Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Isabel Guerrero | -- | 2871 | 2024-03-12 12:10:58 | | | |
| 2 | Sirius Huang | Meta information modification | 2871 | 2024-03-15 09:00:00 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Jiménez-Jiménez, C.; Grobe, K.; Guerrero, I. Glypican-Regulated Transport and Gradient Formation in Drosophila. Encyclopedia. Available online: https://encyclopedia.pub/entry/56161 (accessed on 15 January 2026).
Jiménez-Jiménez C, Grobe K, Guerrero I. Glypican-Regulated Transport and Gradient Formation in Drosophila. Encyclopedia. Available at: https://encyclopedia.pub/entry/56161. Accessed January 15, 2026.
Jiménez-Jiménez, Carlos, Kay Grobe, Isabel Guerrero. "Glypican-Regulated Transport and Gradient Formation in Drosophila" Encyclopedia, https://encyclopedia.pub/entry/56161 (accessed January 15, 2026).
Jiménez-Jiménez, C., Grobe, K., & Guerrero, I. (2024, March 12). Glypican-Regulated Transport and Gradient Formation in Drosophila. In Encyclopedia. https://encyclopedia.pub/entry/56161
Jiménez-Jiménez, Carlos, et al. "Glypican-Regulated Transport and Gradient Formation in Drosophila." Encyclopedia. Web. 12 March, 2024.
Copy Citation
Glypicans (Glps) are a family of heparan sulphate proteoglycans that are attached to the outer plasma membrane leaflet of the producing cell by a glycosylphosphatidylinositol anchor. Glps are involved in the regulation of many signalling pathways, including those that regulate the activities of Wnts, Hedgehog (Hh), Fibroblast Growth Factors (FGFs), and Bone Morphogenetic Proteins (BMPs), among others. In the Hh-signalling pathway, Glps have been shown to be essential for ligand transport and the formation of Hh gradients over long distances, for the maintenance of Hh levels in the extracellular matrix, and for unimpaired ligand reception in distant recipient cells.
glypicans
heparan sulphate proteoglycans
Dally
Dally like
Hedgehog
1. Introduction
Communication between cells is essential for the proper development of all multicellular organisms. Cellular communication is often mediated by the activity of specific signalling molecules called morphogens. During embryogenesis, morphogens are defined as being produced at a localised source and acting at significant distances from the source. As they spread, morphogens are thought to be progressively distributed within a morphogenetic field, which is defined as the area in which recipient cells respond by activating different target genes depending on the level of signal [1]. Therefore, the release of morphogens from producing cells, their graded distribution across the morphogenetic field, and the ability of recipient cells to respond specifically to different ligand concentrations must be tightly regulated to ensure proper tissue formation and function.
Both the graded distribution and receptor-mediated internalisation of morphogens require a family of cell surface-associated heparan sulphate proteoglycans (HSPGs), the glypicans (Glps). Glps have evolved as essential modulators of key regulatory proteins such as Wnts, Hedgehog (Hh), Fibroblast Growth Factor (FGF), Bone Morphogenetic Proteins (BMPs), and the Jak/Stat signalling pathway by acting on signal spreading and receptor activation, which in turn controls signal transduction and fate in target cells. Glps can also act as co-receptors for morphogens, enhancing or inhibiting their binding to primary cell surface receptors [2]. In addition, Glps can regulate the intracellular trafficking and degradation of morphogens, affecting their availability and activity [3][4]. Consistent with the importance of these activities in influencing critical signalling pathways, dysregulated Glp function has also been implicated in various diseases such as cancer, inflammation, and neurodegeneration [5].
