requires good trophoblastic invasion, with a relatively low rate of pre-eclampsia. The average wait of 7–8 months before conception by a newly established couple represents the mechanism by which the human female protects herself from pre-eclampsia by preserving the possibility of reproducing again, perhaps with the same partner [43]. Data from assisted reproductive technology seem to confirm similar associations: pregnancies achieved through a sperm donor have a significantly increased risk of developing pre-eclampsia [44], while pregnancies after double gamete donation are at about a 3 times higher risk of developing pre-eclampsia compared with oocyte donation alone [45] and with standard in vitro fertilization [46].
5. Father–Mother Competition
Modern genetics indicates that for specific genes only one of the two alleles is active, in some cases only the maternal one, in others only the paternal one. This phenomenon is called “genomic imprinting” and explains some diseases that do not follow Mendelian heredity. Experiments in the reproductive field have clarified some aspects of such hereditary mechanisms. Laboratories modified oocytes to create two kinds of embryos: “gynogenetic” with dual maternal genetic heritage and “androgenetic” with only dual paternal heritage. The first type died within a few days due to the lack of extraembryonic tissues (trophoblast); the second one reached the stage of blastocyst but not implantation [47], suggesting a main role for paternal genes. Interestingly, similar phenomena occur in teratoma (or dermoid cysts). In this type of pathological tissue, only duplicate maternal genetic material is present that can generate embryonic tissues derived from all three germ leaves (ectoderm, endoderm, and mesoderm). In fact, in these cysts, hair, skin with its adnexa, glands, and nerves may be present [48]. In contrast, the complete hydatidiform molar contains exclusively duplicate paternal genetic inheritance and is not capable of generating an embryo, only an extraembryonic trophoblast [49]. These data suggest that paternal genes have a pivotal role in promoting placental growth, while maternal genes have a main role in the formation of the embryo. Proper embryo–fetal development requires both maternal and paternal components as well as the selective inactivation of an allele (genomic imprinting), which is a complex phenomenon that must be well regulated for optimal embryo–fetal growth. An interesting theory concerning this matter was postulated as a kind of “maternal-paternal conflict” [47][48][49][50]. Although the common objective of the male and the female is the survival of the species, this should take place as much as possible in polygamous conditions so that as many genes as possible are inherited. Such a view leads to conflict, in that the father’s genes are interested in the greatest possible growth of the fetus and, therefore, of the future infant, through the development of the placental tissue; on the other hand, the mother would be more interested in not spending too many resources for each individual child, so that she can be immediately ready for conception with another male, so she would tend to genetically “slow down” fetal growth. Into this conflicting game would be inserted an imprinting genomic that would regulate fetal growth by drawing on genetic resources in a modulated manner from both parents (Figure 1). It is interesting, from this perspective, to consider the difference between humans and birds, where the amount of nutrition for the embryo is pre-determined in the egg and cannot undergo subsequent modulations.
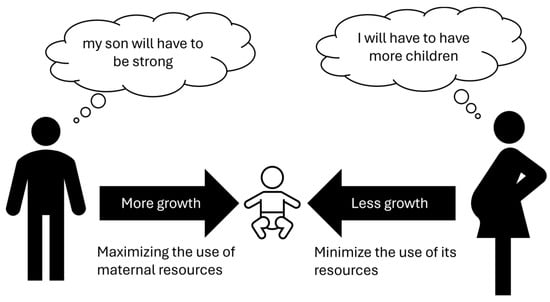
Figure 1. Maternal–paternal conflict theory.
6. The Father, the Fetus, and the Future Offspring
The paternal origins of health and disease (POHaD) concept can be evidenced from the early stages of fetal life because of the influence of genetic and environmental epigenetic modifications of spermatozoa and seminal plasma on fetoplacental development. In the last few decades, several studies have highlighted that paternal age, nutrition, lifestyle, environmental/occupational exposure, body mass index (BMI), and diabetes may have an impact on fetal health and future offspring.
6.1. Paternal Age
Even in the absence of an accepted definition of advanced paternal age (APA) in the current literature, the father’s aging leads to several modifications of sperm cells: decreased sperm quality [51], increased DNA damage [52][53], telomere elongation [54][55][56], centrosome aberrations [57], de novo DNA mutations [58], aneuploidies [59], and epigenetics alterations [51]. These sperm modifications harm fertility, decrease pregnancy rates in assisted reproductive technology, and increase the risk of miscarriage [51], aneuploidies [59], congenital anomalies (cleft palate, limb defects, musculoskeletal anomalies, patent ductus arteriosus) and rare syndromes related to fibroblast growth factor receptor (FGFR) mutations (achondroplasia, thanatophoric dysplasia, osteogenesis imperfecta, Apert syndrome, Pfeiffer syndrome, Crouzon syndrome). Neurofibromatosis and Marfan syndrome have also been consistently reported more frequently in the offspring of older fathers [51][60]. Moreover, compared to younger fathers of 25–29 years, paternal age over 45 years increases the risk of low birth weight (LBW) [61], PTB [62][63], and late stillbirth [64][65][66]. APA also adversely affects the offspring, maybe through an accumulation of de novo mutations in sperm cells [51][67][68][69]. It has been shown that children with older fathers are at higher risk for pediatric malignancies (acute lymphoblastic leukemia, retinoblastoma, breast and central nervous system cancers) [51][58][70][71][72] and neuropsychiatric disorders (bipolarism, autism, schizophrenia) [61][73][74][75]. Parental seniority can also adversely affect the future of offspring, as advanced age increases the likelihood of illness that would force children into early caregiving and increases the likelihood of death that would compromise their economic status and happiness [76]. The problem of parental seniority also occurs in the context of de novo genetic mutation transmission. Monogenic disorders (MDs) that share a combination of epidemiological and molecular features have been grouped into a distinct class of MDs with a high rate of de novo cases, termed paternal age effect disorders (PAEs). In these cases, there is an increased rate of germline mutations, paternal origin of the mutations, advanced paternal age, and involvement in the rat sarcoma virus protein–mitogen-activated protein kinase (RAS-MAPK) pathway. The risk of an aging father having a child with a de novo PAE disorder is at least 1 in 200 [77]. Infants with PAE develop congenital skeletal defects, including facial dysmorphia, craniosynostosis, syndactyly, short stature, hypertelorism, or marfanoid habitus. In addition, some diseases are associated with cardiac malformations, neurological defects, or predisposition to cancer [78]. Pathogenic variants of the FGFR2 gene can lead to syndromes such as Crouzon (OMIM 612247), Pfeiffer (OMIM 612247), and Apert (OMIM 123500). MD PAEs include multiple endocrine neoplasia type 2A (OMIM 171400) and 2B (OMIM 162300), i.e., marfanoid habitus, gastrointestinal tract defects, and increased risk of developing bone marrow and thyroid cancer [79]. Similarly, patients with Noonan and Costello (OMIM 218040) syndromes, caused by the PTPN11 and HRAS genes, have higher risk of childhood cancers, facial dysmorphia, cardiac disorders, neurocognitive delays, and musculoskeletal and cardiac abnormalities [80] (Table 1).
