
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jianping Xu | -- | 4123 | 2024-03-12 10:34:20 | | | |
| 2 | Sirius Huang | -40 word(s) | 4083 | 2024-03-15 08:57:29 | | |
Video Upload Options
Modern efforts to influence materials science with principles of biology have allowed fungal mycelial materials to take a foothold and develop novel solutions for the circular bioeconomy of tomorrow. However, recent studies have shown that the value of tomorrow’s green materials is not determined simply by their environmental viability, but rather by their ability to make the polluting materials of today obsolete. With an inherently strong structure of chitin and β-glucan, the ever-adaptable mycelia of fungi can compete at the highest levels with a litany of materials from leather to polyurethane foam to paper to wood. There are significant efforts to optimize pure mycelial materials (PMMs) through the entire process of species and strain selection, mycelial growth, and fabrication.
1. Towards Functional Fungi
2. Techniques in the Cultivation of Mycelium
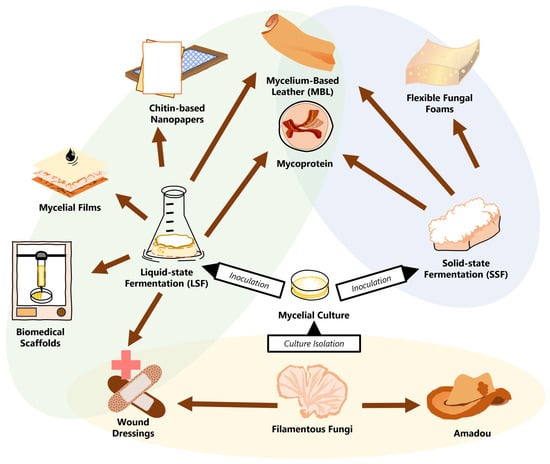
2.1. Solid-State Fermentation
2.2. Liquid-State Fermentation
3. The Growing Profile of Pure Mycelial Materials
3.1. Laying out the Design Space for PMMs
| Materials Property | Definition | Unit |
|---|---|---|
| Density (ρ) | A material’s mass per unit of volume. | kg/m3 |
| Percent elongation (%EL) | A material’s deformation when it fractures due to a tensile load. | % |
| Ultimate tensile strength (σUTS) | The maximum amount of strength a material can withstand under tension. | Megapascals (MPa) |
| Young’s modulus (E) | The modulus of elasticity or the material’s ability to stretch and deform. | Gigapascals (GPa) |
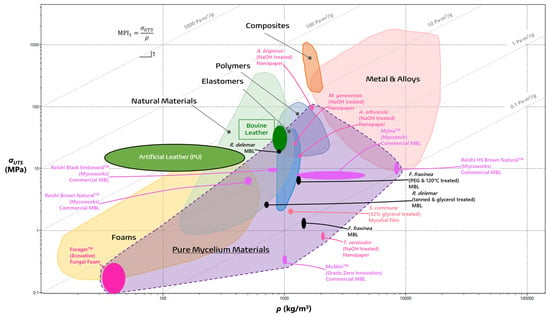
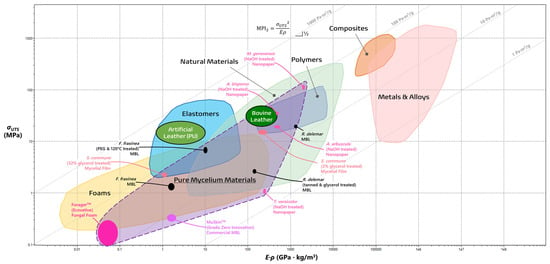
3.2. The Past, Present, and Future of Mycelial Textiles
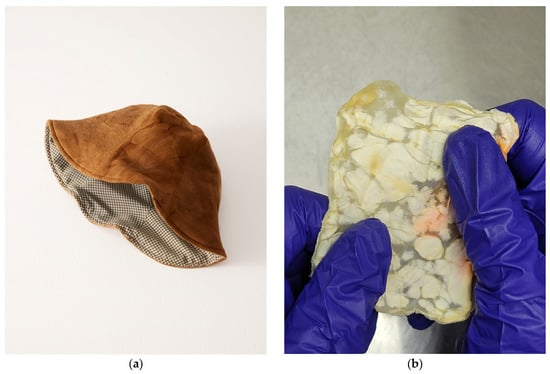
| Textile | ρ (kg/m3) | %EL | σUTS (MPa) | E (GPa) | MPI1 (Pa·m3/g) | MPI2 (Pa·m3/g) | References |
|---|---|---|---|---|---|---|---|
| Fomitella fraxinea MBL (oak & bran substrate) |
1580 | 4.30–4.98 | 1.18–1.62 | 0.00117–0.00157 | 0.0875 | 893 | [19] |
| Fomitella fraxinea MBL polyethylene glycol treated with 120 °C heat press (oak & bran substrate) |