One of the best-studied experimental systems for testing Glp function is the development of the Drosophila melanogaster wing imaginal disc, which gives rise to the adult wing. It is well established that wing development is strongly dependent on unimpaired Hh morphogen production, release, and extracellular spreading. Hh is also known to be involved in stem cell maintenance, axon guidance, cell migration, and oncogenesis in a wide range of organisms [6]. In the developing wing, the production, transport, release, and reception of Hh must therefore be kept under tight spatial and temporal control in order for Hh to fulfil its signalling function. An important feature of all invertebrate and vertebrate Hh family members is their post-translational modification by a covalently C-terminally attached cholesterol [7] and an N-terminally attached palmitic acid during biosynthesis [8]. Both lipids promote a tight association of Hh with the outer cell membrane leaflet, raising the important question of how the dual-lipidated morphogen is released from the plasma membrane and delivered to recipient cells in a robust, yet scalable manner. Over the past two decades, several modes have been proposed to overcome the apparent paradox that a tightly membrane-associated protein can signal to recipient cells over considerable distances: (1) the release of lipidated Hh by micelle formation [9]; (2) association of lipidated Hh with lipoprotein particles (LPP) [10][11]; (3) association of lipidated Hh with exosomes [12][13]; (4) Hh association with a soluble factor called Shifted (Shf) [14][15]; (5) proteolytic processing to release the Hh-signalling domain from both lipidated membrane anchors [16]. All of these modes are expected to convert insoluble Hh into protein complexes that can be transported by diffusion. Another proposed mechanism to regulate Hh distribution is by specialised filopodia (called cytonemes) that transport ligands to receptors on the surface of the signal-receiving cell while still attached to the plasma membrane of the signal-generating cell [17]. It is conceivable that these different modes of Hh release and trafficking operate in different tissues and developmental contexts or may act together in the same tissue to fine-tune Hh biofunction. Given the essential role that Glp expression plays in Hh signalling, all proposed mechanisms of Hh release and relay are expected to depend on Glp expression in the morphogenetic field and/or on producing and receiving cells.
2. Structure, Biochemistry and Metabolism of Glp-HSPGs in the Extracellular Matrix
A general function of the extracellular matrix is to modulate the activities of soluble growth factors and morphogens by sequestration, stabilisation, facilitation or inhibition of transport, and receptor binding. In mammals, cell surface-associated proteins that fulfil these roles include CD44, NG2, neuropilin-1, and the syndecans. Another group of extracellular matrix proteins known to fulfil all these functions for multiple soluble ligands in a context-dependent manner is the Glp protein family. This family has been conserved during animal evolution in both invertebrates and vertebrates [18][19], with six Glps (Glp1 to Glp6) identified in mammals [20] and two (Division abnormally delayed (Dally) and Dally like protein (Dlp)) in Drosophila melanogaster. On the basis of amino acid homology, mammalian Glps can be divided into two distinct groups. The first group includes Glp1, Glp2, Glp4, and Glp6 with 35–63% sequence similarity; the second group includes Glp3 and Glp5, with 54% sequence similarity [19][21], whereas the homology between the two groups is only 17–25%. The two Drosophila Glps Dally and Dlp [22] are representatives of each group: Dally is an ortholog of mammalian Glp3 and 5, and Dlp is an ortholog of Glps1, 2, 4 and 6 [19]. Crystallography and structural analysis support this relationship, revealing similar elongated, alpha-helical folds for Dlp and Glp1, despite the fact that these two Glps share only 25% sequence homology [23]. Furthermore, both structures do not appear to be homologous to any other known protein structure, suggesting unique functional roles for vertebrate and invertebrate Glp core proteins [23]. Glp core proteins are ~60 to 70 kDa in size and share three common structural features (Figure 1A).
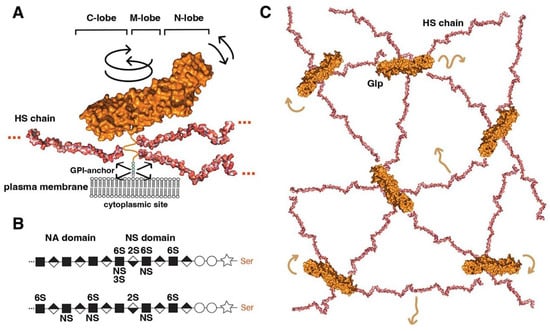
Figure 1. Overview of Glp structure and predicted cell surface distribution. (A) Representation of Glp1 lacking the most C-terminal disordered domain (Glp1DC) [24]. The disordered C-terminal Glp domain (aa Asn474-Ser530) contains the attachment sites of three closely spaced HS chains located close to the folded core (linked to serine residues Ser486, Ser488, and Ser490) and connects the Glp core domain to the GPI anchor. The HS structures were resolved separately [25] and manually added to the schematic. Three different lobes can be assigned to the Glp1DC structure: The cysteine-rich N-lobe, the central or M-lobe, and the C-lobe (also called the protease lobe because it has been described in many Glp family members to be susceptible to processing by furin proteases [26]). The structure of Glp1 is very similar to that of Drosophila melanogaster Dlp, despite only 25% sequence similarity. Note that the GPI membrane anchor and the unstructured flexible C-terminal domain give the core a large degree of freedom to tilt, move laterally, and rotate relative to the membrane (arrows). Shown are protein data bank (pdb) structures 3irl (HS) and 4ad7 (Glp). The structures are not to scale. (B) HS biosynthesis starts with a xylose residue (star) linked to a serine of the proteoglycan protein, followed by two galactose (circles) and a glucuronic acid residue (diamond). The subsequent addition of an N-acetylglucosamine residue (square) to the tetrasaccharide linker region initiates the biosynthesis of HS chains by the HS copolymerase complex. The growing chain (eventually consisting of 50–150 sugar residues) is simultaneously modified by N- and 2O-, 3O-, and 6O-sulphotransferases and an epimerase that generates iduronic acid residues (inverted diamond) from glucuronic acid residues. In vertebrates, high sulphated domains (NS domains) are separated by low-sulphated domains called NA domains (top). In contrast, Drosophila HS consists of a continuous sulphated domain (bottom) [27]. (C) A bird’s eye view of modelled multiple highly dynamic (brown arrows) interaction sites of Glp HS chains with neighbouring Glp core proteins, other HS chains and lipid head groups at the cell surface [28].
