Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Victoria Samanidou | -- | 1704 | 2024-03-11 16:00:20 | | | |
| 2 | Peter Tang | Meta information modification | 1704 | 2024-03-12 02:23:45 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Samanidou, V.; Kabir, A. Applications of Magnet Integrated Fabric Phase Sorptive Extraction. Encyclopedia. Available online: https://encyclopedia.pub/entry/56120 (accessed on 08 February 2026).
Samanidou V, Kabir A. Applications of Magnet Integrated Fabric Phase Sorptive Extraction. Encyclopedia. Available at: https://encyclopedia.pub/entry/56120. Accessed February 08, 2026.
Samanidou, Victoria, Abuzar Kabir. "Applications of Magnet Integrated Fabric Phase Sorptive Extraction" Encyclopedia, https://encyclopedia.pub/entry/56120 (accessed February 08, 2026).
Samanidou, V., & Kabir, A. (2024, March 11). Applications of Magnet Integrated Fabric Phase Sorptive Extraction. In Encyclopedia. https://encyclopedia.pub/entry/56120
Samanidou, Victoria and Abuzar Kabir. "Applications of Magnet Integrated Fabric Phase Sorptive Extraction." Encyclopedia. Web. 11 March, 2024.
Copy Citation
In 2014, Kabir and Furton invented fabric phase sorptive extraction (FPSE), which was considered as a new breakthrough in microextraction technologies at that time, while two years later, the same research group introduced an advantageous innovative configuration of FPSE that combines stirring and extraction mechanism into a single sample preparation device, keeping all benefits originally offered by FPSE. Magnet integrated fabric phase sorptive extraction (MI-FPSE) was eventually emerged as a new, advantageous implementation of FPSE.
green sample preparation
sorptive extraction
fabric phase sorptive extraction
FPSE
magnet integrated fabric phase sorptive extraction
MI-FPSE
1. Introduction
Due to the increasing concern of consumers as well as different regulatory agencies and environmental advocacy groups, Green(er) sample preparation technologies remain as the primary goal of any new sample preparation technology. Separation scientists across the word are working hard to develop new sample preparation technologies that would comply with the Green Analytical Chemistry (GAC) demands [1]. Green Analytical Chemistry, emerged in 2000, is an offshoot of Green Chemistry that advocates for making analytical laboratory practices more environmentally friendly. GAC has formulated 12 principles that include inhibition of waste, use of safer solvents, design for energy efficiency, decrease derivatization, and improve safety of the operator, among others.
To follow this trend, microextraction techniques are continuously evolving to simplify sample preparation workflow that would be better aligned with the green analytical chemistry principles.
In 2014, Kabir and Furton invented fabric phase sorptive extraction (FPSE) [2][3] which was considered as a new breakthrough in microextraction technologies at that time, while two years later, the same research group introduced an advantageous innovative configuration of FPSE that combines stirring and extraction mechanism into a single sample preparation device, keeping all benefits originally offered by FPSE. Magnet integrated fabric phase sorptive extraction (MI-FPSE) was eventually emerged as a new, advantageous implementation of FPSE [4][5]. MI-FPSE, by virtue of its integrated magnet, provides rapid diffusion of the sample matrix and consequently quicker extraction equilibrium. As a result, it reduces the overall time needed for the sample preparation and improves the quality of the analytical data by improving the accuracy and precision of the sample preparation process. MI-FPSE also reassures the need for integrating processes that fulfil all Green Analytical Chemistry requirements [1].
2. Fabric Phase Sorptive Extraction (FPSE)
Fabric phase sorptive extraction was invented to address most of the sample preparation challenges that other sorptive microextraction techniques failed to offer individually. It unites the extraction principles of solid phase microextraction (SPME) (equilibrium-based extraction) and solid phase extraction (SPE) (exhaustive extraction). FPSE has adopted sol-gel coating technology for the sorbent coating on a flexible, porous fabric such as cotton/polyester/fiberglass substrate. Sol-gel coating technology chemically attaches the organic/organic-inorganic hybrid polymer to the fabric substrate by means of a sol-gel linker that results in unprecedented thermal, chemical, and solvent stability to the sorbent coating with pH stability ~1–13. The sol-gel coating technology, unlike classical polymer coating and immobilization technique used in SPME or SBSE, is a highly controllable chemical deposition process that provides unprecedented batch-to-batch reproducibility in the sorbent coating process. It is worthy to mention that extraction reproducibility between different SPME fibers and Twister stir bars are among many challenges the analysts face when using SPME or SBSE as the sample preparation techniques. In addition to superior batch to batch coating reproducibility in FPSE membranes, the porous morphology of the sol-gel sorbent facilitates the rapid permeation of the sample through the sorbent as well as the FPSE membrane and the analytes get extracted onto the sol-gel sorbent coated FPSE membrane via numerous analyte-sorbent intermolecular/ionic interactions. FPSE has developed more than 30 different sorbent chemistries that include polar, non-polar, medium polar, cation exchanger, anion exchanger, mixed mode cation exchanger, mixed mode anion exchanger and zwitterionic multi-mode sorbents. Depending on the nature of the target analytes, FPSE sorbents exploit London dispersion, dipole–dipole interaction, π-π interaction, hydrogen bonding and electrostatic interactions. Only very small volumes of organic solvent (100–500 µL) are sufficient to achieve quantitative analyte back-extraction. The small volumes of the organic solvent as the eluent ensures high preconcentration factor and remove the need of evaporation and sample reconstitution, thus minimizes analyte loss, and increases overall absolute recovery of the analytes [6].