1460 | 13.87–17.91 | 6.28–8.14 | 0.00669–0.00736 | 4.9 | 4990 | [19] |
| Rhizopus delemar MBL (bread substrate) |
884–922 | 1.7–2.3 | 19.04–20.74 | 1.38–1.50 | 2.2 | 304 | [7] |
| Rhizopus delemar MBL, tannin/glycerol treated (bread substrate) |
695–739 | 14.5–18.6 | 2.51–2.93 | 0.199–0.201 | 0.378 | 5.14 | [7] |
| MuSkinTM MBL (Grado Zero Innovation, Firenze, Italy) * |
1000 | 17.3–38.6 | 0.3–0.4 | 0.0018–0.0028 | 0.0346 | 53.5 | [52][62] |
| ReishiTM Brown Natural MBL (MycoWorks, Emeryville, CA, USA) * |
480–540 | 16–36 | 5.6–7.4 | - | 1.27 | - | [46][63] |
| ReishiTM Brown Natural High Strength MBL (MycoWorks) * |
8400 | 55–80 | 8.8–12.5 | - | 0.125 | - | [47][63] |
| ReishiTM Black Emboss MBL (MycoWorks) * |
740–880 | 51–52 | 9.2–10.2 | - | 1.2 | - | [48][63] |
| MyleaTM MBL (Mycotech; Aurora, CO, USA) * |
1330–4440 | 22–35 | 8–11 | - | 0.386 | - | [49][50] |
| Artificial leather (Polyurethane composites) |
340–470 | - | 9.4–24.5 | 0.012–0.036 | 12 | 87,700 | [52][64][65] |
| Bovine leather | 810–1050 | 18–75 | 20–50 | 0.10–0.50 | 3.43 | 4560 | [51] |
3.3. Flexible Fungal Foams
References
- Kendrick, B. Fungi and the History of Mycology. In eLS; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011; ISBN 978-0-470-01590-2.
- Naranjo-Ortiz, M.A.; Gabaldón, T. Fungal Evolution: Diversity, Taxonomy and Phylogeny of the Fungi. Biol. Rev. Camb. Philos. Soc. 2019, 94, 2101–2137.
- Gow, N.A.R.; Latge, J.-P.; Munro, C.A. The Fungal Cell Wall: Structure, Biosynthesis, and Function. Microbiol. Spectr. 2017, 5, FUNK-0035-2016.
- Ainsworth, G.C.; Bisby, G.R.; Kirk, P.M.; Cannon, P.F.; Minter, D.W.; Stalpers, J.A. Ainsworth & Bisby’s Dictionary of the Fungi, 10th ed.; CABI: Wallingford, UK, 2008; ISBN 978-0-85199-826-8.
- Jones, M.; Bhat, T.; Huynh, T.; Kandare, E.; Yuen, R.; Wang, C.H.; John, S. Waste-Derived Low-Cost Mycelium Composite Construction Materials with Improved Fire Safety. Fire Mater. 2018, 42, 816–825.
- Pelletier, M.G.; Holt, G.A.; Wanjura, J.D.; Greetham, L.; McIntyre, G.; Bayer, E.; Kaplan-Bie, J. Acoustic Evaluation of Mycological Biopolymer, an All-Natural Closed Cell Foam Alternative. Ind. Crops Prod. 2019, 139, 111533.