The first structural feature is a globular cysteine-rich N-lobe, which is similar to the cysteine-rich domains found in the Wnt receptor Frizzled and in the Hh-signalling transducer Smoothened [29]. The tertiary structure of the Glp N-lobe is likely to be constant among family members, due to the presence of 14 highly conserved cysteine residues that form stabilising disulphide bonds (Figure 1A). The second and third structural features of Glps are a central (or M) lobe and a C-terminal lobe susceptible to furin processing [26]. The fourth structural feature of all Glps is a disordered linker domain at the C-terminal end that connects the core protein to a glycosylphosphatidylinositol (GPI) anchor [30] for Glp insertion into the outer leaflet of the cell membrane (Figure 1A). This disordered linker, containing more than 50 amino acids, allows Glps to rotate freely and move laterally at the cell surface. The lack of a cytoplasmic domain prevents Glp internalisation by caveolin- and clathrin-coated vesicles. Instead, the GPI-anchor can target the molecule to recycling endosomes in a cdc42-dependent manner in mammals [31].
The fifth structural feature is a short peptide adjacent to the linker region that is decorated with varying numbers (2 to 5) of heparan sulphate (HS) glycosaminoglycan chains (Figure 1A). These chains on the Glp core protein are produced by most vertebrate and invertebrate cell types [32][33][34][35] (Figure 1B). Heparan sulphate (HS) biosynthesis starts with the addition of a tetrasaccharide linker to dedicated serine residues of the core protein in the Golgi compartment, followed by the synthesis of a linear carbohydrate backbone consisting of alternating glucuronic acid or iduronic acid/N-acetylglucosamine disaccharide units by enzymes called the exostosins (Exts) [36]. In Drosophila, the formation of HS glycosaminoglycan chains is catalysed by glycosyltransferases encoded by members of the EXT gene family: tout-velu (ttv) [37], brother of tout-velou (botv), and sister of tout-velou (sotv) [38]. The nascent chains are then modified by one or more of the four N-deacetylase/N-sulphotransferase (Ndst) isoforms identified in vertebrates [39] (called sulfateless (sfl) in Drosophila) and further modified by sulphotransferases and a GlcA-C5 epimerase [40]. The resulting HS chains vary in size from ∼5 to 70 kDa (corresponding to chain lengths ranging from 40 nm to more than 160 nm [41]) and are located within 50 amino acid residues of the membrane anchor. As a result, HS chains are positioned close to the cell membrane, allowing them not only to bind many soluble growth factors, chemokines, cytokines, and morphogens via their strong negative charge to bring them closer to the cell surface but also to bind their receptors and polar lipid head groups on the cell surface [42][43]. All-atom molecular modelling and simulation of GPI-anchored Glp1 with three HS chains in a lipid bilayer to explore their possible dynamics and interactions suggested multi-site interactions between Glps and the plasma membrane (Figure 1C) [28]. These dynamic interactions are facilitated by the unstructured C-terminal Glp domain linking the core domains to the GPI anchor, which gives the molecule a large degree of freedom to tilt, rotate, and move laterally at the cell membrane. In particular, the highly dynamic and flexible HS chains can make contact with neighbouring Glp1 protein cores, with other HS chains in the vicinity and surrounding head groups (Figure 1C). These simulations make it possible to imagine Glp HS chains forming a highly dynamic, negatively charged network at the cell surface for efficient interaction with growth factor/morphogen ligands and with their receptors.