3. Magnet Integrated Fabric Phase Sorptive Extraction (MI-FPSE)
MI-FPSE is an upgraded format of FPSE that was created (a) to improve the extraction reproducibility; (b) to expedite the analyte mass transfer from the bulk of the sample solution; (c) to eliminate the necessity of an external magnet and (d) to use more than one sorbent chemistry simultaneously in the same device in order to expand the extractive efficiency, in the case of various target analytes of different properties present in the sample (neutral, acidic, basic, polar, nonpolar). Due to the precise control of the spinning rate (rpm), MI-FPSE demonstrates higher reproducibility and improved absolute recovery values. The seamless and precisely controllable stirring speed facilitates the rapid permeation of the sample matrix through the FPSE membrane in MI-FPSE. These result in faster analyte mass transfer from the bulk of the solution to the FPSE membrane and consequently improves the rate of extraction in comparison to classical FPSE mode. Additionally, extraction equilibrium is achieved in substantially shorter time [4]. Compared to classical microextraction techniques, another major advantage of MI-FPSE is the ability to change the disk size from ¼” to 2” depending on the sample size. As such, a smaller MI-FPSE device is preferred for biological samples, whereas a large MI-FPSE device is recommended for environmental samples when large volume of sample can be collected easily.
Figure 1 presents the images of FPSE membranes, MI-FPSE devices and the extraction process.
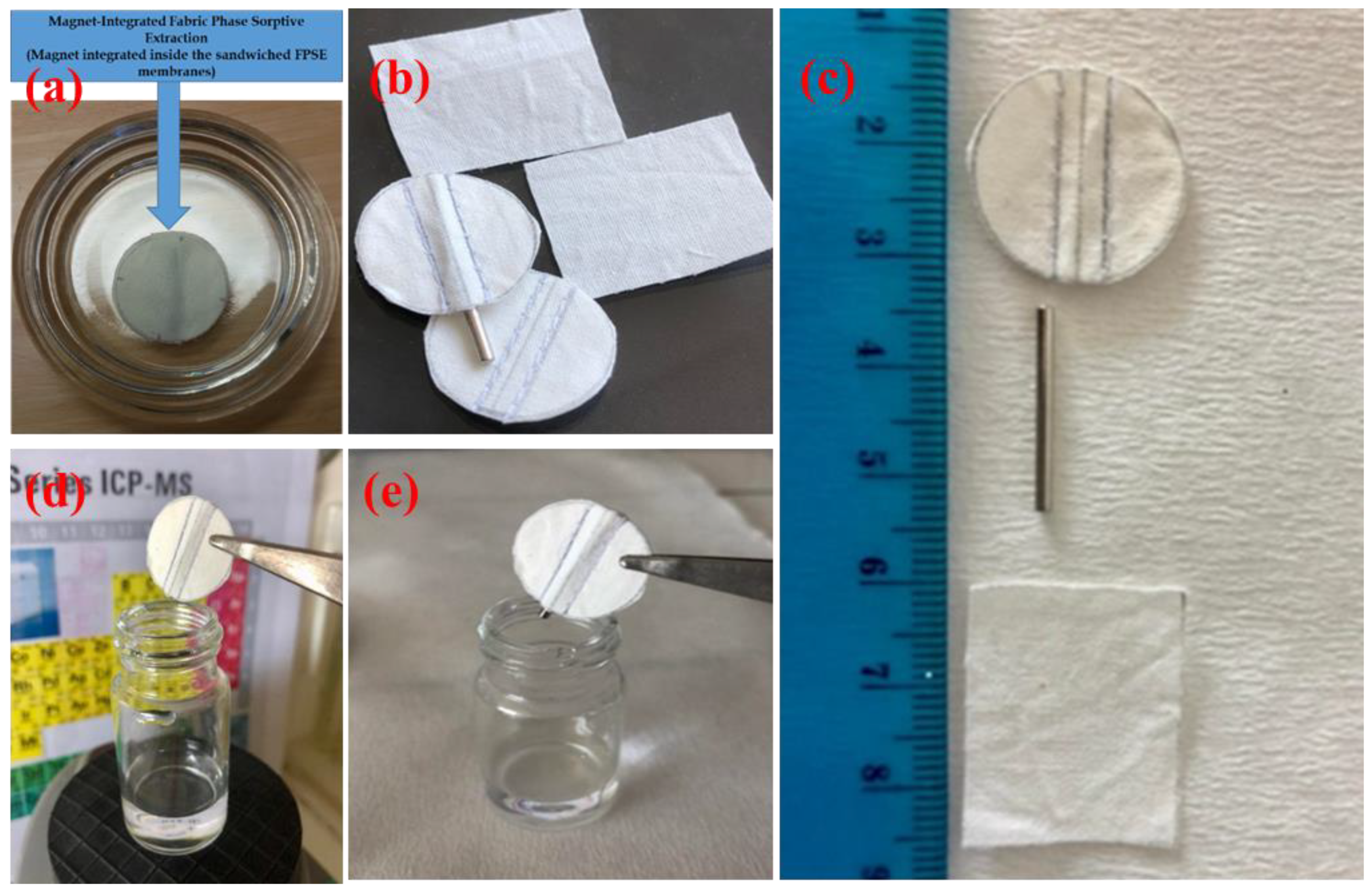
Figure 1. Fabric phase sorptive extraction and magnet integrated fabric phase sorptive extraction (MI-FPSE) membranes: (a) image of an MI-FPSE membrane; (b) side-by side images of classical FPSE membranes and MI-FPSE membranes; (c) size comparison between classical FPSE membrane and MI-FPSE membrane; (d) extraction operation of MI-FPSE; (e) desorption operation in MI-FPSE.
4. Mechanism of Extraction
Unlike other microextraction techniques, MI-FPSE is unique in a sense that it incorporates the extraction mechanism of solid phase microextraction (equilibrium-based extraction) and solid phase extraction (exhaustive extraction) by design. MI-FPSE membranes are porous and permeable. The sol-gel sorbent coating does not obstruct the through pores of the fabric substrate. As a result, when MI-FPSE device spins inside the aqueous sample matrix on a