Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Shaik Ismail Mohammed Thangameeran | -- | 4562 | 2024-03-12 04:37:36 | | | |
| 2 | Camila Xu | Meta information modification | 4562 | 2024-03-13 02:31:38 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Thangameeran, S.I.M.; Wang, P.; Liew, H.; Pang, C. Influence of Alcohol on Intracerebral Hemorrhage. Encyclopedia. Available online: https://encyclopedia.pub/entry/56141 (accessed on 07 February 2026).
Thangameeran SIM, Wang P, Liew H, Pang C. Influence of Alcohol on Intracerebral Hemorrhage. Encyclopedia. Available at: https://encyclopedia.pub/entry/56141. Accessed February 07, 2026.
Thangameeran, Shaik Ismail Mohammed, Po-Kai Wang, Hock-Kean Liew, Cheng-Yoong Pang. "Influence of Alcohol on Intracerebral Hemorrhage" Encyclopedia, https://encyclopedia.pub/entry/56141 (accessed February 07, 2026).
Thangameeran, S.I.M., Wang, P., Liew, H., & Pang, C. (2024, March 12). Influence of Alcohol on Intracerebral Hemorrhage. In Encyclopedia. https://encyclopedia.pub/entry/56141
Thangameeran, Shaik Ismail Mohammed, et al. "Influence of Alcohol on Intracerebral Hemorrhage." Encyclopedia. Web. 12 March, 2024.
Copy Citation
Alcohol, a key part of human culture since ancient times, has various uses ranging from beverages to antiseptics and fuels. Different types of alcoholic beverages such as wine and beer, being the oldest and probably the most widely used drugs, were known for their therapeutic value in addition to the vital part they played in the daily life of people in ancient times. A standard alcohol unit, used to measure drinking consistently, varies globally. The World Health Organization (WHO) suggests that a standard drink contains 10 g of pure ethanol per day, though this varies (8–20 g) across countries.
alcohol consumption
intracerebral hemorrhage
alcoholism
1. Introduction
Alcohol, a key part of human culture since ancient times, has various uses ranging from beverages to antiseptics and fuels. Different types of alcoholic beverages such as wine and beer, being the oldest and probably the most widely used drugs, were known for their therapeutic value in addition to the vital part they played in the daily life of people in ancient times. Ethanol, produced by fermentation, was consumed either in a diluted form or concentrated by distillation and these beverages were often considered divine, featuring in religious ceremonies, mythology, and social meals like the Greek symposia [1].
Despite its role in social events and its increased accessibility, alcohol abuse poses significant public health and economic challenges. In the exploration of alcohol’s impact on health, it is crucial to present a balanced view that encompasses both its potential benefits and risks. While moderate alcohol consumption has been associated with certain cardiovascular benefits, these effects must be critically weighed against the well-documented risks, especially concerning intracerebral hemorrhage (ICH). This includes the exacerbation of risk factors such as hypertension and the direct contribution to secondary brain injury (SBI) mechanisms. The nuanced nature of alcohol’s effects necessitates a careful examination of dose—response relationships and the differential impacts of consumption patterns [2][3][4]. Moderate alcohol consumption may offer neuroprotective benefits through mechanisms like oxidative stress reduction and improved vascular health, whereas heavy drinking exacerbates ICH risk factors [5].
Chronic heavy drinking is linked to increased hypertension and cardiovascular diseases [6][7][8], elevating blood pressure and disrupting blood coagulation [9]. Conversely, moderate drinking is often associated with positive cardiovascular effects [10][11][12] and could benefit cardiac health through certain physiological mechanisms [13][14]. Alcohol consumption also influences risk factors for ICH, such as hypertension, smoking, and the use of certain medications [15][16][17].
2. Standard Drink of Alcohol
A standard alcohol unit, used to measure drinking consistently, varies globally. The World Health Organization (WHO) suggests that a standard drink contains 10 g of pure ethanol per day, though this varies (8–20 g) across countries (Figure 1). Kalinowski and Humphreys (2016) systemically reviewed government guidelines worldwide. The National Institute on Alcohol Abuse and Alcoholism (NIAAA) categorizes alcohol use as moderate (up to 1 drink daily for women and 2 for men), binge drinking (5 or more drinks for men and 4 for women, within about 2 h), and heavy alcohol use (more than 4 drinks per day or 14 per week for men and more than 3 drinks per day or 7 per week for women). In the US, a standard drink is defined as 14 g of pure alcohol [18].
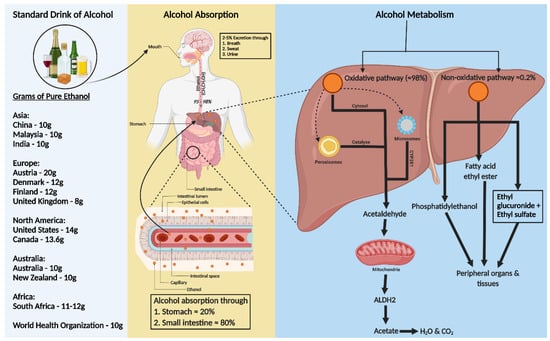
Figure 1. Consumption, absorption, and metabolism of alcohol. Consumption of alcohol (expressed as grams of ethanol per day) among different regions around the world is shown on the left of the figure. Alcohol is primarily absorbed in the small intestine (80%) and stomach (20%), with 2–5% excreted unchanged via breath, sweat, and urine. The rest, 95–98% of ethanol, is metabolized in the liver through two pathways: oxidative and non-oxidative. The oxidative pathway involves alcohol dehydrogenase (ADH) in the cytosol converting ethanol to acetaldehyde and further metabolism to acetate by aldehyde dehydrogenase 2 (ALDH2) in the mitochondria. Cytochrome P450 2E1 (CYP2E1) and catalase also play roles in ethanol metabolism, especially at higher consumption levels. In the non-oxidative pathway, ethanol forms fatty acid ethyl ester (FAEE) or phosphatidyl ethanol, catalyzed by FAEE synthase and phospholipase D. The metabolites from both pathways enter the circulation, impacting peripheral organs.
3. Alcohol Absorption and Metabolism
Ethanol, a water, and lipid-soluble molecule, is easily absorbed through biological membranes. It is distributed quickly throughout the body after consumption, entering the bloodstream primarily via the stomach (20%) and small intestine (80%), with faster absorption in the intestine due to larger surface areas of villi and microvilli (Figure 1) [19][20]. Only 2–5% of alcohol is excreted unchanged through the lungs, skin, and kidneys [21]. Most ethanol is metabolized in the liver, through oxidative and non-oxidative pathways [22][23].
In the liver, alcohol dehydrogenase (ADH) converts ethanol to acetaldehyde, which is then rapidly changed to acetate by aldehyde dehydrogenase 2 (ALDH2) in the mitochondria [23][24]. This acetate enters the Krebs cycle, becoming water and carbon dioxide for elimination [25]. Cytochrome P450 monooxygenases (CYP2E1) and catalase in the peroxisome also contribute to oxidative metabolism, especially after heavy drinking [22][23][24][26][27].
Less than 0.2% of ethanol undergoes non-oxidative metabolism in the liver, forming ethyl glucuronide (EtG) and ethyl sulfate (EtS) [28]. Ethanol can also be metabolized to fatty acid ethyl ester (FAEE) via FAEE synthase and to phosphatidyl ethanol by phospholipase D [29] (Figure 1).
4. Alcohol Induced Hypertension
Research indicates that high alcohol consumption is linked to increased blood pressure, with clinical and preclinical studies confirming this correlation [30][31][32][33]. High alcohol intake activates the sympathetic nervous system and increases corticotropin-releasing factor secretion [34], impairs baroreceptor activity in the brainstem, and raises levels of cortisol and angiotensin, known blood pressure modulators [35][36]. Some studies illuminate the intricate interplay between alcohol consumption and vascular health. Chronic alcohol exposure significantly alters endothelial and smooth muscle function across various tissues, including the buccal mucosa and systemic circulation. This manifests as increased endothelin-1 levels, decreased nitric oxide synthase (NOS) activity, and direct effects on vascular smooth muscle, influencing vasodilation and blood pressure regulation. While moderate alcohol intake may confer some protective cardiovascular effects, chronic and heavy consumption exacerbate vascular dysfunction, highlighting alcohol’s dual role in cardiovascular health [37][38][39]. A review of 30 cross-sectional population studies found small but significant elevations in blood pressure in those consuming three drinks or more per day compared to nondrinkers [40].
Conversely, moderate alcohol consumption shows a potential effect of lowering blood pressure. Studies on individuals consuming up to two drinks per day did not find a correlation between reduced intake and decreased blood pressure, though cutting down alcohol did lower systolic and diastolic blood pressure [41]. The exact mechanism by which moderate alcohol consumption affects blood pressure remains unclear, suggesting a need for further molecular studies.
5. ICH
Spontaneous ICH, causing about 10 to 15% of all strokes with an incidence of 4.3 per 10,000 person-years, significantly impacts global health, particularly in low- and middle-income countries. In 2010, it affected 5.3 million people globally. The burden of both ischemic and hemorrhagic stroke has increased significantly in terms of the absolute number of incidents, deaths, and disability-adjusted life-years (DALYs) lost, with most of the burden in low-income and middle-income countries. High-income countries have seen a reduction in the incidence and mortality of these strokes, indicating the effectiveness of improved healthcare and preventive strategies. However, in low-income and middle-income countries, an increase in the incidence of hemorrhagic stroke and a non-significant increase in ischemic stroke have been noted [42].