- Wijayarathna, E.R.K.B.; Mohammadkhani, G.; Soufiani, A.M.; Adolfsson, K.H.; Ferreira, J.A.; Hakkarainen, M.; Berglund, L.; Heinmaa, I.; Root, A.; Zamani, A. Fungal Textile Alternatives from Bread Waste with Leather-like Properties. Resour. Conserv. Recycl. 2022, 179, 106041.
- Livne, A.; Wösten, H.A.B.; Pearlmutter, D.; Gal, E. Fungal Mycelium Bio-Composite Acts as a CO2-Sink Building Material with Low Embodied Energy. ACS Sustain. Chem. Eng. 2022, 10, 12099–12106.
- Meyer, V.; Basenko, E.Y.; Benz, J.P.; Braus, G.H.; Caddick, M.X.; Csukai, M.; de Vries, R.P.; Endy, D.; Frisvad, J.C.; Gunde-Cimerman, N.; et al. Growing a Circular Economy with Fungal Biotechnology: A White Paper. Fungal Biol. Biotechnol. 2020, 7, 5.
- Cairns, T.C.; Zheng, X.; Zheng, P.; Sun, J.; Meyer, V. Turning Inside Out: Filamentous Fungal Secretion and Its Applications in Biotechnology, Agriculture, and the Clinic. J. Fungi 2021, 7, 535.
- Butu, A.; Rodino, S.; Miu, B.; Butu, M. Mycelium-Based Materials for the Ecodesign of Bioeconomy. Dig. J. Nanomater. Biostructures 2020, 15, 1129–1140.
- Jones, M.; Mautner, A.; Luenco, S.; Bismarck, A.; John, S. Engineered Mycelium Composite Construction Materials from Fungal Biorefineries: A Critical Review. Mater. Des. 2020, 187, 108397.
- Vandelook, S.; Elsacker, E.; Van Wylick, A.; De Laet, L.; Peeters, E. Current State and Future Prospects of Pure Mycelium Materials. Fungal Biol. Biotechnol. 2021, 8, 20.
- Bentangan, M.A.; Nugroho, A.R.; Hartantyo, R.; Ilman, R.Z.; Ajidarma, E.; Nurhadi, M.Y. Mycelium Material, Its Method to Produce and Usage as Leather Substitute. WO2020136448A1, 2 July 2020.
- Derbyshire, E.J.; Delange, J. Fungal Protein—What Is It and What Is the Health Evidence? A Systematic Review Focusing on Mycoprotein. Front. Sustain. Food Syst. 2021, 5, 581682.
- Kaplan-Bie, J.H.; Bonesteel, I.T.; Greetham, L.; McIntyre, G.R. Increased Homogeneity of Mycological Biopolymer Grown into Void Space. WO2019099474A1, 23 May 2019.
- Barabash, A.; Deschenko, V.; Chuk, O. Leather-like Material Biofabrication Using Pleurotus osteatus. Ph.D. Thesis, National Aviation University, Kyiv, Ukraine, 2021.
- Cartabia, M.; Girometta, C.E.; Milanese, C.; Baiguera, R.M.; Buratti, S.; Branciforti, D.S.; Vadivel, D.; Girella, A.; Babbini, S.; Savino, E.; et al. Collection and Characterization of Wood Decay Fungal Strains for Developing Pure Mycelium Mats. J. Fungi 2021, 7, 1008.
- Raman, J.; Kim, D.-S.; Kim, H.-S.; Oh, D.-S.; Shin, H.-J. Mycofabrication of Mycelium-Based Leather from Brown-Rot Fungi. J. Fungi 2022, 8, 317.
- Gandia, A.; van den Brandhof, J.G.; Appels, F.V.W.; Jones, M.P. Flexible Fungal Materials: Shaping the Future. Trends Biotechnol. 2021, 39, 1321–1331.
- Gowthaman, M.K.; Krishna, C.; Moo-Young, M. Fungal Solid State Fermentation—An Overview. Appl. Mycol. Biotechnol. 2001, 1, 305–352.