3. Glp Expression Patterns and Their Influence on Morphogenetic Signalling in Drosophila
Glp expression during Drosophila development is highly dynamic and tissue-specific [44]. The role of Glps in cell signalling has been largely determined in the developing wing disc, which later gives rise to the adult fly wing (Figure 2A). Dally and Dlp show a generalised expression, a positive modulation of Dally and Dlp levels has been observed in two regions of the wing pouch (Figure 2B). Dally expression is down-regulated in cells near the anterior-posterior (A-P) compartment boundary [45]. In contrast, Dlp protein is distributed in most disc cells except for a region centred on the D-V boundary (white stripe in Figure 2B). Importantly, these regions correspond to areas where several signalling factors and their receptors are expressed, suggesting that Dally and Dlp influence the formation of the morphogenetic gradients of Decapentaplegic (Dpp, BMP in vertebrates), Wg (the Drosophila Wnt) and Hh, in addition to the FGF and Jak/Stat pathways in the wing disc.
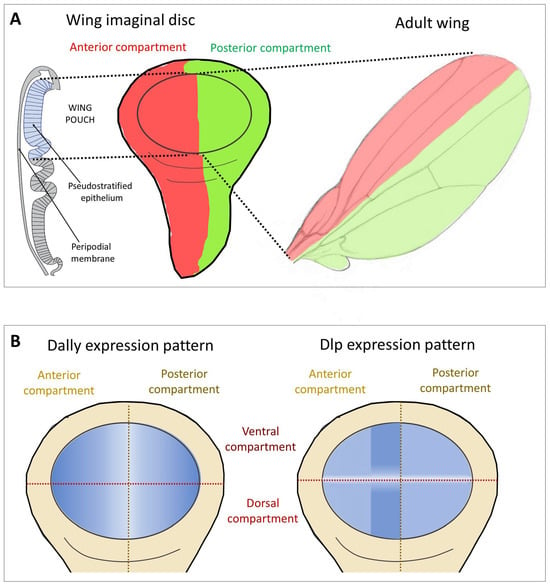
Figure 2. Wing imaginal disc as a model to study the function of Glps in Hh signalling. (A) The imaginal wing disc consists of a sheet of epithelial cells that form a sac-like fold of epithelium in fly larvae, called the wing pouch (marked in blue in the schematic representation of the polarised epithelium of the wing disc in a transverse section shown on the left), overlain by a fluid-filled closed compartment called the peripodial space (shown in white). Hh is produced throughout the posterior (P) compartment of the epithelial layer of the disc (green) and moves into the anterior (A) compartment to bind the Ptc receptor on the same epithelial layer (red). During this movement, Hh is thought to form a gradient of decreasing concentration with increasing distance from its source. Note that Hh movement must be confined to the epithelial layer to prevent morphogen loss into the overlying peripodial space, effectively ruling out free Hh diffusion as the underlying transport mode. Instead, Hh movement is thought to be confined to the epithelial layer by Glps. (B) Expression patterns of the Glps Dally and Dlp in the wing imaginal disc. Note that Dally shows reduced expression levels in the central area of the A-P boundary. Dlp shows higher expression levels in the A compartment adjacent to the P compartment and a marked decrease along the dorsoventral (D-V) axis.
3.1. Dpp
Dpp is expressed at the A-P compartment boundary in the wing disc [46][47], and is induced by the Hh signalling pathway. Dally appears to play a more important role than Dlp in establishing the Dpp gradient [48] through the interaction of Dally core protein with Dpp [48]. Dally binds and stabilises Dpp on the cell surface and has been shown to be involved in both signalling (acting as a co-receptor) and ligand spreading [49][50]. In addition, Dally delays the degradation of the Dpp receptor complex, thereby potentiating Dpp signalling [50]. Recently, it has been shown that Dally HS chains have the function of stabilizing Dpp on the cell surface by antagonizing Dpp internalisation through its receptor Tkv [51]. The secreted protein Pentagon (Pent, also known as Magu) has been described as also being required for Glp activity in the formation of long-range Dpp gradients [4][51][52][53]. Pent has been shown to interact with the HS chains of Dally [4][52], raising the possibility that the interaction of the HS chains of Dally with Pent is critical for Dpp stability and thus for scaling the Dpp gradient. In this context, tunable levels of Pent regulate Glps to maintain an optimal balance between delayed receptor degradation and functional inhibition of the ligand [4].