Table 1. Paternal age effect (PAE) disorders.
| Disorders |
|---|
| Achondroplasia |
| Apert syndrome |
| Cardiofaciocutaneous syndrome |
| Costello syndrome |
| Crouzon syndrome |
| Hypochondroplasia |
| Muenke syndrome |
| Multiple endocrine neoplasia types 2A, 2B |
| Noonan syndrome |
| Pfeiffer syndrome |
| Thanatophoric dysplasia |
6.2. Paternal Nutrition
In animal models, there is growing evidence that the nutritional status of the father, as with that of the mother, strongly influences the health of the fetus and its progeny. How this can happen is still little known, but seems to be based on epigenetic mechanisms (DNA methylation, histone modifications) and/or small non-coding alterations of spermatic RNA (sncRNAs) [81]. In rodents, paternal folate deficiency during the periconceptional period is linked to a higher rate of post-implantation embryo loss, fused and abnormal placentas, fetal abnormalities (craniofacial and limb defects), delayed muscle/skeletal development, and an increased vulnerability to anxiety and depression in the offspring [82][83]. In chickens, folic acid supplementation could also impact spermatozoa mRNA expression and induce transgenerational metabolic changes [84][85]. Fathers on a low-protein diet will have pups who are more likely to develop glucose intolerance, metabolic and cardiovascular dysfunction, an impaired skeletal system, and impaired bone deposition [86][87][88][89] with an increased risk of breast cancer in a mouse model [90]. Moreover, the up-regulation of genes coding for nutrient transporters in the placenta and of genes involved in fetal growth regulation is also reported [89][91]. Paternal food restriction seems to impair paternal fertility and increase dyslipidemia and adiposity prevalence in the offspring [91]. Also, a high-fat diet (HFD) negatively affects embryo development and the health of future progeny in rodents. Paternal HFD exposure leads to a reduced rate of ongoing pregnancy, decreased fetal and placental weights, delayed limb morphology, a decreased crown-rump length, and an increased risk in the offspring of chronic kidney diseases and metabolic syndrome-like characteristics; most recently, this has been found to occur through the transmission of β-cell dysfunction and glucose intolerance in F1 female offspring and altered insulin sensibility in F1 male offspring [83][92][93]. HFD also impairs hippocampal neurogenesis, maybe through increased methylation of the brain-derived neurotrophic factor (BDNF) gene promoter on spermatozoa, with resulting cognitive impairment in the F1 generation [94]. In the end, a paternal high-sugar diet seems to induce the up-regulation of some specific subtypes of tRNA-derived small RNAs (tsRNAs) of sperm cells that alter energy homeostasis in the offspring [81][95].
6.3. Paternal Lifestyle
Paternal “unhealthy habits” during the pre-conception period can profoundly impair the father’s sexual organs, the fetus, and the offspring’s health. Tobacco smoke contains several toxic and mutagenic substances that have a deep impact not only on male fertility but also on the genetics of the non-smoking descendant. The chemicals of cigarettes act as endocrine disruptors and increase the formation of reactive oxygen species (ROS) and secretion of inflammatory cytokines, leading to the development of oxidative stress (OS), DNA damage, and germ cell apoptosis. In addition, tobacco smoke is a mutagen and an aneugen of spermatozoa, where the genetic mutations can be transferred to the progeny [96]. This is why there is evidence that paternal pre-conception cigarette smoking increases the risk of adverse birth outcomes, including spontaneous abortion, preterm birth, aneuploidy, heart malformations (conotruncal defects, septal and left ventricular outflow tract obstructions defects), clefts, anorectal malformations, and childhood leukemia (acute myeloid leukemia/acute myeloblastic leukemia) [97][98][99][100][101]. Moreover, a father smoking before the age of 15 seems to be associated with an increased risk of asthma in his progeny [102]. As for smoking, paternal excessive alcohol intake deteriorates sperm quality and induces epigenetic changes, as well as DNA damage, in the testicular germline and sperm [103]. However, the effects on the fetus and offspring need to be further investigated, as there are different and inconsistent findings in the literature. Some data underline a possible association between paternal smoking and alcohol intake during the prenatal period and impaired offspring mental health (especially hyperactivity/Attention Deficit Hyperactivity Disorder (ADHD)) [104]. Recreational drugs, as with other ʺunhealthy habitsʺ, impair seminal parameters. Paternal cocaine and opioid addiction seems to be associated with LBW, premature deaths before the age of 6, and severe forms of ADHD in the offspring [105][106][107]. Finally, the role of paternal physical activity and caffeine use in the pre-conception period is almost unexplored in the current literature. However, regular exercise in male mice has shown increased insulin sensitivity in their offspring, due to the different methylation status of glucose metabolism genes of paternal sperm and skeletal muscle [108].
6.4. Paternal Environmental/Occupational Exposure
In general, men are more frequently exposed to harmful substances in the workplace and during everyday life than women, especially because of their lack of awareness of the potential reproductive risks. Research concerning mechanisms regarding the impact of environmental and occupational paternal exposure on pregnancy and offspring is growing. There are many professional agents, potentially teratogenic, that, to different degrees and with varying durations of exposure, can induce functional, epigenetic, and genetic changes in sperm cells. These may include chemicals and radiation. However, the current literature is still limited on this specific topic; in fact, most studies concern parental exposures and not just paternal exposures. Occupational exposure, even of only one parent, to organic solvents, phthalates, alkylphenolic compounds, herbicides, and pesticides is associated, respectively, with an increased risk of anencephaly [109], peri-membranous ventricular septal defects and pulmonary valve stenosis, ventricular septal defects [110], astrocytoma [111], and astroglia brain tumors [112]. In fact, there is an increased risk of fetal death from congenital anomalies for the offspring of agricultural workers, due to pesticide exposure [113]. Children of painters and carpenters regularly exposed to solvents are at greater risk of congenital abnormalities (anencephaly, heart defects, neural tube defects) [114][115]. In addition, exposure to ionizing agents appears to be a risk factor for PTB and LBW [116]. Regarding environmental exposure, several toxic agents have been identified in recent decades as endocrine disruptors (CDEs).