magnetic stirrer, it creates a strong vortex that forces aqueous samples to permeate through the MI-FPSE membrane, resulting in rapid analyte mass transfer from the bulk of the aqueous sample to the sol-gel sorbent coated on the membrane. The MI-FPSE membrane behaves like an SPE disk during the extraction process. At the same time, as the FPSE membrane is submerged into the sample matrix, it acts like an SPME fiber in its direct immersion extraction mode. Analytes diffuses from the bulk of the solution towards the MI-FPSE membrane and slowly establishes equilibrium. The combination of the extraction mechanism of SPME and SPE by design is one of the key advantages of MI-FPSE that is absent in other microextraction techniques. In addition, the overall selectivity of MI-FPSE is determined by (i) the substrate surface chemistry (hydrophobic or hydrophilic); (ii) nature of the functional group connected to the sol-gel precursor used in the sol solution formulation; and (iii) nature of the organic/inorganic polymer used in the sol solution. As a result, the selectivity of the MI-FPSE can be fine-tuned based on the properties of the target analytes [6]. This unique advantage is absent in classical microextraction techniques where pristine polymer (such as polydimethyl siloxane, polyethylene glycol) is the only source of the selectivity parameter that cannot be changed or modified.
Sol-gel based sorbents are intrinsically porous with sponge-like internal structure that provides very high specific surface area. As a result, a small volume of organic solvent (100–500 µL) can exhaustively elute the adsorbed analytes from the MI-FPSE membrane in minutes. The low volume of the eluent provides higher preconcentration factor and eliminates the requirement of solvent evaporation and sample reconstitution from the sample preparation workflow. As such, MI-FPSE is considered as a lean sample preparation technique.
A detailed, step-by-step procedure for creating FPSE membranes are presented elsewhere [7]. Several review articles on FPSE can also be consulted for obtaining a thorough insight of this emerging technology [8][9][10].
5. Applications
The first application is a method developed by Alampanos et al. in 2021 [11] and refers to the magnet integrated fabric phase sorptive extraction of selected endocrine disrupting chemicals from human urine and subsequent analysis by high-performance liquid chromatography–photodiode array detection.