3.2. Wg
Glps are thought to primarily help establish the Wg gradient at the D/V axis of the wing disc where they are expressed [45][54][55][56][57][58][59]. Here, Glps play a cell-autonomous role in receiving Wg signals and both Glps help to stabilise Wg at the cell surface [45]. As for Dpp, it has been proposed that the role of Dlp in Wg transport across cells is based on its ability to “transfer” Wg from one Dlp molecule to the next [60][61]. In this case, the core Dlp protein can interact with Wg (described in more detail in the next section), and the HS chains enhance this interaction [60]. To form the gradient, the secreted Wg antagonist Notum [62] and Dlp work together to restrict Wg signalling. This function of Notum can be explained by the conversion of Dlp from a membrane-tethered coreceptor to a secreted antagonist [57][62]. Another proposed mechanism underlying Notum’s suppressive activity on Wg signalling is ligand deacylation, a posttranslational modification that renders Wg inactive [63]. In this mechanism, the role of Glps has been suggested to help the carboxylesterase Notum to co-localize with Wg ligands at the cell surface. In the absence of Notum, Dlp shows a biphasic role in the activity of Wg morphogen in the wing disc: Dlp represses short-range Wg signalling while simultaneously activating long-range signalling [57][58][60][62]. The transition from signalling activator to repressor is determined by the relative expression levels of Dlp and the Wg receptor Frizzled2 (Fz2) [57][60]; thus, the ratios of Wg, Fz2, and Dlp are essential.
Another important property of Wg and Wnt proteins that leads to their association with the Glp core protein is their hydrophobicity as a result of their palmitoylation. Structural analysis has shown that in the presence of palmitoylated peptides, Glps change their conformation to create a hydrophobic space. In this way, Dlp family Glps can accommodate the Wnt/Wg lipid and protect it from the aqueous environment, thus acting as a reservoir from which Wnt/Wg proteins can be delivered to signalling receptors. Glp6 and Glp4, which form the Glp subfamily with the highest homology to Dlp, are also able to bind to the palmitate moiety of Wnt. In contrast, Dally and the mammalian Glps with the highest homology to Dally (Glp3 and Glp5) are unable to interact with palmitate moieties attached to a modified peptide. Based on these observations, it has been proposed that Dlp acts by sequestering the hydrophobic palmitate during Wg spreading and reception in the aqueous extracellular environment [64].
References
- Rogers, K.; Schier, A.F. Morphogen Gradients: From Generation to Interpretation. Annu. Rev. Cell Dev. Biol. 2011, 27, 377–407.
- Fico, A.; Maina, F.; Dono, R. Fine-tuning of cell signaling by glypicans. Cell. Mol. Life Sci. 2011, 68, 923–929.
- Bilioni, A.; Sánchez-Hernández, D.; Callejo, A.; Gradilla, A.-C.; Ibáñez, C.; Mollica, E.; Rodríguez-Navas, M.C.; Simon, E.; Guerrero, I. Balancing Hedgehog, a retention and release equilibrium given by Dally, Ihog, Boi and shifted/DmWif. Dev. Biol. 2013, 376, 198–212.
- Norman, M.; Vuilleumier, R.; Springhorn, A.; Gawlik, J.; Pyrowolakis, G. Pentagone internalises glypicans to fine-tune multiple signalling pathways. eLife 2016, 5, e13301.
- Kolluri, A.; Ho, M. The Role of Glypican-3 in Regulating Wnt, YAP, and Hedgehog in Liver Cancer. Front. Oncol. 2019, 9, 708.
- Zhang, Y.; Beachy, P.A. Cellular and molecular mechanisms of Hedgehog signalling. Nat. Rev. Mol. Cell Biol. 2023, 24, 668–687.
- Porter, J.A.; Young, K.E.; Beachy, P.A. Cholesterol Modification of Hedgehog Signaling Proteins in Animal Development. Science 1996, 274, 255–259.
- Pepinsky, R.B.; Zeng, C.; Wen, D.; Rayhorn, P.; Baker, D.P.; Williams, K.P.; Bixler, S.A.; Ambrose, C.M.; Garber, E.A.; Miatkowski, K.; et al. Identification of a Palmitic Acid-modified Form of Human Sonic hedgehog. J. Biol. Chem. 1998, 273, 14037–14045.
- Zeng, X.; Goetz, J.A.; Suber, L.M.; Scott, W.J., Jr.; Schreiner, C.M.; Robbins, D.J. A freely diffusible form of Sonic hedgehog mediates long-range signalling. Nature 2001, 411, 716–720.
- Panáková, D.; Sprong, H.; Marois, E.; Thiele, C.; Eaton, S. Lipoprotein particles are required for Hedgehog and Wingless signalling. Nature 2005, 435, 58–65.
- Callejo, A.; Culi, J.; Guerrero, I. Patched, the receptor of Hedgehog, is a lipoprotein receptor. Proc. Natl. Acad. Sci. USA 2008, 105, 912–917.
- Gradilla, A.-C.; González, E.; Seijo, I.; Andrés, G.; Bischoff, M.; González-Mendez, L.; Sánchez, V.; Callejo, A.; Ibáñez, C.; Guerra, M.; et al. Exosomes as Hedgehog carriers in cytoneme-mediated transport and secretion. Nat. Commun. 2014, 5, 5649.