ICH is a debilitating condition with only about 20% of survivors managing self-care six months post-ICH and 74% experiencing ongoing symptoms after a year. It has a high 30-day mortality rate of about 40%. ICH typically involves hematoma formation within the brain parenchyma, often in the putamen, and can affect areas like the cerebral lobes, basal ganglia, thalamus, brainstem, and cerebellum [43][44][45]. The condition encompasses two phases: an initial rupture of blood vessels, often due to hypertension or cerebral amyloid angiopathy, leading to primary brain injury from hematoma formation and expansion. Subsequently, SBI follows, involving hematoma clearance, immune response, neuroendocrine activation, and homeostasis response [46][47]. The severity of primary brain injury is linked to the site and volume of the hematoma, its expansion rate, and edema formation [43][48][49][50]. Large hematomas can be fatal due to increased intracranial pressure and potential brain herniation, restricting blood flow to certain brain regions [51].
6. SBI in ICH
SBI is a crucial factor in brain damage following ICH. It results from the volumetric compression of brain tissue and toxic blood components causing edema, blood–brain barrier (BBB) disruption, cell apoptosis, necrosis, and severe inflammation [52]. Hematomas disrupt neurons and glial cells, leading to neurotransmitter release and mitochondrial dysfunction. Coagulation products, particularly thrombin, initiate SBI, exacerbating blood-induced neurotoxicity [15][53][54]. This process activates microglia, subsequently releasing inflammatory cytokines, damaging the BBB, and causing neuronal apoptosis. Similarly, the breakdown of hemoglobin (Hb) activates astrocytes, contributing to neuronal damage through cytokine release [55]. Both microglia and astrocyte activation are mediated by toll-like receptors (TLR) [56] (a graphical representation of SBI events is depicted in Figure 2). In the evolving narrative of alcohol’s impact on ICH, emerging research delineates a J-shaped curve, signifying that while heavy consumption exacerbates ICH risk, moderate drinking may offer neuroprotection by mitigating oxidative stress and inflammation. This dual nature underscores the complexity of alcohol’s effects on SBI, suggesting a critical need for nuanced public health guidelines and personalized clinical strategies [57].
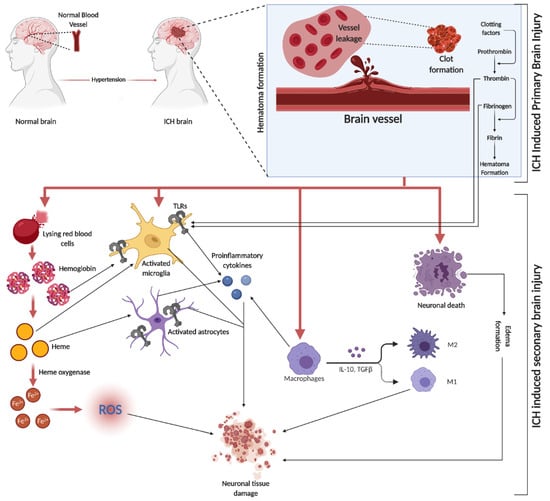
Figure 2. ICH is initiated by the rupture of blood vessels in the brain, leading to red blood cell (RBC) leakage and hematoma formation, facilitated by clotting factors like thrombin, fibrin, and fibrinogen. The subsequent SBI involves erythrocyte lysis, releasing Hb, which is broken down into heme and iron (Fe2+), triggering oxidative stress through various reactive oxygen species (ROS). This process activates microglia and astrocytes, with TLRs playing a role in their activation. The initial event causes direct neuronal damage and edema, further exacerbating tissue damage. Additionally, circulating macrophages infiltrate the injury site, differentiating into pro-inflammatory M1 and anti-inflammatory M2 macrophages, releasing a mix of cytokines.
The relationship between alcohol consumption and ICH presents a complex interplay of factors that influence the onset and progression of this critical condition. Notably, the role of alcohol, ranging from moderate to heavy consumption, delineates varying paths of influence through oxidative stress, neuroendocrine disruptions, and neuroinflammatory responses leading to SBI. While the body of evidence, predominantly sourced from rodent models, offers significant insights into the molecular mechanisms underpinning these effects, the direct translatability of these findings to human pathophysiology remains a subject of considerable debate.
7. Role of Alcohol on SBI-Induced Toxic Products
Following ICH, there is an active clearance of red blood cells (RBCs) by microglia, macrophages, and infiltrating macrophages. RBCs break down into Hb, heme, and iron, with mechanisms like haptoglobin (Hp) binding to Hb to form a less toxic complex, aiding in preventing brain damage. Vascular injury during also leads to thrombin generation, contributing to clot formation and affecting microparticles from platelets and endothelial cells [15][54][56][58][59].
Studies have shown that alcohol consumption influences factors like fibrinogen and Hp, which are both primarily synthesized by the liver. Moderate alcohol intake (15.4 g/day) is associated with reduced fibrinogen levels, potentially lowering clot density and influencing cardiovascular health, particularly hypertension [60]. Similarly, moderate alcohol consumption correlates with lower Hp levels and can decrease fibrinogen levels in individuals post-exercise [61]. Additionally, chronic alcohol exposure in mice downregulated heme oxygenase (HO)-1, a key enzyme in heme catabolism during erythrocyte destruction [62].
8. Effect of Alcohol on Mitochondrial Dysfunction and Oxidative Stress in Brain Injury
Mitochondria, crucial in cellular function, regulate the balance between pro-oxidants and antioxidants, calcium homeostasis, ATP production, and stress responses [59]. After ICH, SBI involves toxic product release, leading to oxidative stress and cell injury. This process includes the breakdown of Hb into products like heme and iron. These products then produce hydroxyl radicals through the Fenton reaction, which exacerbates oxidative damage [63][64].
Oxidative stress, characterized by an overproduction of reactive oxygen species (ROS), surpasses antioxidant defenses, causing cellular damage. Antioxidants like superoxide dismutase (SOD) and glutathione peroxidase (GPx) decrease in models, indicating an imbalance in ROS and antioxidant production [65][66][67][68]. Microglial activation and iron toxicity from injury lead to lipid ROS accumulation and ferroptosis cell death, with proinflammatory cytokines further inducing ROS through enzymes like nicotinamide adenine dinucleotide phosphate (NADPH) oxidase [52][69][70][71][72]. SBI triggers mitochondrial dysfunction, with calcium and iron overload leading to increased ROS and apoptosis. Increased matrix metalloproteinases are associated with oxidative stress and BBB disruption [73][74][75][76][77]. Alcohol consumption, especially chronic heavy use, further exacerbates mitochondrial dysfunction and oxidative stress, impacting bioenergetics and increasing cell apoptosis [78][79][80].
Studies have shown varying effects of alcohol on ICH. Moderate alcohol preconditioning reduced hematoma volume, blood pressure, and oxidative and ER stress in a rat model, whereas high levels exacerbated these conditions [5]. Ethanol exposure was found to induce mitochondrial dysfunction and apoptotic gene regulation in human platelets [81]. These findings highlight the complex role of alcohol in mitochondrial function and oxidative stress in the context of ICH.
9. Effect of Alcohol on Neuroendocrine Axis Activation
Following ICH, the body activates the hypothalamic–pituitary–adrenal (HPA) axis and the sympathetic nervous system (SNS) to manage the stress induced by the injury. This response, involving the release of inflammatory cytokines, creates a feedback mechanism that further stimulates the HPA axis and SNS [82][83][84][85][86].
However, chronic alcohol consumption can impair HPA axis function. Studies have shown that mild alcohol intoxication inhibits the HPA axis response to cortisol [87][88], and active alcoholics exhibit compromised HPA axis activity under basal conditions [88]. Heavy and hazardous drinkers also have diminished cortisol reactivity compared to light or social drinkers [88][89][90]. Even in zebrafish larvae, 1% ethanol exposure led to increased HPA axis hormones [91]. This impairment suggests that heavy alcohol intoxication could hinder the necessary HPA axis response during CNS injuries. Conversely, chronic alcoholics exhibit increased SNS activity, with acute rises in blood alcohol levels also showing heightened SNS activity [92][93].
10. Alcohol and Activation of Complement System during SBI
Complement-mediated brain injury, particularly through the formation of the membrane attack complex (MAC), plays a key role in erythrocyte lysis, leading to delayed brain edema and inflammation after ICH [94]. MAC not only contributes to erythrocyte lysis but also influences cellular functions by releasing cytokines, ROS, and matrix proteins [95][96]. Components of the complement system, like C5a and C3a, are involved in leukocyte chemotaxis and microglial activation, respectively, enhancing inflammatory responses post-ICH [97][98]. The presence of complement C3d and C9 in peri-hematoma regions confirms the activation of the complement system in ICH [99].
Research on fetal alcohol spectrum disorders in rats shows that postnatal alcohol exposure results in the secretion of microglial exosome and the complement C1q, both of which are linked to ethanol-induced neuronal apoptosis and necrosis [100]. These findings underscore the need to understand the relationship between alcohol intoxication, the complement system, and ICH, especially regarding hematoma clearance, edema formation, and inflammation in the context of alcohol consumption.
11. Alcohol and Neuroinflammatory Mechanism Activated in SBI
Neuroinflammation in SBI after ICH is a complex and multifaceted process. It involves the activation of various immune cells, including microglia and astrocytes, and is marked by the release of cytokines and chemokines. These inflammatory mediators contribute significantly to brain tissue damage. The response is not just limited to local effects but also involves systemic reactions, including the neuroendocrine axis and the complement system, which further exacerbate the inflammation and subsequent neuronal damage [101][102][103].
Recent studies have highlighted potential therapeutic targets within these neuroinflammatory pathways. Strategies to modulate this response, including the use of immunotherapies, are being explored. Therapeutic interventions aim to balance the inflammatory response, reducing the damaging effects while promoting beneficial repair mechanisms. This approach could significantly improve outcomes for patients suffering from ICH, as it addresses both the immediate and long-term consequences of the inflammatory response in the brain [101][104] (Refer to Figure 3 for the representative figure of the multifaceted effect of alcohol on ICH components).