TCDD (2,3,7,8-tetrachlorodibenzo-p-dioxin) is an example of EDCs, and its paternal exposure in pups is found to be related to IUGR due to placental dysfunction and alterations in the expression of Insulin-like Growth Factor 2 (IGF2) through epigenetic changes in the male germ cell line in a mouse model [117][118]. Bisphenol-A (BPA) is one of the most used ECDs, with widespread application in the manufacture of plastics and epoxy resins. There is evidence that paternal exposure to BPA induces physiological and functional disruption in male germ cells that impairs the reproductive health of the progeny in mice [116]. Regarding air pollutants, a recent study demonstrates that paternal exposure to PM ₂₅ programs the energy homeostasis of the offspring [119].
6.5. Paternal Body Mass Index
There is increasing awareness that paternal weight before conception can affect not only sperm quality but also genomic imprinting in germ cells, with a great impact on the health of the fetus, the placenta, and the offspring. Recent cohort studies demonstrate that paternal obesity is associated with an increased risk of hypertensive disorders of pregnancy, macrosomia, PTB, and small for gestational age (SGA) [120][121][122]. In addition, studies have demonstrated a higher incidence of asthma; hand, foot, and mouth disease; anemia; dental caries; and obesity in adolescents of obese fathers [120][123][124]. The mechanism underlining these transmissions is still almost unexplored. There is evidence in mice that paternal obesity induces placental hypoxia and sex-specific impairments in placental vascularization and offspring metabolism [125]. Paternal obesity in mice has shown increased endoplasmic reticulum (ER) stress-related protein levels in the fetal liver and altered hepatic expression of gluconeogenic factors at E18.5. These prenatal modifications result in glucose intolerance and impaired energy metabolism in the offspring. In humans, researchers have shown a significant decrease in methylation among newborns of obese fathers in the IGF2 methylated regions (DMRs) in DNA extracted from cord blood leucocytes [126]; hypomethylation at IGF2 DMR is associated with higher circulating IGF2 levels in the offspring [127][128].
6.6. Paternal Diabetes
The complex etiology and the exponential increase in type 2 diabetes (T2DM) highlight the need to find alternative strategies able to combat this public health concern. It is well established in literature that T2DM can be programmed from the early stages of life when there is an adverse intrauterine environment. What still needs to be clarified is whether there is a role of the father in the programming of T2DM, because this specific topic is still almost not investigated. Fornes et al. have shown that paternal T2DM affects fetal overgrowth, placental development, and sex-dependent lipid metabolism in rats. Specifically, they observed that only the placentas of male fetuses from diabetic fathers showed an increase in lipid reserve and in the mRNA expression of enzymes involved in lipid oxidation and transport pathways [129].
References
- Grandone, E.; Tiscia, G.L.; Mastroianno, M.; Larciprete, G.; Kovac, M.; Tamborini Permunian, E.; Lojacono, A.; Barcellona, D.; Bitsadze, V.; Khizroeva, J.; et al. Findings from a multicentre, observational study on reproductive outcomes in women with unexplained recurrent pregnancy loss: The OTTILIA registry. Hum. Reprod. 2021, 36, 2083–2090.
- Pourmasumi, S.; Sabeti, P.; Ghasemi, N. Male factor testing in recurrent pregnancy loss cases: A narrative review. Int. J. Reprod. Biomed. 2022, 20, 447–460.
- Skaletsky, H.; Kuroda-Kawaguchi, T.; Minx, P.J.; Cordum, H.S.; Hillier, L.; Brown, L.G.; Repping, S.; Pyntikova, T.; Ali, J.; Bieri, T.; et al. The male-specific region of the human Y chromosome is a mosaic of discrete sequence classes. Nature 2003, 423, 825–837.
- Evenson, D.P.; Jost, L.K.; Marshall, D.; Zinaman, M.J.; Clegg, E.; Purvis, K.; de Angelis, P.; Claussen, O.P. Utility of the sperm chromatin structure assay as a diagnostic and prognostic tool in the human fertility clinic. Hum. Reprod. 1999, 14, 1039–1049.
- Leporrier, N.; Herrou, M.; Morello, R.; Leymarie, P. Fetuses with Down’s Syndrome detected by prenatal screening are more likely to abort spontaneously than fetuses with Down’s Syndrome not detected by prenatal screening. BJOG 2003, 110, 18–21.
- Thompson, J.S.; Thompson, M.W. Genetics in Medicine, 3rd ed.; Dimock, K., Thiel, M., Thiel, M., Eds.; Saunders Company: Philadelphia, PA, USA, 1980.
- Chien, S.C.; Chen, C.P.; Liou, J.D. Prenatal diagnosis and genetic counseling of uniparental disomy. Taiwan J. Obstet. Gynecol. 2022, 61, 210–215.
- Spanò, M.; Bonde, J.P.; Hjøllund, H.I.; Kolstad, H.A.; Cordelli, E.; Leter, G. Sperm chromatin damage impairs human fertility. The Danish First Pregnancy Planner Study Team. Fertil. Steril. 2000, 73, 43–50.
- Aldrich, C.L.; Stephenson, M.D.; Karrison, T.; Odem, R.R.; Branch, D.W.; Scott, J.R.; Schreiber, J.R.; Ober, C. HLA-G genotypes and pregnancy outcome in couples with unexplained recurrent miscarriage. Mol. Hum. Reprod. 2001, 7, 1167–1172.
- Farahmand, K.; Totonchi, M.; Hashemi, M.; Reyhani Sabet, F.; Kalantari, H.; Gourabi, H.; Mohseni Meybodi, A. Thrombophilic genes alterations as risk factor for recurrent pregnancy loss. J. Matern Fetal Neonatal Med. 2016, 29, 1269–1273.
- Said, J.M.; Higgins, J.R.; Moses, E.K.; Walker, S.P.; Monagle, P.T.; Brennecke, S.P. Inherited thrombophilias and adverse pregnancy outcomes: A case-control study in an Australian population. Acta Obstet. Gynecol. Scand. 2012, 91, 250–255.