- Vyas, N.; Walvekar, A.; Tate, D.; Lakshmanan, V.; Bansal, D.; Cicero, A.L.; Raposo, G.; Palakodeti, D.; Dhawan, J. Vertebrate Hedgehog is secreted on two types of extracellular vesicles with different signaling properties. Sci. Rep. 2014, 4, 7357.
- Gorfinkiel, N.; Sierra, J.; Callejo, A.; Ibañez, C.; Guerrero, I. The Drosophila Ortholog of the Human Wnt Inhibitor Factor Shifted Controls the Diffusion of Lipid-Modified Hedgehog. Dev. Cell 2005, 8, 241–253.
- Glise, B.; Miller, C.A.; Crozatier, M.; Halbisen, M.A.; Wise, S.; Olson, D.J.; Vincent, A.; Blair, S.S. Shifted, the Drosophila Ortholog of Wnt Inhibitory Factor-1, Controls the Distribution and Movement of Hedgehog. Dev. Cell 2005, 8, 255–266.
- Ohlig, S.; Farshi, P.; Pickhinke, U.; Boom, J.v.D.; Höing, S.; Jakuschev, S.; Hoffmann, D.; Dreier, R.; Schöler, H.R.; Dierker, T.; et al. Sonic Hedgehog Shedding Results in Functional Activation of the Solubilized Protein. Dev. Cell 2011, 20, 764–774.
- Bischoff, M.; Gradilla, A.-C.; Seijo, I.; Andrés, G.; Rodríguez-Navas, C.; González-Méndez, L.; Guerrero, I. Cytonemes are required for the establishment of a normal Hedgehog morphogen gradient in Drosophila epithelia. Nature 2013, 15, 1269–1281.
- Filmus, J.; Selleck, S.B. Glypicans: Proteoglycans with a surprise. J. Clin. Investig. 2001, 108, 497–501.
- Filmus, J.; Capurro, M.; Rast, J. Glypicans. Genome Biol. 2008, 9, 224.
- Song, H.H.; Filmus, J. The role of glypicans in mammalian development. Biochim. Biophys. Acta (BBA) Gen. Subj. 2002, 1573, 241–246.
- Veugelers, M.; De Cat, B.; Ceulemans, H.; Bruystens, A.-M.; Coomans, C.; Dürr, J.; Vermeesch, J.; Marynen, P.; David, G. Glypican-6, a New Member of the Glypican Family of Cell Surface Heparan Sulfate Proteoglycans. J. Biol. Chem. 1999, 274, 26968–26977.
- Khare, N.; Baumgartner, S. Dally-like protein, a new Drosophila glypican with expression overlapping with wingless. Mech. Dev. 2000, 99, 199–202.
- Kim, M.-S.; Saunders, A.M.; Hamaoka, B.Y.; Beachy, P.A.; Leahy, D.J. Structure of the protein core of the glypican Dally-like and localization of a region important for hedgehog signaling. Proc. Natl. Acad. Sci. USA 2011, 108, 13112–13117.
- Svensson, G.; Awad, W.; Håkansson, M.; Mani, K.; Logan, D.T. Crystal Structure of N-Glycosylated Human Glypican-1 Core Protein. J. Biol. Chem. 2012, 287, 14040–14051.
- Khan, S.; Gor, J.; Mulloy, B.; Perkins, S.J. Semi-Rigid Solution Structures of Heparin by Constrained X-ray Scattering Modelling: New Insight into Heparin–Protein Complexes. J. Mol. Biol. 2010, 395, 504–521.
- De Cat, B.; Muyldermans, S.-Y.; Coomans, C.; Degeest, G.; Vanderschueren, B.; Creemers, J.; Biemar, F.; Peers, B.; David, G. Processing by proprotein convertases is required for glypican-3 modulation of cell survival, Wnt signaling, and gastrulation movements. J. Cell Biol. 2003, 163, 625–635.
- Kusche-Gullberg, M.; Nybakken, K.; Perrimon, N.; Lindahl, U. Drosophila Heparan Sulfate, a Novel Design. J. Biol. Chem. 2012, 287, 21950–21956.
- Dong, C.; Choi, Y.K.; Lee, J.; Zhang, X.F.; Honerkamp-Smith, A.; Widmalm, G.; Lowe-Krentz, L.J.; Im, W. Structure, Dynamics, and Interactions of GPI-Anchored Human Glypican-1 with Heparan Sulfates in a Membrane. Glycobiology 2020, 31, 593–602.