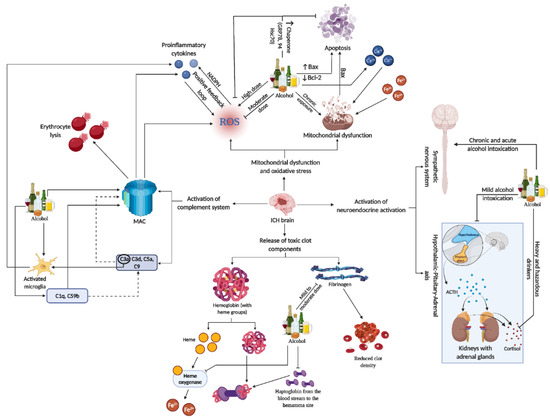
Figure 3. This representative figure delineates the multifaceted interactions between alcohol consumption patterns and brain injury subsequent to ICH. It maps out the cascade from alcohol-induced microglia activation and complement system engagement to the generation of ROS, mitochondrial damage, and apoptosis. It further illustrates systemic effects, including neuroendocrine responses, highlighting the extensive influence of alcohol on both brain pathology and peripheral organ systems in the context of ICH. For a detailed understanding, please refer to Section 11..
11.1. Influence of Alcohol on Microglial Activation and Polarization
Microglia, the central nervous system’s resident macrophages, are activated by the release of toxic components from erythrocyte lysis following a hematoma, primarily through TLRs [105][106]. Their primary function is to clear hematoma debris, largely through pathways involving nuclear factor erythroid 2-related factor 2 (Nrf2) [107], peroxisome proliferator-activated receptor γ (PPARγ) [108], and interleukin-10 (IL-10) mediated regulation of CD-36 [109].
Microglia polarization occurs post-activation, differentiating into pro-inflammatory M1 microglia, which produce IL-6, IL-1β, TNF-α, and increase ROS, and anti-inflammatory M2 microglia, which release cytokines like IL-10 and IL-4, promoting nerve repair through neurotrophic and growth factors [110][111].
In a study using a four-day binge alcohol exposure model where rats received ethanol (5 g/kg), there was a notable increase in both M1 and M2 microglia populations in the hippocampus and entorhinal cortex. The rise in the M1 microglial phenotype was marked by increased expression of surface markers like MHC-II, CD86, and CD32, while the M2 phenotype was indicated by elevated CD206 levels [112]. Ethanol exposure in primary rat and mouse microglial cultures activated microglia and significantly increased proinflammatory cytokines like TNF-α and IL-1β [113]. In TLR4 knockout mice, ethanol exposure was linked to increased IBA1 (a microglial marker) immunoreactivity, with binge or chronic ethanol drinking also raising IBA1 levels [113]. Acute ethanol doses (50 mM) induced TLR4 and TLR2 interaction, releasing inflammatory mediators in microglial cells [113][114][115]. Furthermore, ethanol treatment was found to alter microglial morphology through NF-κB signaling, NADPH oxidase activity, and ROS production [115][116].
11.2. Influence of Alcohol on Astrocyte Activation
Astrocytes are crucial glial cells in the brain, involved in various functions including nutrient metabolism, neurogenesis, and maintaining the BBB. They contribute significantly to brain homeostasis and influence neuronal activity. Astrocytes are also involved in the pathophysiology of several neurodegenerative diseases, such as Alzheimer’s and Huntington’s, where their altered functions can exacerbate disease progression [117][118]. Astrocytes have a significant role in the body’s response to ICH. They express TLRs and release cytokines and chemokines upon activation, a process that becomes particularly prominent in response to brain injuries like ICH. These cells are crucial in mediating the inflammatory response during ICH, where the disruption of the BBB is a key event. This BBB disruption enables peripheral macrophages and neutrophils to infiltrate the injury site, exacerbating the condition. Astrocytes get activated during hematoma lysis by thrombin and hemoglobin through protease-activated receptors (PARs), contributing to an increase in lesion volume [118].
Intermittent heavy ethanol exposure in adolescent rats significantly affects astrocytes in the hippocampus. This exposure leads to increased activation of astroglial hemichannels and pannexons, contributing to neuroinflammation and altered astrocyte arborization. The study demonstrates that ethanol exposure increases the opening of connexin 43 (Cx43) hemichannels and pannexin-1 (Panx1) channels in astrocytes, which correlates with elevated levels of proinflammatory cytokines like IL-1β, TNF-α, and IL-6 in the hippocampus. The findings suggest that these changes in astrocyte activity and neuroinflammation could contribute to the neurotoxic effects of adolescent alcohol consumption [119]. High doses of ethanol elevate the number of GFAP+ astrocytes in the rat cerebral cortex [120] and increase astrocyte density in the prelimbic cortex of alcohol-preferring rats [121]. Astrocytes, when exposed to ethanol, exhibit significant changes in their inflammatory response mechanisms. This exposure leads to the induction of inducible nitric oxide synthase (iNOS) and the upregulation of cyclooxygenase-2 (COX-2). These molecular alterations are not merely indicative of an inflammatory state but are closely associated with the development of brain edema, a critical concern in neurological conditions. The activation of iNOS and COX-2 pathways suggests a direct link between ethanol exposure and the exacerbation of inflammatory responses in the brain, highlighting the potential risks associated with alcohol consumption, particularly in relation to neuroinflammation and brain edema [122][123]. Table 1 details the patterns and dosages of ethanol exposure, ICH models used, and their relation to SBI components.
Table 1. Alcohol exposure and SBI Components. Upward arrow refers upregulation, downward arrow refers downregulation.
| Relationship with SBI | SBI Components | Effects | Ref. |
|---|---|---|---|
| Direct relationship with neuroinflammation | ↓ HO-1 | Reduced heme catabolism thereby increasing the free heme in the brain parenchyma and exacerbating the injury | [59] |
| ↑ Intracellular Calcium | Mitochondrial dysfunction to neuronal apoptosis | [76][77] | |
| ↓ ATP | Increased oxidative stress, mitochondrial dysfunction, and neuronal apoptosis | ||
| ↑ MMP-9 | BBB dysfunction | [78] | |
| Direct relationship wtih neuroinflammation | ↑ ROS-Increased oxidative stress | Increased neuronal tissue damage | [75] |
| ↑ Bax-Pro-apoptotic | Increased platelet apoptosis | [80] | |
| ↓ Bcl-2-Anti-apototic | |||
| Neuroendocrine Axis | ↓ Cortisol Reactivity | Reduced HPA activity to mitigate the ICH injury | [88][89][90] |
| Complement System | ↑ Complement C1q, C5b9 and MAC | Neuronal apoptosis and necrosis at the site of ICH injury | [100] |
| Inflammatory Mediators | ↑ M1 and M2 microglial phenotypes | M1-Pro-inflammatory activities and M2-Anti-inflammatory activities | [112] |
| ↑ Pro-inflammatory cytokines (TNF-α, IL-1β) | Increased neuroinflammation | [113] | |
| ↑ TLR-4 and TLR-2 interaction | Release inflammatory mediators | [115] | |
| ↑ NF-κB signaling and NADPH oxidase | Increased ROS production and changes in microglial morphology thereby increasing neuroinflammation | [116] | |
| ↑ iNOS ↑ COX-2 | Activated immune response | [112] | |
| ↑ Pro-inflammatory cytokines (TNF-α, IL-1β & IL-6), reduced chaperone proteins and diminished anti-inflammatory cytokines | Increased neuroinflammation | [79] | |
| Direct relationship with neuroinflammation | ↓ Fibrinogen | Reduces the clot density | [58] |
| ↓ Oxidative stress | Ameliorated neurological deficits | [79] | |
| Neuroendocrine Axis | ↓ HPA Acvitity | Compromised HPA activity dampens the stress mitigation caused by ICH | [86] |
| ↑ SNS Activity | Exerts already existing SNS activity thereby compromising the SNS activity needed to accord the stress caused by ICH | [92][93] | |
| Inflammatory mediators and stressors | ↓ ER stress | Increased ER homeostasis | [79] |
| ↑ Chaperone proteins (GRP78, GRP94 & Hsc70) | Reduced ER stress thereby reducing oxidative stress and neuronal apoptosis | ||
| ↓ Pro-inflammatory cytokines (TNF-α, IL-1β & IL-6) | Reduced neuroinflammation | ||
| Restored anti-inflammatory cytokine (IL-10) |
11.3. Relationship between and Neurotrophic Factors (NTFs) and Influence of Alcohol on NTFs
Neurotrophic factors (NTFs) are crucial in regulating neuronal life and death, playing a key role in neurogenesis, synaptogenesis, and neuroprotection against apoptosis [124]. There are three major families of NTFs: the neurotrophin family including nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), and NT-4; the glial-cell derived neurotrophic factor (GDNF) family; and the neuropoietic or interleukin-6 family. These NTFs are potential treatments for neurological diseases due to their regenerative capabilities [125][126][127][128].
After ICH, microglia and macrophages produce NTFs, particularly neurotrophins, to initiate repair. Studies have shown that BDNF can reduce hematoma volume, promote neural regeneration, and improve behavioral outcomes in models [129][130][131][132]. Similarly, NGF has been found to reduce inflammation and improve neurological function in patients [133][134]. The neurotrophic receptor p75 (p75NTR) is crucial in these processes, facilitating neurotrophins activities and mediating apoptosis. Models have shown that upregulated p75NTR is associated with increased apoptosis and inflammation, while its knockdown has protective effects [135][136].
Alcohol exposure, impacting both paternal and maternal sides, is known to significantly disrupt neurotrophin signaling pathways, crucial for neuronal development and regeneration. Prenatal alcohol exposure, in particular, has been shown to impair the synthesis of vital neurotrophins such as NGF and BDNF, critical for healthy brain development. This impairment is also associated with altered expression of neurotrophic receptors, leading to cellular damage. The subsequent increase in pro-inflammatory cytokines and ROS adds to the neurodevelopmental complications [137][138]. In adults with alcohol dependence, there is an observed disruption in the maturation process of BDNF, which is essential for neuronal survival, differentiation, and plasticity. The alteration in the conversion of pro-BDNF to mature BDNF and an increase in the levels of the p75NTR suggest a complex interplay between alcohol and brain chemistry that could have long-term implications on brain function and behavior [139]. Excessive alcohol consumption has also been linked to changes in BDNF signaling in brain regions associated with habit formation and addiction, such as the dorsolateral striatum. This change in signaling through p75NTR emphasizes the extensive and profound impact that alcohol has on neurotrophins and their receptors, which may contribute to the neuropathological changes observed in alcohol use disorders [140].