- Govindaiah, V.; Naushad, S.M.; Prabhakara, K.; Krishna, P.C.; Radha Rama Devi, A. Association of parental hyperhomocysteinemia and C677T Methylene tetrahydrofolate reductase (MTHFR) polymorphism with recurrent pregnancy loss. Clin. Biochem. 2009, 42, 380–386.
- Jivraj, S.; Rai, R.; Regan, L. Genetic thrombophilic mutations among couples with recurrent miscarriage. Hum. Reprod. 2006, 21, 1161–1165.
- Toth, B.; Vocke, F.; Rogenhofer, N.; Friese, K.; Thaler, C.J.; Lohse, P. Paternal thrombophilic gene mutations are not associated with recurrent miscarriage. Am. J. Reprod. Immunol. 2008, 60, 325–332.
- Chandley, A.C. Chromosome anomalies and Y chromosome microdeletions as causal factors in male infertility. Hum. Reprod. 1998, 13, 45–50.
- Sharma, R.; Agarwal, A.; Rohra, V.K.; Assidi, M.; Abu-Elmagd, M.; Turki, R.F. Effects of increased paternal age on sperm quality, reproductive outcome and associated epigenetic risks to offspring. Reprod. Biol. Endocrinol. 2015, 19, 13–35.
- Robertson, S.A.; Sharkey, D.J. Seminal fluid and fertility in women. Fertil. Steril. 2016, 106, 511–519.
- Hassold, T.; Hunt, P. Maternal age and chromosomally abnormal pregnancies: What we know and what we wish we knew. Curr. Opin. Pediatr. 2009, 21, 703–708.
- Chan, P.T.K.; Robaire, B. Advanced Paternal Age and Future Generations. Front. Endocrinol. 2022, 13, 897101.
- Cioppi, F.; Casamonti, E.; Krausz, C. Age-Dependent De Novo Mutations during Spermatogenesis and Their Consequences. Adv. Exp. Med. Biol. 2019, 1166, 29–46.
- Hoek, J.; Schoenmakers, S.; van Duijn, L.; Willemsen, S.P.; van Marion, E.S.; Laven, J.S.E.; Baart, E.B.; Steegers-Theunissen, R.P.M. A higher preconceptional paternal body mass index influences fertilization rate and preimplantation embryo development. Andrology 2022, 10, 486–494.
- Olesen, A.W.; Basso, O.; Olsen, J. Risk of recurrence of prolonged pregnancy. BMJ 2003, 326, 476.
- Gomez-Lopez, N.; StLouis, D.; Lehr, M.A.; Sanchez-Rodriguez, E.N.; Arenas-Hernandez, M. Immune cells in term and preterm labor. Cell. Mol. Immunol. 2014, 11, 571–581.
- Clausson, B.; Lichtenstein, P.; Cnattingius, S. Genetic influence on birthweight and gestational length determined by studies in offspring of twins. BJOG 2000, 107, 375–381.
- Li, D.K. Changing paternity and the risk of preterm delivery in the subsequent pregnancy. Epidemiology 1999, 10, 148–152.
- Green, C.A.; Johnson, J.D.; Vladutiu, C.J.; Manuck, T.A. The association between maternal and paternal race and preterm birth. Am. J. Obstet. Gynecol. MFM 2021, 3, 100353.
- Uzun, A.; Schuster, J.S.; Stabila, J.; Zarate, V.; Tollefson, G.A.; Agudelo, A.; Kothiyal, P.; Wong, W.S.W.; Padbury, J. Protein interaction networks define the genetic architecture of preterm birth. Sci. Rep. 2022, 12, 438.
- Giannubilo, S.R.; Tranquilli, A.L. Anticoagulant therapy during pregnancy for maternal and fetal acquired and inherited thrombophilia. Curr. Med. Chem. 2012, 19, 4562–4571.
- Davenport, W.B.; Kutteh, W.H. Inherited thrombophilias and adverse pregnancy outcomes: A review of screening patterns and recommendations. Obstet. Gynecol. Clin. N. Am. 2014, 41, 133–144.
- Dizon-Townson, D.S.; Meline, L.; Nelson, L.M.; Varner, M.; Ward, K. Fetal carriers of the factor V Leiden mutation are prone to miscarriage and placental infarction. Am. J. Obstet. Gynecol. 1997, 177, 402–405.
- Thorarensen, O.; Ryan, S.; Hunter, J.; Younkin, D.P.; Factor, V. Leiden mutation: An unrecognized cause of hemiplegic cerebral palsy, neonatal stroke, and placental thrombosis. Ann. Neurol. 1997, 42, 372–375.
- Dekker, G.; Robillard, P.Y.; Roberts, C. The etiology of preeclampsia: The role of the father. J. Reprod. Immunol. 2011, 89, 126–132.
- Riley, J.K. Trophoblast immune receptors in maternal-fetal tolerance. Immunol. Investig. 2008, 37, 395–426.
- Tranquilli, A.L.; Saccucci, F.; Giannubilo, S.R.; Cecati, M.; Nocchi, L.; Lorenzi, S.; Emanuelli, M. Unexplained fetal loss: The fetal side of thrombophilia. Fertil. Steril. 2010, 94, 378–380.
- Udry, S.; Aranda, F.M.; Latino, J.O.; de Larrañaga, G.F. Paternal factor V Leiden and recurrent pregnancy loss: A new concept behind fetal genetics? J. Thromb. Haemost. 2014, 12, 666–669.
- Tranquilli, A.L.; Emanuelli, M. The thrombophilic fetus. Med. Hypotheses 2006, 67, 1226–1229.
- Leistra-Leistra, M.J.; Timmer, A.; van Spronsen, F.J.; Geven, W.B.; van der Meer, J.; Erwich, J.J. Fetal thrombotic vasculopathy in the placenta: A thrombophilic connection between pregnancy complications and neonatal thrombosis? Placenta 2004, 25, S102–S105.
- Ichiyama, M.; Ohga, S.; Ochiai, M.; Fukushima, K.; Ishimura, M.; Torio, M.; Urata, M.; Hotta, T.; Kang, D.; Hara, T. Fetal hydrocephalus and neonatal stroke as the first presentation of protein C deficiency. Brain Dev. 2016, 38, 253–256.
- Mészáros, B.; Kukor, Z.; Valent, S. Recent Advances in the Prevention and Screening of Preeclampsia. J. Clin. Med. 2023, 12, 6020.