Manousi et al. in 2022 [4] proposed the use of magnet integrated fabric phase sorptive extraction as a stand-alone extraction device to monitor benzoyl urea insecticides in water samples by high performance liquid chromatography-diode array detection (HPLC-DAD). Benzoyl urea insecticides, a group of pesticides often used in agriculture, that are persistent in environmental samples and therefore their monitoring is necessary to prevent adverse effects to human health and the environmental ecosystem. In this work, the application potential of MI-FPSE was assessed for the first time in environmental pollution monitoring. More specifically MI-FPSE was employed for the extraction and preconcentration of several benzoyl urea insecticides including diflubenzuron, triflumuron, hexaflumuron, lufenuron and chlorfluazuron from environmental water samples followed by their chromatographic determination by high performance liquid chromatography-diode array detection (HPLC-DAD). The most important parameters that may affect the performance of the newly developed methodology were comprehensively optimized and the MI-FPSE-HPLC-DAD method was validated.
Since the method favours handling of relatively high sample volume, high preconcentration factors can be achieved easily.
The validated method was then successfully employed for the analysis of several environmental samples including tap, mineral, river, and lake water samples. Additionally, the ComplexGAPI [12] evaluator tool was used to demonstrate the green potential of developed method.
Last but not the least, in 2022 Manousi et al. [5] demonstrated also the applicability of MI-FPSE in food analysis. In this work, MI-FPSE using a sol–gel poly(tetrahydrofuran) coated FPSE cellulose membrane was applied for the first time to selectively extract six triazine herbicides from herbal infusions prior to their determination by HPLC. After optimization of all crucial parameters that affect adsorption and desorption steps, the developed method was validated in terms of accuracy, precision, linearity, limits of detection (LODs) and limits of quantification (LOQs). Finally, the methodology was successfully applied for the analysis of several herbal infusion samples.
References
- Gałuszka, A.; Migaszewski, Z.; Namiesnik, J. The 12 principles of green analytical chemistry and the SIGNIFICANCE mnemonic of green analytical practices. TrAC Trends Anal. Chem. 2013, 50, 78–84.
- Kumar, R.; Gaurav; Heena; Malik, A.K.; Kabir, A.; Furton, K.G. Efficient analysis of selected estrogens using fabric phase sorptive extraction and high performance liquid chromatography-fluorescence detection. J. Chromatogr. A 2014, 1359, 16–25.
- Kabir, A.; Furton, K.G. Fabric Phase Sorptive Extractors; United States Patents and Trademark Office: Alexandria, VA, USA, 2016.
- Manousi, N.; Alampanos, V.; Ferracane, A.; Efstratiadis, G.; Kabir, A.; Furton, K.G.; Tranchida, P.Q.; Zachariadis, G.A.; Mondello, L.; Rosenberg, E.; et al. Magnet integrated fabric phase sorptive extraction as a stand-alone extraction device for the monitoring of benzoyl urea insecticides in water samples by HPLC-DAD. J. Chromatogr. A 2022, 1672, 463026.
- Manousi, N.; Kabir, A.; Furton, K.G.; Zachariadis, G.A.; Rosenberg, E. Expanding the applicability of magnet integrated fabric phase sorptive extraction in food analysis: Extraction of triazine herbicides from herbal infusion samples. Microchem. J. 2022, 179, 107524.
- Kabir, A.; Furton, K.G.; Malik, A. Innovations in sol-gel microextraction phases for solvent-free sample preparation in analytical chemistry. TrAC Trends Anal. Chem. 2013, 45, 197–218.
- Kabir, A.; Mesa, R.; Jurmain, J.; Furton, K.G. Fabric Phase Sorptive Extraction Explained. Separations 2017, 4, 21.
- Zilfidou, E.; Kabir, A.; Furton, K.G.; Samanidou, V. Fabric Phase Sorptive Extraction: Current State of the Art and Future Perspectives. Separations 2018, 5, 40.
- Kazantzi, V.; Anthemidis, A. Fabric Sol–gel Phase Sorptive Extraction Technique: A Review. Separations 2017, 4, 20.
- Kabir, A.; Locatelli, M.; Ulusoy, H.I. Recent Trends in Microextraction Techniques Employed in Analytical and Bioanalytical Sample Preparation. Separations 2017, 4, 36.
- Alampanos, V.; Kabir, A.; Furton, K.; Samanidou, V. Magnet integrated fabric phase sorptive extraction of selected endocrine disrupting chemicals from human urine followed by high-performance liquid chromatography – photodiode array analysis. J. Chromatogr. A 2021, 1654, 462459.
- Płotka-Wasylka, J.; Wojnowski, W. Complementary green analytical procedure index (ComplexGAPI) and software. Green Chem. 2021, 23, 8657–8665.
More
Information
Subjects:
Chemistry, Analytical
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
520
Revisions:
2 times
(View History)
Update Date:
12 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