11.4. Beneficial Effects of Antioxidant Polyphenols
The growing body of research on plant-based compounds underscores their pivotal role in promoting human health and preventing chronic diseases. Recent studies have focused on the intricate ways in which dietary phytochemicals, such as polyphenols found in fruits, vegetables, and grains, interact with the gut microbiota, influencing metabolic, cardiovascular, and neurodegenerative disease outcomes [141][142]. These interactions have been shown to modulate inflammatory pathways, enhance antioxidant defense mechanisms, and improve gut barrier function, which are crucial for maintaining homeostasis and preventing disease progression [143].
Moreover, investigations into specific compounds like resveratrol and quercetin have illuminated their potential neuroprotective effects, particularly in the context of acute neurological injuries and chronic neurodegeneration. These natural antioxidants are found to mitigate oxidative stress, suppress inflammatory responses, and inhibit apoptosis in neuronal cells, offering promising therapeutic avenues for conditions such as ischemic stroke and intracerebral hemorrhage. The mechanisms behind these effects involve the activation of signaling pathways like Nrf2 and suppression of pro-inflammatory mediators, highlighting the complex interplay between dietary components and cellular health [144][145][146][147][148].
The capacity of polyphenols to modulate key signaling pathways has been linked to the reduction of oxidative stress, mitigation of inflammatory responses, and the promotion of neuronal survival and recovery post-stroke. These findings are supported by a range of studies utilizing animal models of middle cerebral artery occlusion (MCAO), which have been instrumental in delineating the mechanisms through which polyphenols exert their beneficial effects on the ischemic brain [149][150].
In addition to neuroprotection, the role of dietary antioxidants in combating oxidative stress and inflammation extends to the prevention and management of other chronic conditions, including cardiovascular diseases and cancer. The modulation of oxidative stress through dietary interventions is suggested to influence genetic and epigenetic mechanisms, with implications for disease risk and progression. This aspect of nutritional science opens new frontiers in personalized medicine, where dietary strategies could be tailored to individual genetic profiles to optimize health outcomes. The impact of these findings is profound, suggesting that incorporating a variety of phytochemical-rich foods into the diet could serve as a cost-effective and accessible strategy for enhancing public health [151][152][153].
Furthermore, the research underscores the therapeutic potential of polyphenols in the context of ischemic stroke, suggesting that dietary interventions enriched with polyphenolic compounds could offer a complementary approach to traditional stroke treatments. By focusing on the neuroprotective effects of polyphenols, studies have highlighted their ability to not only reduce the extent of brain damage following ischemia but also to enhance the recovery processes, thereby improving functional outcomes [150][154][155][156]. Despite the promising data, the translation of these findings into clinical practice necessitates further investigation into the optimal delivery methods, dosages, and combinations of polyphenols that maximize their efficacy and bioavailability. As the scientific community continues to explore the therapeutic avenues offered by polyphenols, their integration into preventive and treatment regimens for ischemic stroke represents a promising and innovative approach to reducing the burden of this debilitating condition.
11.5. Epidemiological Evidence Linking Alcohol and ICH
Epidemiological studies have established a clear correlation between factors like hypertension, alcohol use, smoking, and certain medications [41][42]. Alcohol, particularly, is a significant risk factor. Heavy alcohol consumption elevates blood pressure and impairs platelet aggregation, increasing the risk of hypertension [5][8]. Binge drinking, especially in young adults, is linked to increased stroke risk [157][158][159][160]. Studies like the ethnic/racial variations of intracerebral hemorrhage (ERICH) [161] and multicenter study on cerebral hemorrhage in Italy (MUCH)-Italy [162] specifically indicate that heavy drinking escalates risk, with the latter showing a significant impact on older individuals and an association with deep ICH. The ERICH study revealed that while heavy alcohol consumption increases risk, moderate consumption might be protective [161]. Similarly, one other study found that heavy drinking (>4 drinks/day) is more associated with hemorrhagic stroke types [163]. In contrast, moderate alcohol intake, in some studies, shows a reduced risk of cardiovascular disease and strokes [31][164][165]. However, this is contradicted by evidence linking moderate and heavy alcohol intake with increased spontaneous risk in hypertensive individuals. Alcohol’s role in pathophysiology involves exacerbating vascular damage and SBI [69][166][167][168][169][170]. Chronic alcohol consumption leads to hypertension and affects blood pressure [170][171]. Alcohol’s role in oxidative stress, endothelial dysfunction, and increased hematoma volume has been demonstrated in various animal models and clinical studies [172][173][174][175][176].
References
- Rosso, A.M. Beer and wine in antiquity: Beneficial remedy or punishment imposed by the Gods? Acta Med. Hist. Adriat. 2012, 10, 237–262.
- McGinnis, J.M.; Foege, W.H. Actual causes of death in the United States. JAMA 1993, 270, 2207–2212.
- Li, T.K.; Hewitt, B.G.; Grant, B.F. Alcohol use disorders and mood disorders: A National Institute on Alcohol Abuse and Alcoholism perspective. Biol. Psychiatry 2004, 56, 718–720.
- Room, R.; Babor, T.; Rehm, J. Alcohol and public health. Lancet 2005, 365, 519–530.
- Lin, P.B.; Wang, P.K.; Pang, C.Y.; Hu, W.F.; Tsai, A.P.; Oblak, A.L.; Liew, H.K. Moderate Ethanol Pre-treatment Mitigates ICH-Induced Injury via ER Stress Modulation in Rats. Front. Mol. Neurosci. 2021, 14, 682775.
- Beilin, L.J.; Puddey, I.B. Alcohol and hypertension: An update. Hypertension 2006, 47, 1035–1038.
- Klatsky, A.L. Alcohol-associated hypertension: When one drinks makes a difference. Hypertension 2004, 44, 805–806.
- Sesso, H.D.; Cook, N.R.; Buring, J.E.; Manson, J.E.; Gaziano, J.M. Alcohol consumption and the risk of hypertension in women and men. Hypertension 2008, 51, 1080–1087.
- Pletsch, G.R.; Boehme, A.K.; Albright, K.C.; Burns, C.; Beasley, T.M.; Martin-Schild, S. Low-density lipoprotein and intracerebral hematoma expansion in daily alcohol users. Cerebrovasc. Dis. Extra 2014, 4, 1–8.
- Rehm, J.; Rehn, N.; Room, R.; Monteiro, M.; Gmel, G.; Jernigan, D.; Frick, U. The global distribution of average volume of alcohol consumption and patterns of drinking. Eur. Addict. Res. 2003, 9, 147–156.
- Rehm, J.; Room, R.; Graham, K.; Monteiro, M.; Gmel, G.; Sempos, C.T. The relationship of average volume of alcohol consumption and patterns of drinking to burden of disease: An overview. Addiction 2003, 98, 1209–1228.
- Rehm, J.; Sempos, C.T.; Trevisan, M. Alcohol and cardiovascular disease—More than one paradox to consider. Average volume of alcohol consumption, patterns of drinking and risk of coronary heart disease—A review. J. Cardiovasc. Risk 2003, 10, 15–20.
- Collins, M.A.; Neafsey, E.J.; Mukamal, K.J.; Gray, M.O.; Parks, D.A.; Das, D.K.; Korthuis, R.J. Alcohol in moderation, cardioprotection, and neuroprotection: Epidemiological considerations and mechanistic studies. Alcohol. Clin. Exp. Res. 2009, 33, 206–219.
- Mukamal, K.J.; Chung, H.; Jenny, N.S.; Kuller, L.H.; Longstreth, W.T., Jr.; Mittleman, M.A.; Burke, G.L.; Cushman, M.; Beauchamp, N.J., Jr.; Siscovick, D.S. Alcohol use and risk of ischemic stroke among older adults: The cardiovascular health study. Stroke 2005, 36, 1830–1834.
- Huang, F.P.; Xi, G.; Keep, R.F.; Hua, Y.; Nemoianu, A.; Hoff, J.T. Brain edema after experimental intracerebral hemorrhage: Role of hemoglobin degradation products. J. Neurosurg. 2002, 96, 287–293.
- Monforte, R.; Estruch, R.; Graus, F.; Nicolas, J.M.; Urbano-Marquez, A. High ethanol consumption as risk factor for intracerebral hemorrhage in young and middle-aged people. Stroke 1990, 21, 1529–1532.
- Taylor, J.R.; Combs-Orme, T. Alcohol and strokes in young adults. Am. J. Psychiatry 1985, 142, 116–118.
- Kalinowski, A.; Humphreys, K. Governmental standard drink definitions and low-risk alcohol consumption guidelines in 37 countries. Addiction 2016, 111, 1293–1298.
- Paton, A. Alcohol in the body. BMJ 2005, 330, 85–87.
- Jones, A.W.; Jonsson, K.A.; Kechagias, S. Effect of high-fat, high-protein, and high-carbohydrate meals on the pharmacokinetics of a small dose of ethanol. Br. J. Clin. Pharmacol. 1997, 44, 521–526.
- Holford, N.H. Clinical pharmacokinetics of ethanol. Clin. Pharmacokinet. 1987, 13, 273–292.
- Zakhari, S. Overview: How is alcohol metabolized by the body? Alcohol Res. Health 2006, 29, 245–254.
- Zakhari, S. Special remarks from the National Institute on Alcohol Abuse and Alcoholism. J. Gastroenterol. Hepatol. 2006, 21 (Suppl. 3), S2.