- Tossetta, G.; Fantone, S.; Giannubilo, S.R.; Marinelli Busilacchi, E.; Ciavattini, A.; Castellucci, M.; Di Simone, N.; Mattioli-Belmonte, M.; Marzioni, D. Pre-eclampsia onset and SPARC: A possible involvement in placenta development. J. Cell. Physiol. 2019, 234, 6091–6098.
- Tossetta, G.; Avellini, C.; Licini, C.; Giannubilo, S.R.; Castellucci, M.; Marzioni, D. High temperature requirement A1 and fibronectin: Two possible players in placental tissue remodelling. Eur. J. Histochem. 2016, 60, 2724.
- Deen, M.E.; Ruurda, L.G.; Wang, J.; Dekker, G.A. Risk factors for preeclampsia in multiparous women: Primipaternity versus the birth interval hypothesis. J. Matern Fetal Neonatal Med. 2006, 19, 79–84.
- Tubbergen, P.; Lachmeijer, A.M.; Althuisius, S.M.; Vlak, M.E.; van Geijn, H.P.; Dekker, G.A. Change in paternity: A risk factor for preeclampsia in multiparous women? J. Reprod. Immunol. 1999, 45, 81–88.
- Lie, R.T.; Rasmussen, S.; Brunborg, H.; Gjessing, H.K.; Lie-Nielsen, E.; Irgens, L.M. Fetal and maternal contributions to risk of pre-eclampsia: Population based study. BMJ 1998, 316, 1343–1347.
- Ancuța, E.; Zamfir, R.; Martinescu, G.; Crauciuc, D.V.; Ancuța, C. The Complement System, T Cell Response, and Cytokine Shift in Normotensive versus Pre-Eclamptic and Lupus Pregnancy. J. Clin. Med. 2021, 10, 5722.
- Wyatt, M.A.; Baumgarten, S.C.; Weaver, A.L.; Van Oort, C.C.; Fedyshyn, B.; Ruano, R.; Shenoy, C.C.; Enninga, E.A.L. Evaluating Markers of Immune Tolerance and Angiogenesis in Maternal Blood for an Association with Risk of Pregnancy Loss. J. Clin. Med. 2021, 10, 3579.
- Robillard, P.Y.; Dekker, G.A.; Hulsey, T.C. Evolutionary adaptations to pre-eclampsia/eclampsia in humans: Low fecundability rate, loss of oestrus, prohibitions of incest and systematic polyandry. Am. J. Reprod. Immunol. 2002, 47, 104–111.
- Dekker, G. The partner’s role in the etiology of preeclampsia. J. Reprod. Immunol. 2002, 57, 203–215.
- González-Comadran, M.; Urresta Avila, J.; Saavedra Tascón, A.; Jimenéz, R.; Solà, I.; Brassesco, M.; Carreras, R.; Checa, M.Á. The impact of donor insemination on the risk of preeclampsia: A systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 182, 160–166.
- Blazquez, A.; García, D.; Vassena, R.; Figueras, F.; Rodriguez, A. Risk of preeclampsia in pregnancies resulting from double gamete donation and from oocyte donation alone. Pregnancy Hypertens 2018, 13, 133–137.
- Fishel Bartal, M.; Sibai, B.M.; Bart, Y.; Shina, A.; Mazaki-Tovi, S.; Eisen, I.S.; Hendler, I.; Baum, M.; Schiff, E. The Impact of Sperm and Egg Donation on the Risk of Pregnancy Complications. Am. J. Perinatol. 2019, 36, 205–211.
- Latham, K.-E. X chromosome imprinting and inactivation in the early mammalian embryo. Trends Genet. 1996, 12, 134–138.
- Ramalingam, P. Germ Cell Tumors of the Ovary: A Review. Semin. Diagn. Pathol. 2023, 40, 22–36.
- Strickland, A.L.; Gwin, K. Gestational trophoblastic disease-rare, sometimes dramatic, and what we know so far. Semin. Diagn. Pathol. 2022, 39, 228–237.
- Moore, T.; Haig, D. Genomic imprinting in mammalian development: A parental tug-of-war. Trends Genet. 1991, 7, 45–49.
- Kaltsas, A.; Moustakli, E.; Zikopoulos, A.; Georgiou, I.; Dimitriadis, F.; Symeonidis, E.N.; Markou, E.; Michaelidis, T.M.; Tien, D.M.B.; Giannakis, I.; et al. Impact of Advanced Paternal Age on Fertility and Risks of Genetic Disorders in Offspring. Genes 2023, 14, 486.
- Singh, N.P.; Muller, C.H.; Berger, R.E. Effects of age on DNA double-strand breaks and apoptosis in human sperm. Fertil. Steril. 2003, 80, 1420–1430.
- Oliveira, J.B.A.; Petersen, C.G.; Mauri, A.L.; Vagnini, L.D.; Baruffi, R.L.R.; Franco, J.G., Jr. The effects of age on sperm quality: An evaluation of 1,500 semen samples. JBRA Assist. Reprod. 2014, 18, 34–41.
- Kimura, M.; Cherkas, L.F.; Kato, B.S.; Demissie, S.; Hjelmborg, J.B.; Brimacombe, M.; Cupples, A.; Hunkin, J.L.; Gardner, J.P.; Lu, X.; et al. Offspring’s leukocyte telomere length, paternal age, and telomere elongation in sperm. PLoS Genet. 2008, 4, e37.
- Aston, K.I.; Hunt, S.C.; Susser, E.; Kimura, M.; Factor-Litvak, P.; Carrell, D.; Aviv, A. Divergence of sperm and leukocyte age-dependent telomere dynamics: Implications for male-driven evolution of telomere length in humans. Mol. Hum. Reprod. 2012, 18, 517–522.
- Ferlin, A.; Rampazzo, E.; Rocca, M.S.; Keppel, S.; Frigo, A.C.; De Rossi, A.; Foresta, C. In young men sperm telomere length is related to sperm number and parental age. Hum. Reprod. 2013, 28, 3370–3376.
- Ohshima, S.; Seyama, A. Cellular aging and centrosome aberrations. Ann. N. Y. Acad. Sci. 2010, 1197, 108–117.
- Kong, A.; Frigge, M.L.; Masson, G.; Besenbacher, S.; Sulem, P.; Magnusson, G.; Gudjonsson, S.A.; Sigurdsson, A.; Jonasdottir, A.; Jonasdottir, A.; et al. Rate of de novo mutations and the importance of father’s age to disease risk. Nature 2012, 488, 471–475.