- Grimm, D.; Wösten, H.A.B. Mushroom Cultivation in the Circular Economy. Appl. Microbiol. Biotechnol. 2018, 102, 7795–7803.
- Hölker, U.; Lenz, J. Solid-State Fermentation—Are There Any Biotechnological Advantages? Curr. Opin. Microbiol. 2005, 8, 301–306.
- Machida, M.; Yamada, O.; Gomi, K. Genomics of Aspergillus Oryzae: Learning from the History of Koji Mold and Exploration of Its Future. DNA Res. 2008, 15, 173–183.
- Rahardjo, Y.S.P. Fungal Mats in Solid-State Fermentation; Wageningen University: Wageningen, The Netherlands, 2005.
- Fazenda, M.L.; Seviour, R.; McNeil, B.; Harvey, L.M. Submerged Culture Fermentation of “Higher Fungi”: The Macrofungi. In Advances in Applied Microbiology; Academic Press: Cambridge, MA, USA, 2008; Volume 63, pp. 33–103.
- Kaplan-Bie, J.H. Solution Based Post-Processing Methods for Mycological Biopolymer Material and Mycological Product Made Thereby. WO2018183735A1, 4 October 2018.
- Ross, P.; Scullin, M.; Wenner, N.; Chase, J.; Miller, Q.; Saltidos, R.; McGaughy, P. Mycelium Growth Bed with Perforation Layer and Related Method for Creating a Uniform Sheet of Mycelium from a Solid-State Medium. US20200196541A1, 25 June 2020.
- Williams, E.; Cenian, K.; Golsteijn, L.; Morris, B.; Scullin, M.L. Life Cycle Assessment of MycoWorks’ ReishiTM: The First Low-Carbon and Biodegradable Alternative Leather. Environ. Sci. Eur. 2022, 34, 120.
- Thakur, M.P. Advances in Mushroom Production: Key to Food, Nutritional and Employment Security: A Review. Indian. Phytopathol. 2020, 73, 377–395.
- Atlast Food Co. Partners with Whitecrest Mushrooms Ltd. to Scale Production of MyBacon® Strips. Available online: https://myforestfoods.com/myblog/blog-whitecrest (accessed on 19 February 2024).
- César, E.; Canche-Escamilla, G.; Montoya, L.; Ramos, A.; Duarte-Aranda, S.; Bandala, V.M. Characterization and Physical Properties of Mycelium Films Obtained from Wild Fungi: Natural Materials for Potential Biotechnological Applications. J. Polym. Environ. 2021, 29, 4098–4105.
- Jones, M.; Weiland, K.; Kujundzic, M.; Theiner, J.; Kählig, H.; Kontturi, E.; John, S.; Bismarck, A.; Mautner, A. Waste-Derived Low-Cost Mycelium Nanopapers with Tunable Mechanical and Surface Properties. Biomacromolecules 2019, 20, 3513–3523.
- Petre, A.; Ene, M.; Vamanu, E. Submerged Cultivation of Inonotus Obliquus Mycelium Using Statistical Design of Experiments and Mathematical Modeling to Increase Biomass Yield. Appl. Sci. 2021, 11, 4104.
- Cui, Y.Q.; van der Lans, R.G.J.M.; Luyben, K.C.a.M. Effect of Agitation Intensities on Fungal Morphology of Submerged Fermentation. Biotechnol. Bioeng. 1997, 55, 715–726.
- Tang, Y.-J.; Zhu, L.-W.; Li, H.-M.; Li, D.-S. Submerged Culture of Mushrooms in Bioreactors—Challenges, Current State-of-the-Art, and Future Prospects. Food Technol. Biotechnol. 2007, 45, 221–229.
- Bakratsas, G.; Polydera, A.; Nilson, O.; Chatzikonstantinou, A.V.; Xiros, C.; Katapodis, P.; Stamatis, H. Mycoprotein Production by Submerged Fermentation of the Edible Mushroom Pleurotus ostreatus in a Batch Stirred Tank Bioreactor Using Agro-Industrial Hydrolysate. Foods 2023, 12, 2295.