- Edenberg, H.J. The genetics of alcohol metabolism: Role of alcohol dehydrogenase and aldehyde dehydrogenase variants. Alcohol Res. Health 2007, 30, 5–13.
- Lieber, C.S. Hepatic, metabolic and toxic effects of ethanol: 1991 update. Alcohol. Clin. Exp. Res. 1991, 15, 573–592.
- Tanaka, E.; Terada, M.; Misawa, S. Cytochrome P450 2E1: Its clinical and toxicological role. J. Clin. Pharm. Ther. 2000, 25, 165–175.
- Tsutsumi, M.; Lasker, J.M.; Takahashi, T.; Lieber, C.S. In vivo induction of hepatic P4502E1 by ethanol: Role of increased enzyme synthesis. Arch. Biochem. Biophys. 1993, 304, 209–218.
- Palmer, R.B. A review of the use of ethyl glucuronide as a marker for ethanol consumption in forensic and clinical medicine. Semin. Diagn. Pathol. 2009, 26, 18–27.
- Staufer, K.; Yegles, M. Biomarkers for detection of alcohol consumption in liver transplantation. World J. Gastroenterol. 2016, 22, 3725–3734.
- Gyntelberg, F.; Meyer, J. Relationship between blood pressure and physical fitness, smoking and alcohol consumption in Copenhagen males aged 40–59. Acta Med. Scand. 1974, 195, 375–380.
- Bos, S.; Grobbee, D.E.; Boer, J.M.; Verschuren, W.M.; Beulens, J.W. Alcohol consumption and risk of cardiovascular disease among hypertensive women. Eur. J. Cardiovasc. Prev. Rehabil. 2010, 17, 119–126.
- Husain, K.; Ansari, R.A.; Ferder, L. Alcohol-induced hypertension: Mechanism and prevention. World J. Cardiol. 2014, 6, 245–252.
- McFadden, C.B.; Brensinger, C.M.; Berlin, J.A.; Townsend, R.R. Systematic review of the effect of daily alcohol intake on blood pressure. Am. J. Hypertens. 2005, 18 Pt 1, 276–286.
- Rupp, H.; Brilla, C.G.; Maisch, B. . Herz 1996, 21, 258–264.
- Jing, L.; Li, W.M.; Zhou, L.J.; Li, S.; Kou, J.J.; Song, J. Expression of renin-angiotensin system and peroxisome proliferator-activated receptors in alcoholic cardiomyopathy. Alcohol. Clin. Exp. Res. 2008, 32, 1999–2007.
- Potter, J.F.; Watson, R.D.; Skan, W.; Beevers, D.G. The pressor and metabolic effects of alcohol in normotensive subjects. Hypertension 1986, 8, 625–631.
- Altura, B.M.; Altura, B.T. Microvascular and vascular smooth muscle actions of ethanol, acetaldehyde, and acetate. Fed. Proc. 1982, 41, 2447–2451.
- Slomiany, B.L.; Piotrowski, J.; Slomiany, A. Alterations in buccal mucosal endothelin-1 and nitric oxide synthase with chronic alcohol ingestion. Biochem. Mol. Biol. Int. 1998, 45, 681–688.
- Puddey, I.B.; Zilkens, R.R.; Croft, K.D.; Beilin, L.J. Alcohol and endothelial function: A brief review. Clin. Exp. Pharmacol. Physiol. 2001, 28, 1020–1024.
- MacMahon, S. Alcohol consumption and hypertension. Hypertension 1987, 9, 111–121.
- Roerecke, M.; Kaczorowski, J.; Tobe, S.W.; Gmel, G.; Hasan, O.S.M.; Rehm, J. The effect of a reduction in alcohol consumption on blood pressure: A systematic review and meta-analysis. Lancet Public Health 2017, 2, e108–e120.
- Krishnamurthi, R.V.; Feigin, V.L.; Forouzanfar, M.H.; Mensah, G.A.; Connor, M.; Bennett, D.A.; Moran, A.E.; Sacco, R.L.; Anderson, L.M.; Truelsen, T.; et al. Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990–2010: Findings from the Global Burden of Disease Study 2010. Lancet Glob. Health 2013, 1, e259–e281.
- Qureshi, A.I.; Tuhrim, S.; Broderick, J.P.; Batjer, H.H.; Hondo, H.; Hanley, D.F. Spontaneous intracerebral hemorrhage. N. Engl. J. Med. 2001, 344, 1450–1460.
- Qureshi, A.I.; Mendelow, A.D.; Hanley, D.F. Intracerebral haemorrhage. Lancet 2009, 373, 1632–1644.
- Xi, G.; Keep, R.F.; Hoff, J.T. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006, 5, 53–63.
- Takebayashi, S.; Kaneko, M. Electron microscopic studies of ruptured arteries in hypertensive intracerebral hemorrhage. Stroke 1983, 14, 28–36.
- Zhu, H.; Wang, Z.; Yu, J.; Yang, X.; He, F.; Liu, Z.; Che, F.; Chen, X.; Ren, H.; Hong, M.; et al. Role and mechanisms of cytokines in the secondary brain injury after intracerebral hemorrhage. Prog. Neurobiol. 2019, 178, 101610.
- Appelboom, G.; Bruce, S.S.; Hickman, Z.L.; Zacharia, B.E.; Carpenter, A.M.; Vaughan, K.A.; Duren, A.; Hwang, R.Y.; Piazza, M.; Lee, K.; et al. Volume-dependent effect of perihaematomal oedema on outcome for spontaneous intracerebral haemorrhages. J. Neurol. Neurosurg. Psychiatry 2013, 84, 488–493.
- Dowlatshahi, D.; Demchuk, A.M.; Flaherty, M.L.; Ali, M.; Lyden, P.L.; Smith, E.E.; Collaboration, V. Defining hematoma expansion in intracerebral hemorrhage: Relationship with patient outcomes. Neurology 2011, 76, 1238–1244.
- LoPresti, M.A.; Bruce, S.S.; Camacho, E.; Kunchala, S.; Dubois, B.G.; Bruce, E.; Appelboom, G.; Connolly, E.S., Jr. Hematoma volume as the major determinant of outcomes after intracerebral hemorrhage. J. Neurol. Sci. 2014, 345, 3–7.
- Mayer, S.A.; Lignelli, A.; Fink, M.E.; Kessler, D.B.; Thomas, C.E.; Swarup, R.; Van Heertum, R.L. Perilesional blood flow and edema formation in acute intracerebral hemorrhage: A SPECT study. Stroke 1998, 29, 1791–1798.
- Li, Q.; Han, X.; Lan, X.; Gao, Y.; Wan, J.; Durham, F.; Cheng, T.; Yang, J.; Wang, Z.; Jiang, C.; et al. Inhibition of neuronal ferroptosis protects hemorrhagic brain. JCI Insight 2017, 2, e90777.
- Keep, R.F.; Hua, Y.; Xi, G. Intracerebral haemorrhage: Mechanisms of injury and therapeutic targets. Lancet Neurol. 2012, 11, 720–731.
- Madangarli, N.; Bonsack, F.; Dasari, R.; Sukumari-Ramesh, S. Intracerebral Hemorrhage: Blood Components and Neurotoxicity. Brain Sci. 2019, 9, 316.
- Senn, R.; Elkind, M.S.; Montaner, J.; Christ-Crain, M.; Katan, M. Potential role of blood biomarkers in the management of nontraumatic intracerebral hemorrhage. Cerebrovasc. Dis. 2014, 38, 395–409.
- Gurley, C.; Nichols, J.; Liu, S.; Phulwani, N.K.; Esen, N.; Kielian, T. Microglia and Astrocyte Activation by Toll-Like Receptor Ligands: Modulation by PPAR-gamma Agonists. PPAR Res. 2008, 2008, 453120.
- Chen, C.J.; Brown, W.M.; Moomaw, C.J.; Langefeld, C.D.; Osborne, J.; Worrall, B.B.; Woo, D.; Koch, S.; Investigators, E. Alcohol use and risk of intracerebral hemorrhage. Neurology 2017, 88, 2043–2051.
- Aronowski, J.; Zhao, X. Molecular pathophysiology of cerebral hemorrhage: Secondary brain injury. Stroke 2011, 42, 1781–1786.
- Bai, Q.; Liu, J.; Wang, G. Ferroptosis, a Regulated Neuronal Cell Death Type After Intracerebral Hemorrhage. Front. Cell Neurosci. 2020, 14, 591874.
- Rautenbach, P.H.; Nienaber-Rousseau, C.; Pieters, M. The association of alcohol with circulating total fibrinogen and plasma clot density is mediated by fibrinogen and FXIII genotypes. Thromb. J. 2020, 18, 35.
- el-Sayed, M.S.; Eastland, P.; Lin, X.; Rattu, A.M. The effect of moderate alcohol ingestion on blood coagulation and fibrinolysis at rest and in response to exercise. J. Sports Sci. 1999, 17, 513–520.
- Gerjevic, L.N.; Lu, S.; Chaky, J.P.; Harrison-Findik, D.D. Regulation of heme oxygenase expression by alcohol, hypoxia and oxidative stress. World J. Biol. Chem. 2011, 2, 252–260.
- Bertero, E.; Maack, C. Calcium Signaling and Reactive Oxygen Species in Mitochondria. Circ. Res. 2018, 122, 1460–1478.
- Zille, M.; Karuppagounder, S.S.; Chen, Y.; Gough, P.J.; Bertin, J.; Finger, J.; Milner, T.A.; Jonas, E.A.; Ratan, R.R. Neuronal Death After Hemorrhagic Stroke In Vitro and In Vivo Shares Features of Ferroptosis and Necroptosis. Stroke 2017, 48, 1033–1043.
- Sinha, K.; Das, J.; Pal, P.B.; Sil, P.C. Oxidative stress: The mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch. Toxicol. 2013, 87, 1157–1180.