- Griffin, D.K.; Abruzzo, M.A.; Millie, E.A.; Feingold, E.; Hassold, T.J. Sex ratio in normal and disomic sperm: Evidence that the extra chromosome 21 preferentially segregates with the Y chromosome. Am. J. Hum. Genet. 1996, 59, 1108–1113.
- Nybo Andersen, A.M.; Urhoj, S.K. Is advanced paternal age a health risk for the offspring? Fertil. Steril. 2017, 107, 312–318.
- Alio, A.P.; Salihu, H.M.; McIntosh, C.; August, E.M.; Weldeselasse, H.; Sanchez, E.; Mbah, A.K. The effect of paternal age on fetal birth outcomes. Am. J. Men’s Health 2012, 6, 427–435.
- Zhu, J.L.; Madsen, K.M.; Vestergaard, M.; Basso, O.; Olsen, J. Paternal age and preterm birth. Epidemiology 2005, 16, 259–262.
- Astolfi, P.; De Pasquale, A.; Zonta, L.A. Paternal age and preterm birth in Italy, 1990 to 1998. Epidemiology 2006, 17, 218–221.
- Cartlidge, P.H.; Stewart, J.H. Effect of changing the stillbirth definition on evaluation of perinatal mortality rates. Lancet 1995, 346, 486–488.
- Nybo Andersen, A.M.; Hansen, K.D.; Andersen, P.K.; Davey Smith, G. Advanced paternal age and risk of fetal death: A cohort study. Am. J. Epidemiol. 2004, 160, 1214–1222.
- Astolfi, P.; De Pasquale, A.; Zonta, L.-A. Late paternity and stillbirth risk. Hum. Reprod. 2004, 19, 2497–2501.
- Crow, J.F. Age and sex effects on human mutation rates: An old problem with new complexities. J. Radiat. Res. 2006, 47, B75–B82.
- Byrne, M.; Agerbo, E.; Ewald, H.; Eaton, W.W.; Mortensen, P.B. Parental age and risk of schizophrenia: A case-control study. Arch. Gen. Psychiatry 2003, 60, 673–678.
- Sipos, A.; Rasmussen, F.; Harrison, G.; Tynelius, P.; Lewis, G.; Leon, D.A.; Gunnell, D. Paternal age and schizophrenia: A population based cohort study. BMJ 2004, 329, 1070.
- Moll, A.C.; Imhof, S.M.; Kuik, D.J.; Bouter, L.M.; Den Otter, W.; Bezemer, P.D.; Koten, J.W.; Tan, K.E. High parental age is associated with sporadic hereditary retinoblastoma: The Dutch retinoblastoma register 1862–1994. Hum. Genet. 1996, 98, 109–112.
- Heck, J.E.; Lombardi, C.A.; Meyers, T.J.; Cockburn, M.; Wilhelm, M.; Ritz, B. Perinatal characteristics and retinoblastoma. Cancer Causes Control 2012, 23, 1567–1575.
- Yip, B.H.; Pawitan, Y.; Czene, K. Parental age and risk of childhood cancers: A population-based cohort study from Sweden. Int. J. Epidemiol. 2006, 35, 1495–1503.
- Torrey, E.F.; Buka, S.; Cannon, T.D.; Goldstein, J.M.; Seidman, L.J.; Liu, T.; Hadley, T.; Rosso, I.M.; Bearden, C.; Yolken, R.H. Paternal age as a risk factor for schizophrenia: How important is it? Schizophr. Res. 2009, 114, 1–5.
- Frans, E.M.; McGrath, J.J.; Sandin, S.; Lichtenstein, P.; Reichenberg, A.; Långström, N.; Hultman, C.M. Advanced paternal and grandpaternal age and schizophrenia: A three-generation perspective. Schizophr. Res. 2011, 133, 120–124.
- Kothari, A.; Thayalan, K.; Dulhunty, J.; Callaway, L. The forgotten father in obstetric medicine. Obstet. Med. 2019, 12, 57–65.
- Braverman, A.M. Old, older and too old: Age limits for medically assisted fatherhood? Fertil. Steril. 2017, 107, 329–333.
- Glaser, R.L.; Jabs, E.W. Dear old dad. Sci. Aging Knowl. Environ. 2004, 2004, re1.
- Maher, G.J.; Ralph, H.K.; Ding, Z.; Koelling, N.; Mlcochova, H.; Giannoulatou, E.; Dhami, P.; Paul, D.S.; Stricker, S.H.; Beck, S.; et al. Selfish mutations dysregulating RAS-MAPK signaling are pervasive in aged human testes. Genome Res. 2018, 28, 1779–1790.
- Liu, Q.; Tong, D.; Yuan, W.; Liu, G.; Yuan, G.; Lan, W.; Zhang, D.; Zhang, J.; Huang, Z.; Zhang, Y.; et al. Different RET gene mutation-induced multiple endocrine neoplasia type 2A in 3 Chinese families. Medicine 2017, 96, e5967.
- Kratz, C.P.; Franke, L.; Peters, H.; Kohlschmidt, N.; Kazmierczak, B.; Finckh, U.; Bier, A.; Eichhorn, B.; Blank, C.; Kraus, C.; et al. Cancer spectrum and frequency among children with Noonan, Costello, and cardio-facio-cutaneous syndromes. Br. J. Cancer 2015, 112, 1392–1397.
- Dimofski, P.; Meyre, D.; Dreumont, N.; Leininger-Muller, B. Consequences of Paternal Nutrition on Offspring Health and Disease. Nutrients 2021, 13, 2818.
- de Castro Barbosa, T.; Ingerslev, L.R.; Alm, P.S.; Versteyhe, S.; Massart, J.; Rasmussen, M.; Donkin, I.; Sjögren, R.; Mudry, J.M.; Vetterli, L.; et al. High-fat diet reprograms the epigenome of rat spermatozoa and transgenerationally affects metabolism of the offspring. Mol. Metab. 2015, 5, 184–197.
- Binder, N.K.; Hannan, N.J.; Gardner, D.K. Paternal diet-induced obesity retards early mouse embryo development, mitochondrial activity and pregnancy health. PLoS ONE 2012, 7, e52304.
- Wu, S.; Guo, W.; Yan, T.; Zhou, J.; Li, Y.; Zhu, Y.; Duan, Y.; Yang, X. Spermatozoal mRNAs expression implicated in embryonic development were influenced by dietary folate supplementation of breeder roosters by altering spermatozoal piRNA expression profiles. Theriogenology 2019, 138, 102–110.