- Pei, J.; Grishin, N.V. Cysteine-rich domains related to Frizzled receptors and Hedgehog-interacting proteins. Protein Sci. 2012, 21, 1172–1184.
- Lin, X. Functions of heparan sulfate proteoglycans in cell signaling during development. Development 2004, 131, 6009–6021.
- Sabharanjak, S.; Sharma, P.; Parton, R.G.; Mayor, S. GPI-Anchored Proteins Are Delivered to Recycling Endosomes via a Distinct cdc42-Regulated, Clathrin-Independent Pinocytic Pathway. Dev. Cell 2002, 2, 411–423.
- Esko, J.D.; Zhang, L. Influence of core protein sequence on glycosaminoglycan assembly. Curr. Opin. Struct. Biol. 1996, 6, 663–670.
- Sugahara, K.; Kitagawa, H. Recent advances in the study of the biosynthesis and functions of sulfated glycosaminoglycans. Curr. Opin. Struct. Biol. 2000, 10, 518–527.
- Duncan, G.; McCormick, C.; Tufaro, F. The link between heparan sulfate and hereditary bone disease: Finding a function for the EXT family of putative tumor suppressor proteins. J. Clin. Investig. 2001, 108, 511–516.
- Esko, J.D.; Lindahl, U. Molecular diversity of heparan sulfate. J. Clin. Investig. 2001, 108, 169–173.
- Sarrazin, S.; Lamanna, W.C.; Esko, J.D. Heparan Sulfate Proteoglycans. Cold Spring Harb. Perspect. Biol. 2011, 3, a004952.
- The, I.; Bellaiche, Y.; Perrimon, N. Hedgehog Movement Is Regulated through tout velu–Dependent Synthesis of a Heparan Sulfate Proteoglycan. Mol. Cell 1999, 4, 633–639.
- Han, C.; Belenkaya, T.Y.; Khodoun, M.; Tauchi, M.; Lin, X.; Lin, X. Distinct and collaborative roles of Drosophila EXT family proteins in morphogen signalling and gradient formation. Development 2004, 131, 1563–1575.
- Aikawa, J.; Grobe, K.; Tsujimoto, M.; Esko, J.D. Multiple Isozymes of Heparan Sulfate/Heparin GlcNAc N-Deacetylase/N-Sulfotransferase: Structure and Activity of the Fourth Member, NDST4. J. Biol. Chem. 2001, 276, 5876–5882.
- Lindahl, U.; Kusche-Gullberg, M.; Kjellén, L. Regulated Diversity of Heparan Sulfate. J. Biol. Chem. 1998, 273, 24979–24982.
- Duchesne, L.; Octeau, V.; Bearon, R.N.; Beckett, A.; Prior, I.A.; Lounis, B.; Fernig, D.G. Transport of Fibroblast Growth Factor 2 in the Pericellular Matrix Is Controlled by the Spatial Distribution of Its Binding Sites in Heparan Sulfate. PLoS Biol. 2012, 10, e1001361.
- Park, P.W.; Reizes, O.; Bernfield, M. Cell surface heparan sulfate proteoglycans: Selective regulators of ligand-receptor en-counters. J. Biol. Chem. 2000, 275, 29923–29926.
- Gallagher, J.T. Heparan sulfate: Growth control with a restricted sequence menu. J. Clin. Investig. 2001, 108, 357–361.
- Saad, K.; Otto, A.; Theis, S.; Kennerley, N.; Munsterberg, A.; Luke, G.; Patel, K. Detailed expression profile of all six Glypicans and their modifying enzyme Notum during chick embryogenesis and their role in dorsal-ventral patterning of the neural tube. Gene 2017, 609, 38–51.
- Han, C.; Yan, D.; Belenkaya, T.Y.; Lin, X. Drosophila glypicans Dally and Dally-like shape the extracellular Wingless morphogen gradient in the wing disc. Development 2005, 132, 667–679.
- Belenkaya, T.Y.; Han, C.; Yan, D.; Opoka, R.J.; Khodoun, M.; Liu, H.; Lin, X. Drosophila Dpp Morphogen Movement Is Independent of Dynamin-Mediated Endocytosis but Regulated by the Glypican Members of Heparan Sulfate Proteoglycans. Cell 2004, 119, 231–244.
- Hamaratoglu, F.; Affolter, M.; Pyrowolakis, G. Dpp/BMP signaling in flies: From molecules to biology. Semin. Cell Dev. Biol. 2014, 32, 128–136.