- Szilvay, G.; Barrantes, M.A. Mycelium Leather: Sustainable Alternative for Leather. Available online: https://www.vttresearch.com/en/news-and-ideas/alternative-leather-and-synthetic-leather-vtt-succeeded-demonstrating-continuous (accessed on 19 February 2024).
- Bae, B.; Kim, M.; Kim, S.; Ro, H.-S. Growth Characteristics of Polyporales Mushrooms for the Mycelial Mat Formation. Mycobiology 2021, 49, 280–284.
- Ashby, M.F. Materials Selection in Mechanical Design, 5th ed.; Butterworth-Heinemann: Amsterdam, The Netherlands; Cambridge, MA, USA, 2017; ISBN 978-0-08-100599-6.
- Ashby, M.F. Overview No. 80: On the Engineering Properties of Materials. Acta Metall. 1989, 37, 1273–1293.
- Ashby, M.F.; Cebon, D. Materials Selection in Mechanical Design. J. Phys. Arch. 1993, 3, C7-1–C7-9.
- Ashby, M.F.; Gibson, L.J.; Wegst, U.; Olive, R. The Mechanical Properties of Natural Materials. I. Material Property Charts. Proc. R. Soc. Lond. A 1995, 450, 123–140.
- Jones, M.; Gandia, A.; John, S.; Bismarck, A. Leather-like Material Biofabrication Using Fungi. Nat. Sustain. 2021, 4, 9–16.
- Appels, F.V.W.; van den Brandhof, J.G.; Dijksterhuis, J.; de Kort, G.W.; Wösten, H.A.B. Fungal Mycelium Classified in Different Material Families Based on Glycerol Treatment. Commun. Biol. 2020, 3, 334.
- Lin, J.; Sadowy, S. Vartest Report on Mycoworks REISHITM Brown Natural; Vartest Laboratories: New York, NY, USA, 2020.
- Lin, J.; Sadowy, S. Vartest Report on Mycoworks REISHITM Brown Natural—High Strength; Vartest Laboratories: New York, NY, USA, 2020.
- Lin, J.; Sadowy, S. Vartest Report on Mycoworks REISHITM Black Emboss; Vartest Laboratories: New York, NY, USA, 2020.
- Mylea Technical Data Sheet. Available online: https://mycl.bio/storage/app/media/mylea/Mylea%20Technical%20Data%20Sheet.pdf (accessed on 10 May 2023).
- Kuijk, A. Biofabricated Mycelium Mylea Leather Sheets~HauteMatter. Available online: https://www.hautematter.com/product-deck/mylea (accessed on 5 October 2023).
- GRANTA EduPack; ANSYS, Inc.: Cambridge, UK; Available online: https://www.ansys.com/materials (accessed on 20 January 2024).
- Meyer, M.; Dietrich, S.; Schulz, H.; Mondschein, A. Comparison of the Technical Performance of Leather, Artificial Leather, and Trendy Alternatives. Coatings 2021, 11, 226.
- Quito, A. Mushroom Leather: How Fungi Became Fashionable. Available online: https://qz.com/emails/quartz-obsession/1849446383/mushroom-leather-how-fungi-became-fashionable (accessed on 7 December 2022).
- Papp, N.; Rudolf, K.; Bencsik, T.; Czégényi, D. Ethnomycological Use of Fomes fomentarius (L.) Fr. and Piptoporus betulinus (Bull.) P. Karst. in Transylvania, Romania. Genet. Resour. Crop Evol. 2017, 64, 101–111.
- Pegler, D.N. Useful Fungi of the World: Amadou and Chaga. Mycologist 2001, 15, 153–154.
- Hahn, J. Traditional Transylvanian Mushroom Leather Wraps Seating Collection by Mari Koppanen. Available online: https://www.dezeen.com/2022/02/02/mari-koppanen-fomes-amadou-seating-design/ (accessed on 7 December 2022).
- Kalitukha, L.; Sari, M. Fascinating Vital Mushrooms. Tinder Fungus (Fomes fomentarius (L.) Fr.) as a Dietary Supplement. Int. J. Res. Stud. Sci. Eng. Technol. 2019, 6, 1–9.