- Wu, S.; Guo, W.; Li, X.; Liu, Y.; Li, Y.; Lei, X.; Yao, J.; Yang, X. Paternal chronic folate supplementation induced the transgenerational inheritance of acquired developmental and metabolic changes in chickens. Proc. Biol. Sci. 2019, 286, 20191653.
- Carone, B.R.; Fauquier, L.; Habib, N.; Shea, J.M.; Hart, C.E.; Li, R.; Bock, C.; Li, C.; Gu, H.; Zamore, P.D.; et al. Paternally induced transgenerational environmental reprogramming of metabolic gene expression in mammals. Cell 2010, 143, 1084–1096.
- Watkins, A.J.; Sinclair, K.D. Paternal low protein diet affects adult offspring cardiovascular and metabolic function in mice. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H1444–H1452.
- Watkins, A.J.; Dias, I.; Tsuro, H.; Allen, D.; Emes, R.D.; Moreton, J.; Wilson, R.; Ingram, R.J.M.; Sinclair, K.D. Paternal diet programs offspring health through sperm- and seminal plasma-specific pathways in mice. Proc. Natl. Acad. Sci. USA 2018, 115, 10064–10069.
- Watkins, A.J.; Sirovica, S.; Stokes, B.; Isaacs, M.; Addison, O.; Martin, R.A. Paternal low protein diet programs preimplantation embryo gene expression, fetal growth and skeletal development in mice. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1371–1381.
- da Cruz, R.S.; Carney, E.J.; Clarke, J.; Cao, H.; Cruz, M.I.; Benitez, C.; Jin, L.; Fu, Y.; Cheng, Z.; Wang, Y.; et al. Paternal malnutrition programs breast cancer risk and tumor metabolism in offspring. Breast Cancer Res. 2018, 20, 99.
- McPherson, N.O.; Fullston, T.; Kang, W.X.; Sandeman, L.Y.; Corbett, M.A.; Owens, J.A.; Lane, M. Paternal under-nutrition programs metabolic syndrome in offspring which can be reversed by antioxidant/vitamin food fortification in fathers. Sci. Rep. 2016, 6, 27010.
- Binder, N.K.; Mitchell, M.; Gardner, D.K. Parental diet-induced obesity leads to retarded early mouse embryo development and altered carbohydrate utilisation by the blastocyst. Reprod. Fertil. Dev. 2012, 24, 804–812.
- Masuyama, H.; Mitsui, T.; Eguchi, T.; Tamada, S.; Hiramatsu, Y. The effects of paternal high-fat diet exposure on offspring metabolism with epigenetic changes in the mouse adiponectin and leptin gene promoters. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E236–E245.
- Zhou, Y.; Zhu, H.; Wu, H.Y.; Jin, L.Y.; Chen, B.; Pang, H.Y.; Ming, Z.H.; Cheng, Y.; Zhou, C.L.; Guo, M.X.; et al. Diet-Induced Paternal Obesity Impairs Cognitive Function in Offspring by Mediating Epigenetic Modifications in Spermatozoa. Obesity 2018, 26, 1749–1757.
- Nätt, D.; Kugelberg, U.; Casas, E.; Nedstrand, E.; Zalavary, S.; Henriksson, P.; Nijm, C.; Jäderquist, J.; Sandborg, J.; Flinke, E.; et al. Human sperm displays rapid responses to diet. PLoS Biol. 2019, 17, e3000559.
- Deng, K.; Liu, Z.; Lin, Y.; Mu, D.; Chen, X.; Li, J.; Li, N.; Deng, Y.; Li, X.; Wang, Y.; et al. Periconceptional paternal smoking and the risk of congenital heart defects: A case-control study. Birth Defects Res. Part A Clin. Mol. Teratol. 2013, 97, 210–216.
- Omolaoye, T.S.; El Shahawy, O.; Skosana, B.T.; Boillat, T.; Loney, T.; du Plessis, S.S. The mutagenic effect of tobacco smoke on male fertility. Environ. Sci. Pollut. Res. 2022, 29, 62055–62066.
- Frederiksen, L.E.; Erdmann, F.; Wesseling, C.; Winther, J.F.; Mora, A.M. Parental tobacco smoking and risk of childhood leukemia in Costa Rica: A population-based case-control study. Environ. Res. 2020, 180, 108827.
- Orsi, L.; Rudant, J.; Ajrouche, R.; Leverger, G.; Baruchel, A.; Nelken, B.; Pasquet, M.; Michel, G.; Bertrand, Y.; Ducassou, S.; et al. Parental smoking, maternal alcohol, coffee and tea consumption during pregnancy, and childhood acute leukemia: The ESTELLE study. Cancer Causes Control 2015, 26, 1003–1017.
- Wang, L.; Deng, Y.; Yang, Y.; Liu, F.; Xu, Q.; Peng, Z.; He, Y.; Wang, Y.; Xu, J.; Zhang, H.; et al. Paternal smoking and preterm birth: A population-based retrospective cohort study among non-smoking women aged 20–49 years in rural China. Reprod. Health 2022, 19, 72.
- Wang, L.; Yang, Y.; Liu, F.; Yang, A.; Xu, Q.; Wang, Q.; Shen, H.; Zhang, Y.; Yan, D.; Peng, Z.; et al. Paternal smoking and spontaneous abortion: A population-based retrospective cohort study among non-smoking women aged 20–49 years in rural China. J. Epidemiol. Community Health 2018, 72, 783–789.
- Accordini, S.; Calciano, L.; Johannessen, A.; Portas, L.; Benediktsdóttir, B.; Bertelsen, R.J.; Bråbäck, L.; Carsin, A.E.; Dharmage, S.C.; Dratva, J.; et al. Ageing Lungs in European Cohorts (ALEC) Study. A three-generation study on the association of tobacco smoking with asthma. Int. J. Epidemiol. 2018, 47, 1106–1117.
- Cambiasso, M.Y.; Gotfryd, L.; Stinson, M.G.; Birolo, S.; Salamone, G.; Romanato, M.; Calvo, J.C.; Fontana, V.A. Paternal alcohol consumption has intergenerational consequences in male offspring. J. Assist. Reprod. Genet. 2022, 39, 441–459.
- Easey, K.E.; Sharp, G.C. The impact of paternal alcohol, tobacco, caffeine use and physical activity on offspring mental health: A systematic review and meta-analysis. Reprod. Health 2021, 18, 214.