- Nakamura, T.; Keep, R.F.; Hua, Y.; Nagao, S.; Hoff, J.T.; Xi, G. Iron-induced oxidative brain injury after experimental intracerebral hemorrhage. Acta Neurochir. Suppl. 2006, 96, 194–198.
- Nakamura, T.; Keep, R.F.; Hua, Y.; Hoff, J.T.; Xi, G. Oxidative DNA injury after experimental intracerebral hemorrhage. Brain Res. 2005, 1039, 30–36.
- Mates, J.M.; Segura, J.A.; Alonso, F.J.; Marquez, J. Oxidative stress in apoptosis and cancer: An update. Arch. Toxicol. 2012, 86, 1649–1665.
- Zia, M.T.; Csiszar, A.; Labinskyy, N.; Hu, F.; Vinukonda, G.; LaGamma, E.F.; Ungvari, Z.; Ballabh, P. Oxidative-nitrosative stress in a rabbit pup model of germinal matrix hemorrhage: Role of NAD(P)H oxidase. Stroke 2009, 40, 2191–2198.
- Qi, J.; Kim, J.W.; Zhou, Z.; Lim, C.W.; Kim, B. Ferroptosis Affects the Progression of Nonalcoholic Steatohepatitis via the Modulation of Lipid Peroxidation-Mediated Cell Death in Mice. Am. J. Pathol. 2020, 190, 68–81.
- Duan, X.; Wen, Z.; Shen, H.; Shen, M.; Chen, G. Intracerebral Hemorrhage, Oxidative Stress, and Antioxidant Therapy. Oxid. Med. Cell Longev. 2016, 2016, 1203285.
- Montoliu, C.; Valles, S.; Renau-Piqueras, J.; Guerri, C. Ethanol-induced oxygen radical formation and lipid peroxidation in rat brain: Effect of chronic alcohol consumption. J. Neurochem. 1994, 63, 1855–1862.
- Chen, W.; Guo, C.; Feng, H.; Chen, Y. Mitochondria: Novel Mechanisms and Therapeutic Targets for Secondary Brain Injury After Intracerebral Hemorrhage. Front. Aging Neurosci. 2020, 12, 615451.
- Mariani, E.; Polidori, M.C.; Cherubini, A.; Mecocci, P. Oxidative stress in brain aging, neurodegenerative and vascular diseases: An overview. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2005, 827, 65–75.
- Sidlauskaite, E.; Gibson, J.W.; Megson, I.L.; Whitfield, P.D.; Tovmasyan, A.; Batinic-Haberle, I.; Murphy, M.P.; Moult, P.R.; Cobley, J.N. Mitochondrial ROS cause motor deficits induced by synaptic inactivity: Implications for synapse pruning. Redox Biol. 2018, 16, 344–351.
- Zheng, J.; Shi, L.; Liang, F.; Xu, W.; Li, T.; Gao, L.; Sun, Z.; Yu, J.; Zhang, J. Sirt3 Ameliorates Oxidative Stress and Mitochondrial Dysfunction After Intracerebral Hemorrhage in Diabetic Rats. Front. Neurosci. 2018, 12, 414.
- Liew, H.K.; Cheng, H.Y.; Huang, L.C.; Li, K.W.; Peng, H.F.; Yang, H.I.; Lin, P.B.; Kuo, J.S.; Pang, C.Y. Acute Alcohol Intoxication Aggravates Brain Injury Caused by Intracerebral Hemorrhage in Rats. J. Stroke Cerebrovasc. Dis. 2016, 25, 15–25.
- Hoek, J.B.; Cahill, A.; Pastorino, J.G. Alcohol and mitochondria: A dysfunctional relationship. Gastroenterology 2002, 122, 2049–2063.
- Manzo-Avalos, S.; Saavedra-Molina, A. Cellular and mitochondrial effects of alcohol consumption. Int. J. Environ. Res. Public Health 2010, 7, 4281–4304.
- Abdul Muneer, P.M.; Alikunju, S.; Szlachetka, A.M.; Haorah, J. The mechanisms of cerebral vascular dysfunction and neuroinflammation by MMP-mediated degradation of VEGFR-2 in alcohol ingestion. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1167–1177.
- Liu, L.; Chen, M.; Zhao, L.; Zhao, Q.; Hu, R.; Zhu, J.; Yan, R.; Dai, K. Ethanol Induces Platelet Apoptosis. Alcohol. Clin. Exp. Res. 2017, 41, 291–298.
- Elenkov, I.J.; Wilder, R.L.; Chrousos, G.P.; Vizi, E.S. The sympathetic nerve—An integrative interface between two supersystems: The brain and the immune system. Pharmacol. Rev. 2000, 52, 595–638.
- Liesz, A.; Middelhoff, M.; Zhou, W.; Karcher, S.; Illanes, S.; Veltkamp, R. Comparison of humoral neuroinflammation and adhesion molecule expression in two models of experimental intracerebral hemorrhage. Exp. Transl. Stroke Med. 2011, 3, 11.
- Olsson, T.; Marklund, N.; Gustafson, Y.; Nasman, B. Abnormalities at different levels of the hypothalamic-pituitary-adrenocortical axis early after stroke. Stroke 1992, 23, 1573–1576.
- Pennypacker, K.R. Targeting the peripheral inflammatory response to stroke: Role of the spleen. Transl. Stroke Res. 2014, 5, 635–637.
- Zhang, J.; Shi, K.; Li, Z.; Li, M.; Han, Y.; Wang, L.; Zhang, Z.; Yu, C.; Zhang, F.; Song, L.; et al. Organ- and cell-specific immune responses are associated with the outcomes of intracerebral hemorrhage. FASEB J. 2018, 32, 220–229.
- Waltman, C.; Blevins, L.S., Jr.; Boyd, G.; Wand, G.S. The effects of mild ethanol intoxication on the hypothalamic-pituitary-adrenal axis in nonalcoholic men. J. Clin. Endocrinol. Metab. 1993, 77, 518–522.
- Dai, X.; Thavundayil, J.; Santella, S.; Gianoulakis, C. Response of the HPA-axis to alcohol and stress as a function of alcohol dependence and family history of alcoholism. Psychoneuroendocrinology 2007, 32, 293–305.
- Schepis, T.S.; Rao, U.; Yadav, H.; Adinoff, B. The limbic-hypothalamic-pituitary-adrenal axis and the development of alcohol use disorders in youth. Alcohol. Clin. Exp. Res. 2011, 35, 595–605.
- Clarke, T.K.; Treutlein, J.; Zimmermann, U.S.; Kiefer, F.; Skowronek, M.H.; Rietschel, M.; Mann, K.; Schumann, G. HPA-axis activity in alcoholism: Examples for a gene-environment interaction. Addict. Biol. 2008, 13, 1–14.
- Du, W.; Chen, X.; Shi, M.; Bian, F.; Zhao, Z. Ethanol affects behavior and HPA axis activity during development in zebrafish larvae. Sci. Rep. 2020, 10, 21402.
- van de Borne, P.; Mark, A.L.; Montano, N.; Mion, D.; Somers, V.K. Effects of alcohol on sympathetic activity, hemodynamics, and chemoreflex sensitivity. Hypertension 1997, 29, 1278–1283.
- Johnson, R.H.; Eisenhofer, G.; Lambie, D.G. The effects of acute and chronic ingestion of ethanol on the autonomic nervous system. Drug Alcohol Depend. 1986, 18, 319–328.
- Xi, G.; Keep, R.F.; Hoff, J.T. Erythrocytes and delayed brain edema formation following intracerebral hemorrhage in rats. J. Neurosurg. 1998, 89, 991–996.
- Hansch, G.M. The complement attack phase: Control of lysis and non-lethal effects of C5b-9. Immunopharmacology 1992, 24, 107–117.
- Taylor, P.; Botto, M.; Walport, M. The complement system. Curr. Biol. 1998, 8, R259–R261.
- Wang, M.; Xia, F.; Wan, S.; Hua, Y.; Keep, R.F.; Xi, G. Role of Complement Component 3 in Early Erythrolysis in the Hematoma After Experimental Intracerebral Hemorrhage. Stroke 2021, 52, 2649–2660.
- Yang, S.; Nakamura, T.; Hua, Y.; Keep, R.F.; Younger, J.G.; He, Y.; Hoff, J.T.; Xi, G. The role of complement C3 in intracerebral hemorrhage-induced brain injury. J. Cereb. Blood Flow. Metab. 2006, 26, 1490–1495.
- Hua, Y.; Xi, G.; Keep, R.F.; Hoff, J.T. Complement activation in the brain after experimental intracerebral hemorrhage. J. Neurosurg. 2000, 92, 1016–1022.
- Mukherjee, S.; Cabrera, M.A.; Boyadjieva, N.I.; Berger, G.; Rousseau, B.; Sarkar, D.K. Alcohol Increases Exosome Release from Microglia to Promote Complement C1q-Induced Cellular Death of Proopiomelanocortin Neurons in the Hypothalamus in a Rat Model of Fetal Alcohol Spectrum Disorders. J. Neurosci. 2020, 40, 7965–7979.
- Xue, M.; Yong, V.W. Neuroinflammation in intracerebral haemorrhage: Immunotherapies with potential for translation. Lancet Neurol. 2020, 19, 1023–1032.
- Wang, J.; Dore, S. Inflammation after intracerebral hemorrhage. J. Cereb. Blood Flow Metab. 2007, 27, 894–908.
- Wang, J. Preclinical and clinical research on inflammation after intracerebral hemorrhage. Prog. Neurobiol. 2010, 92, 463–477.
- Mracsko, E.; Veltkamp, R. Neuroinflammation after intracerebral hemorrhage. Front. Cell Neurosci. 2014, 8, 388.