- Blanchette, R.A.; Haynes, D.T.; Held, B.W.; Niemann, J.; Wales, N. Fungal Mycelial Mats Used as Textile by Indigenous People of North America. Mycologia 2021, 113, 261–267.
- Amadou Tulip Hat—EDEN Power Corp. Available online: https://web.archive.org/web/20220829223510/https://edenpowercorp.com/collections/fungus-yeasts-molds-and-mushrooms/products/pre-order-amadou-tulip-hat (accessed on 24 May 2023).
- Hordon How to Make Amadou Tinder. Available online: https://www.beaverbushcraft.co.uk/page_4140610.html (accessed on 24 May 2023).
- Deeg, K.; Gima, Z.T.; Smith, A.; Stoica, O.C.; Tran, K. Greener Solutions: Improving Performance of Mycelium-Based Leather. Final Report to MycoWorks. 2017. Available online: https://bcgctest.files.wordpress.com/2018/03/gs_2017_mycoworks_finalreport.pdf (accessed on 20 January 2024).
- Bustillos, J.; Loganathan, A.; Agrawal, R.; Gonzalez, B.A.; Perez, M.G.; Ramaswamy, S.; Boesl, B.; Agarwal, A. Uncovering the Mechanical, Thermal, and Chemical Characteristics of Biodegradable Mushroom Leather with Intrinsic Antifungal and Antibacterial Properties. ACS Appl. Bio Mater. 2020, 3, 3145–3156.
- Scullin, M.; Rigas, N. A Story of Superior Quality. ReishiTM. 2020. Available online: https://www.madewithreishi.com/stories/ (accessed on 20 January 2024).
- Roh, E.K. Mechanical Properties and Preferences of Natural and Artificial Leathers, and Their Classification with a Focus on Leather for Bags. J. Eng. Fibers Fabr. 2020, 15, 155892502096882.
- Eun, J.H.; Lee, J.S. Study on the NCO Index and Base Knitted Fabric Substrates on the Thermal, Chemical, and Mechanical Properties of Solvent-Less Formulations Polyurethane Artificial Leather. J. Eng. Fibers Fabr. 2020, 15, 1558925020916458.
- Ettinger, J. Balenciaga Goes “Livestock-Free” With a New €9,000 Coat Made From Mycelium. Ethos. 2022. Available online: https://the-ethos.co/balenciaga-mycelium-leather-coat/ (accessed on 20 January 2024).
- Davidson, L. Ecovative Announces Partners to Bring Mycelium Foam to the Fashion Industry. Available online: https://www.businesswire.com/news/home/20220322005069/en/Ecovative-Announces-Partners-To-Bring-Mycelium-Foam-to-the-Fashion-Industry (accessed on 8 December 2022).
- Greetham, L.; McIntyre, G.R.; Bayer, E.; Winiski, J.; Araldi, S. Mycological Biopolymers Grown in Void Space Tooling. Patent US11277979B2, 22 March 2022. Available online: https://patents.google.com/patent/US20150033620A1/en (accessed on 20 January 2024).
- Compostable Foam: Breathable, Insulating, Water-Repellant|Ecovative. Available online: https://www.ecovative.com/pages/foams (accessed on 23 November 2023).
- Pelletier, M.G.; Holt, G.A.; Wanjura, J.D.; Bayer, E.; McIntyre, G. An Evaluation Study of Mycelium Based Acoustic Absorbers Grown on Agricultural By-Product Substrates. Ind. Crops Prod. 2013, 51, 480–485.
- Asdrubali, F.; Schiavoni, S.; Horoshenkov, K.V. A Review of Sustainable Materials for Acoustic Applications. Build. Acoust. 2012, 19, 283–311.
- Bird, E.T.; Bowden, A.E.; Seeley, M.K.; Fullwood, D.T. Materials Selection of Flexible Open-Cell Foams in Energy Absorption Applications. Mater. Des. 2018, 137, 414–421.