- Fang, S.Y.; Huang, N.; Tsay, J.H.; Chang, S.H.; Chen, C.Y. Excess mortality in children born to opioid-addicted parents: A national register study in Taiwan. Drug Alcohol Depend. 2018, 183, 118–126.
- Lindblad, F.; Ringbäck Weitoft, G.; Hjern, A. Maternal and paternal psychopathology increases risk of offspring ADHD equally. Epidemiol. Psychiatr. Sci. 2011, 20, 367–372.
- Montagnoli, C.; Ruggeri, S.; Cinelli, G.; Tozzi, A.E.; Bovo, C.; Bortolus, E.; Zanconato, G. Anything New about Paternal Contribution to Reproductive Outcomes? A Review of the Evidence. World J. Men’s Health 2021, 39, 626–644.
- Costa-Júnior, J.M.; Ferreira, S.M.; Kurauti, M.A.; Bernstein, D.L.; Ruano, E.G.; Kameswaran, V.; Schug, J.; Freitas-Dias, R.; Zoppi, C.C.; Boschero, A.C.; et al. Paternal exercise improves the metabolic health of offspring via epigenetic modulation of the germline. Int. J. Mol. Sci. 2021, 23, 1.
- Aguilar-Garduño, C.; Lacasaña, M.; Blanco-Muñoz, J.; Borja-Aburto, V.H.; García, A.M. Parental occupational exposure to organic solvents and anencephaly in Mexico. Occup. Environ. Med. 2010, 67, 32–37.
- Smarr, M.M.; Grantz, K.L.; Sundaram, R.; Maisog, J.M.; Kannan, K.; Louis, G.M. Parental urinary biomarkers of preconception exposure to bisphenol A and phthalates in relation to birth outcomes. Environ. Health 2015, 14, 73.
- Wang, C.; Zhan, Y.; Wang, F.; Li, H.; Xie, L.; Liu, B.; Li, Y.; Mu, D.; Zheng, H.; Zhou, K.; et al. Parental occupational exposures to endocrine disruptors and the risk of simple isolated congenital heart defects. Pediatr. Cardiol. 2015, 36, 1024–1037.
- Van Maele-Fabry, G.; Hoet, P.; Lison, D. Parental occupational exposure to pesticides as risk factor for brain tumors in children and young adults: A systematic review and meta-analysis. Environ. Int. 2013, 56, 19–31.
- Regidor, E.; Ronda, E.; Garcia, A.M.; Dominiguez, V. Paternal exposure to agricoltural pesticides and cause specific fetal death. Occup. Environ. Med. 2004, 61, 334–339.
- Messerlian, C.; Mustieles, V.; Minguez-Alarcon, L.; Ford, J.B.; Calafat, A.M.; Souter, I.; Williams, P.L.; Hauser, R. Environment and Reproductive Health (EARTH) Study Team. Preconception and prenatal urinary concentrations of phenols and birth size of singleton infants born to mothers and fathers from the Environment and Reproductive Health (EARTH) study. Environ. Int. 2018, 114, 60–68.
- Chen, S.; Yang, Y.; Qv, Y.; Zou, Y.; Zhu, H.; Gong, F.; Zou, Y.; Yang, H.; Wang, L.; Lian, B.Q.; et al. Paternal exposure to medical-related radiation associated with low birthweight infants: A large population-based, retrospective cohort study in rural China. Medicine 2018, 97, e9565.
- Ding, T.; Lambert, L.A.; Aronoff, D.M.; Osteen, K.G.; Bruner-Tran, K.L. Sex-Dependent Influence of Developmental Toxicant Exposure on Group B Streptococcus-Mediated Preterm Birth in a Murine Model. Reprod. Sci. 2018, 25, 662–673.
- He, Y.; Xie, X.X.; Tang, W.B.; Ma, X. Maternal and paternal obesity and adverse pregnancy outcomes in China: A cohort study. Lancet 2017, 390, S52.
- Johnson, W.; Pereira, S.M.P.; Costa, S.; Baker, J.L.; Norris, T. The associations of maternal and paternal obesity with latent patterns of offspring BMI development between 7 and 17 years of age: Pooled analyses of cohorts born in 1958 and 2001 in the united kingdom. Int. J. Obes. 2023, 47, 39–50.
- Li, J.; Qiu, J.; Lv, L.; Mao, B.; Huang, L.; Yang, T.; Wang, C.; Liu, Q. Paternal factors and adverse birth outcomes in lanzhou, China. BMC Preg. Childbirth 2021, 21, 19.
- Jazwiec, P.A.; Patterson, V.S.; Ribeiro, T.A.; Yeo, E.; Kennedy, K.M.; Mathias, P.C.F.; Petrik, J.J.; Sloboda, D.M. Paternal obesity induces placental hypoxia and sex-specific impairments in placental vascularization and offspring metabolism. Biol. Reprod. 2022, 107, 574–589.
- Soubry, A.; Schildkraut, J.M.; Murtha, A.; Wang, F.; Huang, Z.; Bernal, A.; Kurtzberg, J.; Jirtle, R.L.; Murphy, S.K.; Hoyo, C. Paternal obesity is associated with IGF2 hypomethylation in newborns: Results from a Newborn Epigenetics Study (NEST) cohort. BMC Med. 2013, 11, 29.
- Murphy, S.K.; Huang, Z.; Hoyo, C. Differentially methylated regions of imprinted genes in prenatal, perinatal and postnatal human tissues. PLoS ONE 2012, 7, e40924.
- Hoyo, C.; Fortner, K.; Murtha, A.P.; Schildkraut, J.M.; Soubry, A.; Demark-Wahnefried, W.; Jirtle, R.L.; Kurtzberg, J.; Forman, M.R.; Overcash, F.; et al. Association of cord blood methylation fractions at imprinted insulin-like growth factor 2 (IGF2), plasma IGF2, and birth weight. Cancer Causes Control 2012, 23, 635–645.
- Fornes, D.; Heinecke, F.; Gatti, C.R.; Roberti, S.L.; White, V.; Jawerbaum, A.; Capobianco, E. Paternal diabetes programs sex-dependent placental alterations and fetal overgrowth. J. Endocrinol. 2022, 254, 37–49.
More
Information
Subjects:
Obstetrics & Gynaecology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
490
Revisions:
2 times
(View History)
Update Date:
14 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