- Lin, S.; Yin, Q.; Zhong, Q.; Lv, F.L.; Zhou, Y.; Li, J.Q.; Wang, J.Z.; Su, B.Y.; Yang, Q.W. Heme activates TLR4-mediated inflammatory injury via MyD88/TRIF signaling pathway in intracerebral hemorrhage. J. Neuroinflamm. 2012, 9, 46.
- Smiley, S.T.; King, J.A.; Hancock, W.W. Fibrinogen stimulates macrophage chemokine secretion through toll-like receptor 4. J. Immunol. 2001, 167, 2887–2894.
- Zhao, X.; Sun, G.; Ting, S.M.; Song, S.; Zhang, J.; Edwards, N.J.; Aronowski, J. Cleaning up after ICH: The role of Nrf2 in modulating microglia function and hematoma clearance. J. Neurochem. 2015, 133, 144–152.
- Chang, C.F.; Wan, J.; Li, Q.; Renfroe, S.C.; Heller, N.M.; Wang, J. Alternative activation-skewed microglia/macrophages promote hematoma resolution in experimental intracerebral hemorrhage. Neurobiol. Dis. 2017, 103, 54–69.
- Li, Q.; Lan, X.; Han, X.; Durham, F.; Wan, J.; Weiland, A.; Koehler, R.C.; Wang, J. Microglia-derived interleukin-10 accelerates post-intracerebral hemorrhage hematoma clearance by regulating CD36. Brain Behav. Immun. 2021, 94, 437–457.
- Guo, S.; Wang, H.; Yin, Y. Microglia Polarization From M1 to M2 in Neurodegenerative Diseases. Front. Aging Neurosci. 2022, 14, 815347.
- Orihuela, R.; McPherson, C.A.; Harry, G.J. Microglial M1/M2 polarization and metabolic states. Br. J. Pharmacol. 2016, 173, 649–665.
- Peng, H.; Geil Nickell, C.R.; Chen, K.Y.; McClain, J.A.; Nixon, K. Increased expression of M1 and M2 phenotypic markers in isolated microglia after four-day binge alcohol exposure in male rats. Alcohol. 2017, 62, 29–40.
- Alfonso-Loeches, S.; Pascual-Lucas, M.; Blanco, A.M.; Sanchez-Vera, I.; Guerri, C. Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. J. Neurosci. 2010, 30, 8285–8295.
- Fernandez-Lizarbe, S.; Montesinos, J.; Guerri, C. Ethanol induces TLR4/TLR2 association, triggering an inflammatory response in microglial cells. J. Neurochem. 2013, 126, 261–273.
- Fernandez-Lizarbe, S.; Pascual, M.; Guerri, C. Critical role of TLR4 response in the activation of microglia induced by ethanol. J. Immunol. 2009, 183, 4733–4744.
- Qin, L.; Crews, F.T. NADPH oxidase and reactive oxygen species contribute to alcohol-induced microglial activation and neurodegeneration. J. Neuroinflamm. 2012, 9, 5.
- Siracusa, R.; Fusco, R.; Cuzzocrea, S. Astrocytes: Role and Functions in Brain Pathologies. Front. Pharmacol. 2019, 10, 1114.
- Scimemi, A. Astrocytes and the Warning Signs of Intracerebral Hemorrhagic Stroke. Neural Plast. 2018, 2018, 7301623.
- Gomez, G.I.; Falcon, R.V.; Maturana, C.J.; Labra, V.C.; Salgado, N.; Rojas, C.A.; Oyarzun, J.E.; Cerpa, W.; Quintanilla, R.A.; Orellana, J.A. Heavy Alcohol Exposure Activates Astroglial Hemichannels and Pannexons in the Hippocampus of Adolescent Rats: Effects on Neuroinflammation and Astrocyte Arborization. Front. Cell Neurosci. 2018, 12, 472.
- Dalcik, H.; Yardimoglu, M.; Filiz, S.; Gonca, S.; Dalcik, C.; Erden, B.F. Chronic ethanol-induced glial fibrillary acidic protein (GFAP) immunoreactivity: An immunocytochemical observation in various regions of adult rat brain. Int. J. Neurosci. 2009, 119, 1303–1318.
- Miguel-Hidalgo, J.J. Withdrawal from free-choice ethanol consumption results in increased packing density of glutamine synthetase-immunoreactive astrocytes in the prelimbic cortex of alcohol-preferring rats. Alcohol Alcohol. 2006, 41, 379–385.
- Blanco, A.M.; Pascual, M.; Valles, S.L.; Guerri, C. Ethanol-induced iNOS and COX-2 expression in cultured astrocytes via NF-kappa B. Neuroreport 2004, 15, 681–685.
- Temel, S.G.; Kahveci, Z. Cyclooxygenase-2 expression in astrocytes and microglia in human oligodendroglioma and astrocytoma. J. Mol. Histol. 2009, 40, 369–377.
- Poyhonen, S.; Er, S.; Domanskyi, A.; Airavaara, M. Effects of Neurotrophic Factors in Glial Cells in the Central Nervous System: Expression and Properties in Neurodegeneration and Injury. Front. Physiol. 2019, 10, 486.
- Razavi, S.; Nazem, G.; Mardani, M.; Esfandiari, E.; Salehi, H.; Esfahani, S.H. Neurotrophic factors and their effects in the treatment of multiple sclerosis. Adv. Biomed. Res. 2015, 4, 53.
- Bothwell, M. NGF, BDNF, NT3, and NT4. Handb. Exp. Pharmacol. 2014, 220, 3–15.
- Castilla-Cortazar, I.; Iturrieta, I.; Garcia-Magarino, M.; Puche, J.E.; Martin-Estal, I.; Aguirre, G.A.; Femat-Roldan, G.; Cantu-Martinez, L.; Munoz, U. Neurotrophic Factors and Their Receptors Are Altered by the Mere Partial IGF-1 Deficiency. Neuroscience 2019, 404, 445–458.
- Sousa-Victor, P.; Jasper, H.; Neves, J. Trophic Factors in Inflammation and Regeneration: The Role of MANF and CDNF. Front. Physiol. 2018, 9, 1629.
- Bi, R.; Fang, Z.; You, M.; He, Q.; Hu, B. Microglia Phenotype and Intracerebral Hemorrhage: A Balance of Yin and Yang. Front. Cell Neurosci. 2021, 15, 765205.
- Li, J.; Xiao, L.; He, D.; Luo, Y.; Sun, H. Mechanism of White Matter Injury and Promising Therapeutic Strategies of MSCs After Intracerebral Hemorrhage. Front. Aging Neurosci. 2021, 13, 632054.
- Lin, T.C.; Tsai, Y.C.; Chen, Y.A.; Young, T.H.; Wu, C.C.; Chiang, Y.H.; Kao, C.H.; Huang, A.P.; Hsu, Y.H.; Chen, K.Y.; et al. Brain-derived neurotrophic factor contributes to neurogenesis after intracerebral hemorrhage: A rodent model and human study. Front. Cell Neurosci. 2023, 17, 1170251.
- Bahlakeh, G.; Rahbarghazi, R.; Mohammadnejad, D.; Abedelahi, A.; Karimipour, M. Current knowledge and challenges associated with targeted delivery of neurotrophic factors into the central nervous system: Focus on available approaches. Cell Biosci. 2021, 11, 181.
- Barker, P.A.; Mantyh, P.; Arendt-Nielsen, L.; Viktrup, L.; Tive, L. Nerve Growth Factor Signaling and Its Contribution to Pain. J. Pain Res. 2020, 13, 1223–1241.
- Minnone, G.; De Benedetti, F.; Bracci-Laudiero, L. NGF and Its Receptors in the Regulation of Inflammatory Response. Int. J. Mol. Sci. 2017, 18, 1028.
- Malik, S.C.; Sozmen, E.G.; Baeza-Raja, B.; Le Moan, N.; Akassoglou, K.; Schachtrup, C. In vivo functions of p75(NTR): Challenges and opportunities for an emerging therapeutic target. Trends Pharmacol. Sci. 2021, 42, 772–788.
- Li, Q.; Hu, Y.Z.; Gao, S.; Wang, P.F.; Hu, Z.L.; Dai, R.P. ProBDNF and its receptors in immune-mediated inflammatory diseases: Novel insights into the regulation of metabolism and mitochondria. Front. Immunol. 2023, 14, 1155333.
- Carito, V.; Ceccanti, M.; Ferraguti, G.; Coccurello, R.; Ciafre, S.; Tirassa, P.; Fiore, M. NGF and BDNF Alterations by Prenatal Alcohol Exposure. Curr. Neuropharmacol. 2019, 17, 308–317.
- Ceci, F.M.; Ferraguti, G.; Petrella, C.; Greco, A.; Ralli, M.; Iannitelli, A.; Carito, V.; Tirassa, P.; Chaldakov, G.N.; Messina, M.P.; et al. Nerve Growth Factor in Alcohol Use Disorders. Curr. Neuropharmacol. 2021, 19, 45–60.
- Zhou, L.; Xiong, J.; Ruan, C.S.; Ruan, Y.; Liu, D.; Bao, J.J.; Zhou, X.F. ProBDNF/p75NTR/sortilin pathway is activated in peripheral blood of patients with alcohol dependence. Transl. Psychiatry 2018, 7, 2.
- Darcq, E.; Morisot, N.; Phamluong, K.; Warnault, V.; Jeanblanc, J.; Longo, F.M.; Massa, S.M.; Ron, D. The Neurotrophic Factor Receptor p75 in the Rat Dorsolateral Striatum Drives Excessive Alcohol Drinking. J. Neurosci. 2016, 36, 10116–10127.
- Wang, X.; Qi, Y.; Zheng, H. Dietary Polyphenol, Gut Microbiota, and Health Benefits. Antioxidants 2022, 11, 1212.
- Iqbal, I.; Wilairatana, P.; Saqib, F.; Nasir, B.; Wahid, M.; Latif, M.F.; Iqbal, A.; Naz, R.; Mubarak, M.S. Plant Polyphenols and Their Potential Benefits on Cardiovascular Health: A Review. Molecules 2023, 28, 6403.