- Simon, E.; Jiménez-Jiménez, C.; Seijo-Barandiarán, I.; Aguilar, G.; Sánchez-Hernández, D.; Aguirre-Tamaral, A.; González-Méndez, L.; Ripoll, P.; Guerrero, I. Glypicans define unique roles for the Hedgehog co-receptors boi and ihog in cytoneme-mediated gradient formation. eLife 2021, 10.
- Fujise, M.; Takeo, S.; Kamimura, K.; Matsuo, T.; Aigaki, T.; Izumi, S.; Nakato, H. Dally regulates Dpp morphogen gradient formation in the Drosophila wing. Development 2003, 130, 1515–1522.
- Akiyama, T.; Kamimura, K.; Firkus, C.; Takeo, S.; Shimmi, O.; Nakato, H. Dally regulates Dpp morphogen gradient formation by stabilizing Dpp on the cell surface. Dev. Biol. 2008, 313, 408–419.
- Simon, N.; Safyan, A.; Pyrowolakis, G.; Matsuda, S. Dally is not essential for Dpp spreading or internalization but for Dpp stability by antagonizing Tkv-mediated Dpp internalization. eLife 2024, 12, RP86663.
- Vuilleumier, R.; Springhorn, A.; Patterson, L.; Koidl, S.; Hammerschmidt, M.; Affolter, M.; Pyrowolakis, G. Control of Dpp morphogen signalling by a secreted feedback regulator. Nature 2010, 12, 611–617.
- Ben-Zvi, D.; Pyrowolakis, G.; Barkai, N.; Shilo, B.-Z. Expansion-Repression Mechanism for Scaling the Dpp Activation Gradient in Drosophila Wing Imaginal Discs. Curr. Biol. 2011, 21, 1391–1396.
- Lin, X.; Perrimon, N. Dally cooperates with Drosophila Frizzled 2 to transduce Wingless signalling. Nature 1999, 400, 281–284.
- Tsuda, M.; Kamimura, K.; Nakato, H.; Archer, M.; Staatz, W.; Fox, B.; Humphrey, M.; Olson, S.; Futch, T.; Kaluza, V.; et al. The cell-surface proteoglycan Dally regulates Wingless signalling in Drosophila. Nature 1999, 400, 276–280.
- Baeg, G.-H.; Lin, X.; Khare, N.; Baumgartner, S.; Perrimon, N. Heparan sulfate proteoglycans are critical for the organization of the extracellular distribution of Wingless. Development 2001, 128, 87–94.
- Baeg, G.-H.; Selva, E.M.; Goodman, R.M.; Dasgupta, R.; Perrimon, N. The Wingless morphogen gradient is established by the cooperative action of Frizzled and Heparan Sulfate Proteoglycan receptors. Dev. Biol. 2004, 276, 89–100.
- Kirkpatrick, C.A.; Dimitroff, B.D.; Rawson, J.M.; Selleck, S.B. Spatial Regulation of Wingless Morphogen Distribution and Signaling by Dally-like Protein. Dev. Cell 2004, 7, 513–523.
- Franch-Marro, X.; Marchand, O.; Piddini, E.; Ricardo, S.; Alexandre, C.; Vincent, J.-P. Glypicans shunt the Wingless signal between local signalling and further transport. Development 2005, 132, 659–666.
- Yan, D.; Wu, Y.; Feng, Y.; Lin, S.-C.; Lin, X. The Core Protein of Glypican Dally-Like Determines Its Biphasic Activity in Wingless Morphogen Signaling. Dev. Cell 2009, 17, 470–481.
- Waghmare, I.; Page-McCaw, A. Regulation of Wnt distribution and function by Drosophila glypicans. J. Cell Sci. 2022, 135, jcs259405.
- Kreuger, J.; Perez, L.; Giraldez, A.J.; Cohen, S.M. Opposing Activities of Dally-like Glypican at High and Low Levels of Wingless Morphogen Activity. Dev. Cell 2004, 7, 503–512.
- Kakugawa, S.; Langton, P.F.; Zebisch, M.; Howell, S.A.; Chang, T.-H.; Liu, Y.; Feizi, T.; Bineva, G.; O’reilly, N.; Snijders, A.P.; et al. Notum deacylates Wnt proteins to suppress signalling activity. Nature 2015, 519, 187–192.
- McGough, I.J.; Vecchia, L.; Bishop, B.; Malinauskas, T.; Beckett, K.; Joshi, D.; O’Reilly, N.; Siebold, C.; Jones, E.Y.; Vincent, J.-P. Glypicans shield the Wnt lipid moiety to enable signalling at a distance. Nature 2020, 585, 85–90.
More
Information
Subjects:
Developmental Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
448
Revisions:
2 times
(View History)
Update Date:
15 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