- Santhiravel, S.; Bekhit, A.E.A.; Mendis, E.; Jacobs, J.L.; Dunshea, F.R.; Rajapakse, N.; Ponnampalam, E.N. The Impact of Plant Phytochemicals on the Gut Microbiota of Humans for a Balanced Life. Int. J. Mol. Sci. 2022, 23, 8124.
- Zhou, Y.; Zhang, S.; Fan, X. Role of Polyphenols as Antioxidant Supplementation in Ischemic Stroke. Oxid. Med. Cell Longev. 2021, 2021, 5471347.
- Lan, X.; Han, X.; Li, Q.; Wang, J. (−)-Epicatechin, a Natural Flavonoid Compound, Protects Astrocytes Against Hemoglobin Toxicity via Nrf2 and AP-1 Signaling Pathways. Mol. Neurobiol. 2017, 54, 7898–7907.
- Zhang, Y.; Yi, B.; Ma, J.; Zhang, L.; Zhang, H.; Yang, Y.; Dai, Y. Quercetin promotes neuronal and behavioral recovery by suppressing inflammatory response and apoptosis in a rat model of intracerebral hemorrhage. Neurochem. Res. 2015, 40, 195–203.
- Liu, J.; He, J.; Huang, Y.; Hu, Z. Resveratrol has an Overall Neuroprotective Role in Ischemic Stroke: A Meta-Analysis in Rodents. Front. Pharmacol. 2021, 12, 795409.
- Bonsack, F.; Alleyne, C.H., Jr.; Sukumari-Ramesh, S. Resveratrol Attenuates Neurodegeneration and Improves Neurological Outcomes after Intracerebral Hemorrhage in Mice. Front. Cell Neurosci. 2017, 11, 228.
- Pacifici, F.; Rovella, V.; Pastore, D.; Bellia, A.; Abete, P.; Donadel, G.; Santini, S.; Beck, H.; Ricordi, C.; Daniele, N.D.; et al. Polyphenols and Ischemic Stroke: Insight into One of the Best Strategies for Prevention and Treatment. Nutrients 2021, 13, 1967.
- Panickar, K.S.; Anderson, R.A. Effect of polyphenols on oxidative stress and mitochondrial dysfunction in neuronal death and brain edema in cerebral ischemia. Int. J. Mol. Sci. 2011, 12, 8181–8207.
- Carito, V.; Venditti, A.; Bianco, A.; Ceccanti, M.; Serrilli, A.M.; Chaldakov, G.; Tarani, L.; De Nicolo, S.; Fiore, M. Effects of olive leaf polyphenols on male mouse brain NGF, BDNF and their receptors TrkA, TrkB and p75. Nat. Prod. Res. 2014, 28, 1970–1984.
- Ferraguti, G.; Terracina, S.; Petrella, C.; Greco, A.; Minni, A.; Lucarelli, M.; Agostinelli, E.; Ralli, M.; de Vincentiis, M.; Raponi, G.; et al. Alcohol and Head and Neck Cancer: Updates on the Role of Oxidative Stress, Genetic, Epigenetics, Oral Microbiota, Antioxidants, and Alkylating Agents. Antioxidants 2022, 11, 145.
- Carito, V.; Ceccanti, M.; Cestari, V.; Natella, F.; Bello, C.; Coccurello, R.; Mancinelli, R.; Fiore, M. Olive polyphenol effects in a mouse model of chronic ethanol addiction. Nutrition 2017, 33, 65–69.
- Nabavi, S.F.; Dean, O.M.; Turner, A.; Sureda, A.; Daglia, M.; Nabavi, S.M. Oxidative stress and post-stroke depression: Possible therapeutic role of polyphenols? Curr. Med. Chem. 2015, 22, 343–351.
- Abdelsalam, S.A.; Renu, K.; Zahra, H.A.; Abdallah, B.M.; Ali, E.M.; Veeraraghavan, V.P.; Sivalingam, K.; Ronsard, L.; Ammar, R.B.; Vidya, D.S.; et al. Polyphenols Mediate Neuroprotection in Cerebral Ischemic Stroke-An Update. Nutrients 2023, 15, 1107.
- Li, Z.; Zhao, T.; Shi, M.; Wei, Y.; Huang, X.; Shen, J.; Zhang, X.; Xie, Z.; Huang, P.; Yuan, K.; et al. Polyphenols: Natural food grade biomolecules for treating neurodegenerative diseases from a multi-target perspective. Front. Nutr. 2023, 10, 1139558.
- Beseler, C.L.; Taylor, L.A.; Kraemer, D.T.; Leeman, R.F. A latent class analysis of DSM-IV alcohol use disorder criteria and binge drinking in undergraduates. Alcohol. Clin. Exp. Res. 2012, 36, 153–161.
- Beseler, C.L.; Taylor, L.A.; Leeman, R.F. An item-response theory analysis of DSM-IV alcohol-use disorder criteria and “binge” drinking in undergraduates. J. Stud. Alcohol. Drugs 2010, 71, 418–423.
- Bhochhibhoya, A.; Hayes, L.; Branscum, P.; Taylor, L. The Use of the Internet for Prevention of Binge Drinking Among the College Population: A Systematic Review of Evidence. Alcohol Alcohol. 2015, 50, 526–535.
- Viner, R.M.; Taylor, B. Adult outcomes of binge drinking in adolescence: Findings from a UK national birth cohort. J. Epidemiol. Community Health 2007, 61, 902–907.
- Woo, D.; Rosand, J.; Kidwell, C.; McCauley, J.L.; Osborne, J.; Brown, M.W.; West, S.E.; Rademacher, E.W.; Waddy, S.; Roberts, J.N.; et al. The Ethnic/Racial Variations of Intracerebral Hemorrhage (ERICH) study protocol. Stroke 2013, 44, e120–e125.
- Poli, L.; Grassi, M.; Zedde, M.; Marcheselli, S.; Silvestrelli, G.; Sessa, M.; Zini, A.; Paciaroni, M.; Azzini, C.; Gamba, M.; et al. Anticoagulants Resumption after Warfarin-Related Intracerebral Haemorrhage: The Multicenter Study on Cerebral Hemorrhage in Italy (MUCH-Italy). Thromb. Haemost. 2018, 118, 572–580.
- Larsson, S.C.; Wallin, A.; Wolk, A.; Markus, H.S. Differing association of alcohol consumption with different stroke types: A systematic review and meta-analysis. BMC Med. 2016, 14, 178.
- Fewel, M.E.; Thompson, B.G., Jr.; Hoff, J.T. Spontaneous intracerebral hemorrhage: A review. Neurosurg. Focus 2003, 15, E1.
- Juvela, S.; Hillbom, M.; Palomaki, H. Risk factors for spontaneous intracerebral hemorrhage. Stroke 1995, 26, 1558–1564.
- Lyden, P.D.; Zivin, J.A.; Soll, M.; Sitzer, M.; Rothrock, J.F.; Alksne, J. Intracerebral hemorrhage after experimental embolic infarction. Anticoagulation. Arch. Neurol. 1987, 44, 848–850.
- Martinez-Quinones, P.; McCarthy, C.G.; Watts, S.W.; Klee, N.S.; Komic, A.; Calmasini, F.B.; Priviero, F.; Warner, A.; Chenghao, Y.; Wenceslau, C.F. Hypertension Induced Morphological and Physiological Changes in Cells of the Arterial Wall. Am. J. Hypertens. 2018, 31, 1067–1078.
- Piano, M.R. Alcohol’s Effects on the Cardiovascular System. Alcohol. Res. 2017, 38, 219–241.
- Briasoulis, A.; Agarwal, V.; Messerli, F.H. Alcohol consumption and the risk of hypertension in men and women: A systematic review and meta-analysis. J. Clin. Hypertens. (Greenwich) 2012, 14, 792–798.
- Piano, M.R.; Thur, L.A.; Hwang, C.L.; Phillips, S.A. Effects of Alcohol on the Cardiovascular System in Women. Alcohol Res. 2020, 40, 12.
- Mori, T.A.; Burke, V.; Beilin, L.J.; Puddey, I.B. Randomized Controlled Intervention of the Effects of Alcohol on Blood Pressure in Premenopausal Women. Hypertension 2015, 66, 517–523.
- Dalle-Donne, I.; Rossi, R.; Colombo, R.; Giustarini, D.; Milzani, A. Biomarkers of oxidative damage in human disease. Clin. Chem. 2006, 52, 601–623.
- Ceni, E.; Mello, T.; Galli, A. Pathogenesis of alcoholic liver disease: Role of oxidative metabolism. World J. Gastroenterol. 2014, 20, 17756–17772.
- Tan, Y.; Li, X.; Prabhu, S.D.; Brittian, K.R.; Chen, Q.; Yin, X.; McClain, C.J.; Zhou, Z.; Cai, L. Angiotensin II plays a critical role in alcohol-induced cardiac nitrative damage, cell death, remodeling, and cardiomyopathy in a protein kinase C/nicotinamide adenine dinucleotide phosphate oxidase-dependent manner. J. Am. Coll. Cardiol. 2012, 59, 1477–1486.
- Huang, L.C.; Liew, H.K.; Cheng, H.Y.; Kuo, J.S.; Hsu, W.L.; Pang, C.Y. Brain Magnetic Resonance Imaging of Intracerebral Hemorrhagic Rats after Alcohol Consumption. J. Stroke Cerebrovasc. Dis. 2018, 27, 3493–3502.
- Cheng, H.Y.; Huang, L.C.; Peng, H.F.; Kuo, J.S.; Liew, H.K.; Pang, C.Y. Delayed formation of hematomas with ethanol preconditioning in experimental intracerebral hemorrhage rats. Ci Ji Yi Xue Za Zhi 2018, 30, 5–9.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
617
Revisions:
2 times
(View History)
Update Date:
13 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




